Separation of mixture of Thymol and Menthol Identification





- Slides: 10

Separation of mixture of Thymol and Menthol Identification of each

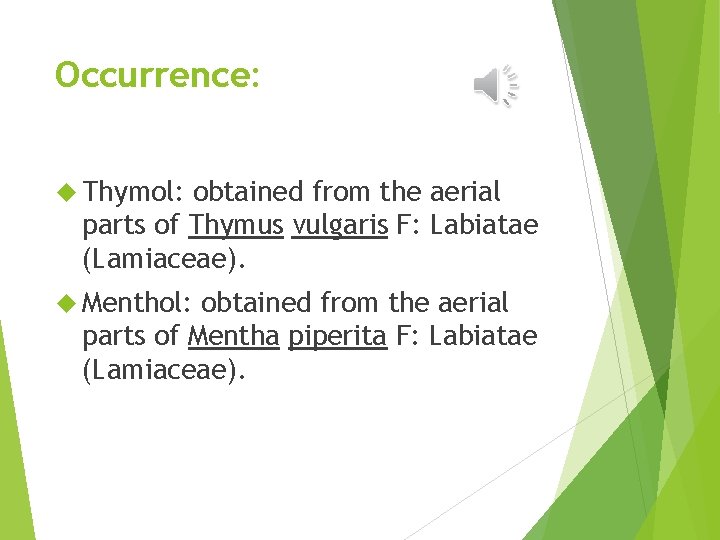
Occurrence: Thymol: obtained from the aerial parts of Thymus vulgaris F: Labiatae (Lamiaceae). Menthol: obtained from the aerial parts of Mentha piperita F: Labiatae (Lamiaceae).

Uses: Thymol is antiseptic, disinfectant and preservative. Menthol is germicidal, used to treat itching of the surface, cellular inflammations, and local pains.

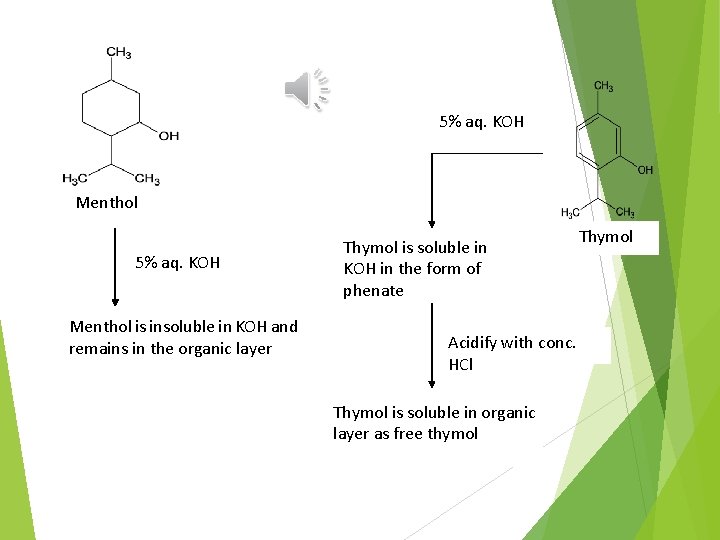
Principle: Separation depends on the fact that thymol forms water soluble phenate with alkali potassium hydroxide being a phenol, while menthol which is terpene alcohol is insoluble in alkali and remains in the organic layer.

5% aq. KOH Menthol is insoluble in KOH and remains in the organic layer Thymol is soluble in KOH in the form of phenate Acidify with conc. HCl Thymol is soluble in organic layer as free thymol Thymol

Separatory funnel

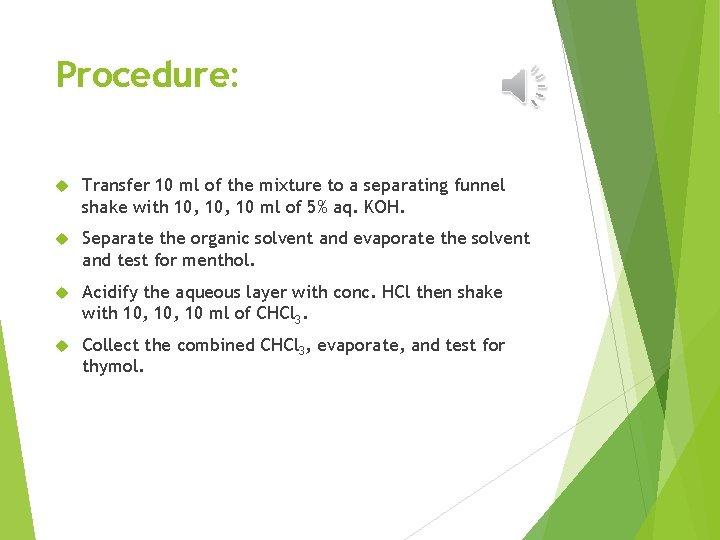
Procedure: Transfer 10 ml of the mixture to a separating funnel shake with 10, 10 ml of 5% aq. KOH. Separate the organic solvent and evaporate the solvent and test for menthol. Acidify the aqueous layer with conc. HCl then shake with 10, 10 ml of CHCl 3. Collect the combined CHCl 3, evaporate, and test for thymol.

Thymol crystals Menthol crystals

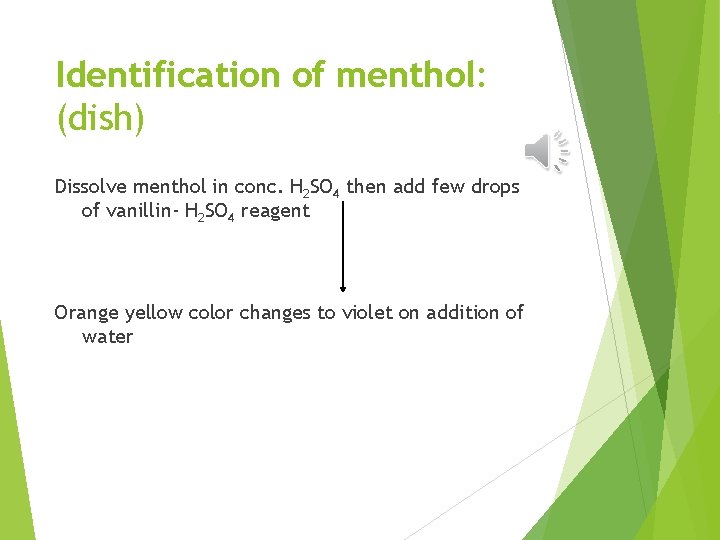
Identification of menthol: (dish) Dissolve menthol in conc. H 2 SO 4 then add few drops of vanillin- H 2 SO 4 reagent Orange yellow color changes to violet on addition of water

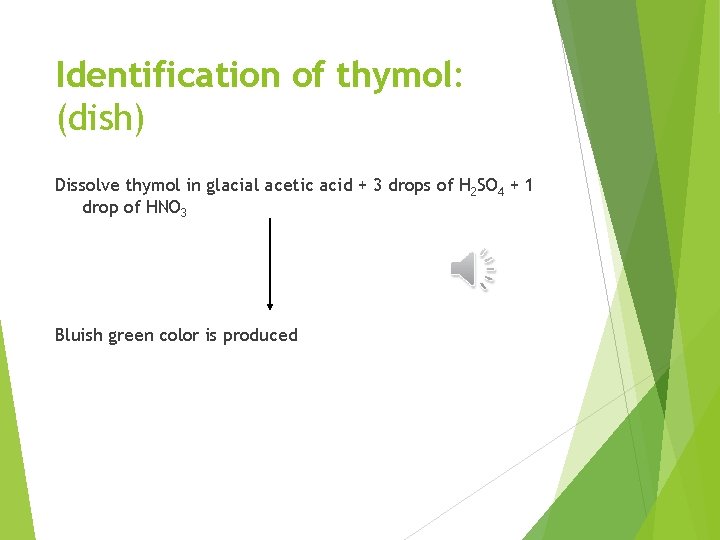
Identification of thymol: (dish) Dissolve thymol in glacial acetic acid + 3 drops of H 2 SO 4 + 1 drop of HNO 3 Bluish green color is produced
 In the mixing of thymol and menthol
In the mixing of thymol and menthol Homogeneous mixture and heterogeneous mixture class 9
Homogeneous mixture and heterogeneous mixture class 9 Schudmengsel
Schudmengsel Why is adding menthol to cigarettes dangerous?
Why is adding menthol to cigarettes dangerous? Basis polietilenglikol inkompatibel dengan bahan obat ini
Basis polietilenglikol inkompatibel dengan bahan obat ini Stemless funnel function
Stemless funnel function Separation of mixture
Separation of mixture Evaporation separating mixtures
Evaporation separating mixtures Salol thymol phase diagram
Salol thymol phase diagram Equilibrium involving thiocyanatoiron (iii) ion
Equilibrium involving thiocyanatoiron (iii) ion Positive identification
Positive identification