Medication Safety An Ounce of Prevention H Gwen








































- Slides: 54

Medication Safety: An Ounce of Prevention H. Gwen Bartlett, BS Pharmacy, Pharm. D, BCPS, BCCCP Assistant Professor of Pharmacy Practice Cardiology Specialty Husson University Bangor, ME 1

Disclosure I have no relevant financial or non-financial conflicts of interest to disclose. 2

“Dying from a disease is sometimes unavoidable; dying from a medicine is unacceptable. ” • Lepakhin V. Geneva 2005 3

Learning Objectives l l Distinguish between a preventable verses a nonpreventable adverse drug event. Describe the role that a latent failure (blunt end) may play in an active failure (sharp end) with respect to medication errors. Evaluate how the recent national initiatives focused on improving medication safety for high-risk patient populations might impact your practice. Summarize the potential impact of low health literacy on medication safety. 4

Patients (our customers, friends, family and…. ourselves) Are At Risk! l l l Between 44, 000 and 98, 000 deaths are attributed to medical errors in U. S. hospitals 7, 000 deaths annually attributable to medication errors Serious and PREVENTABLE errors are occurring Kohn KT, Corrigan JM, Donaldson MS, To Err Is Human: Building a Safer Health System. National Academies Press; Washington, DC 1999; http: //www. nap. edu/openbook. php? record_id=9728 Accessed 9/1/15 5

Why is Medication Safety Important to Pharmacy Practice? l l l 2014 Pharmacy Technician Certification Board → 1 hr CEU in Patient Safety for recertification Since 2006, included in Rx school standards 9 states currently require Patient Safety CEU for annual pharmacist relicensure (i. e. , Delaware, District of Columbia, Florida, Iowa, Maryland, New Mexico, Pennsylvania, New York, and Ohio effective 1/15/16) http: //www. nap. edu/catalog/10681/health-professions-education-a-bridge-to-quality https: //www. ptcb. org/about-ptcb/news-room/news-landing/2014/04/21/ptcb-adds-new-patient-safety-cerequirement-for-recertification#. Ve 1 k 9 h. HBz. RY Accessed 9/1/15 6

Medication-Related Errors: Consistently Rank at the Top of Medical Errors l Reiterated significance of medication errors l l 1 medication error per patient per day of hospitalization Naming (look-alike, soundalike), labeling, packaging account for: l 33% medication errors l 30% fatalities Aspden P, et al; Preventing Medication Errors; Washington DC; National Academies Press; Washington, DC 7 2007 http: //www. nap. edu/catalog/11623/preventing-medication-errors-quality-chasm-series Accessed 9/1/15

The Numbers Speak l l 3 billion prescriptions written annually In 2006, 82% of U. S. adults reported taking at least 1 medication l l 29% take 5 or more Over age 65: l l l 57 -59% take 4 -9 and 17 -19% take 10 or more 1. 5 million preventable ADEs/yr → cost ~ $4 billion National Action Plan for Adverse Drug Event Prevention U. S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. (2014); Washington, DC. http: //health. gov/hcq/pdfs/ADE-Action-Plan-508 c. pdf Accessed 9/1/2015 8

Medication Error “Any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of a health care professional, patient, or consumer……. . ” National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP); http: //www. nccmerp. org/about-medication-errors Accessed 9/5/15 9

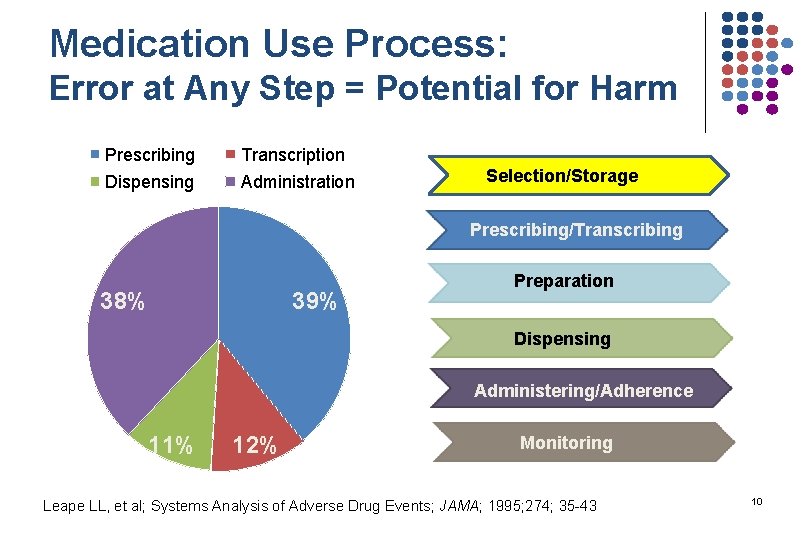
Medication Use Process: Error at Any Step = Potential for Harm Prescribing Transcription Dispensing Administration Selection/Storage Prescribing/Transcribing 38% 39% Preparation Dispensing Administering/Adherence 11% 12% Monitoring Leape LL, et al; Systems Analysis of Adverse Drug Events; JAMA; 1995; 274; 35 -43 10

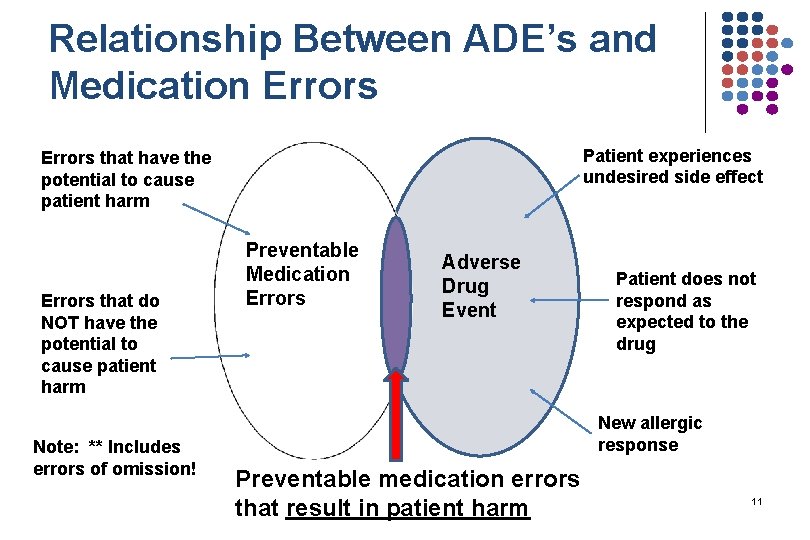
Relationship Between ADE’s and Medication Errors Patient experiences undesired side effect Errors that have the potential to cause patient harm Errors that do NOT have the potential to cause patient harm Note: ** Includes errors of omission! Preventable Medication Errors Adverse Drug Event Patient does not respond as expected to the drug New allergic response Preventable medication errors that result in patient harm 11

Close Calls Are Wake Up Calls! l Near misses are preventable errors that don’t make it to the patient l Safety nets worked l Near misses should be reported (but seldom are). Those with potential for severe outcomes should be examined: root cause analysis l Learn from other’s mistakes → teach others from them 12

Preventable or Non-preventable or Near Miss? 1. Hyperkalemia (serum K+ 5. 2 m. Eq/L) on routine follow-up basic metabolic panel in a 48 y. o. female with an 8 year history of Type II DM initiated on lisinopril 10 mg PO daily 2 weeks ago following a second screening albuminuria level greater than 300 mg/day. 2. Trimethoprim/sulfamethoxazole ordered for presumed uncomplicated UTI in a 54 y. o. female, admitted for dofetilide loading, which precipitated a pharmacist contacting the prescriber to recommend a change in therapy to nitrofurantoin 100 mg PO BID. 3. A left popliteal proximal DVT identified during bilateral lower extremity ultrasound in a 38 y. o. male with idiopathic dilated cardiomyopathy admitted for an acute exacerbation of heart failure 5 days ago and placed on bed rest without DVT prophylaxis. 13

Learning Objectives l l Distinguish between a preventable verses a nonpreventable adverse drug event. Describe the role that a latent failure (blunt end) may play in an active failure (sharp end) with respect to medication errors. Evaluate how the recent national initiatives focused on improving medication safety for high-risk patient populations might impact your practice. Summarize the potential impact of low health literacy on medication safety. 14

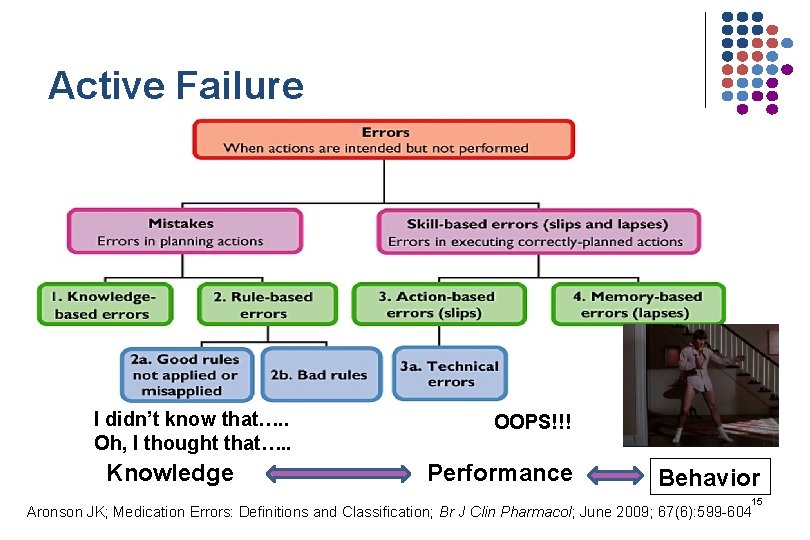
Active Failure I didn’t know that…. . Oh, I thought that…. . Knowledge OOPS!!! Performance Behavior 15 Aronson JK; Medication Errors: Definitions and Classification; Br J Clin Pharmacol; June 2009; 67(6): 599 -604

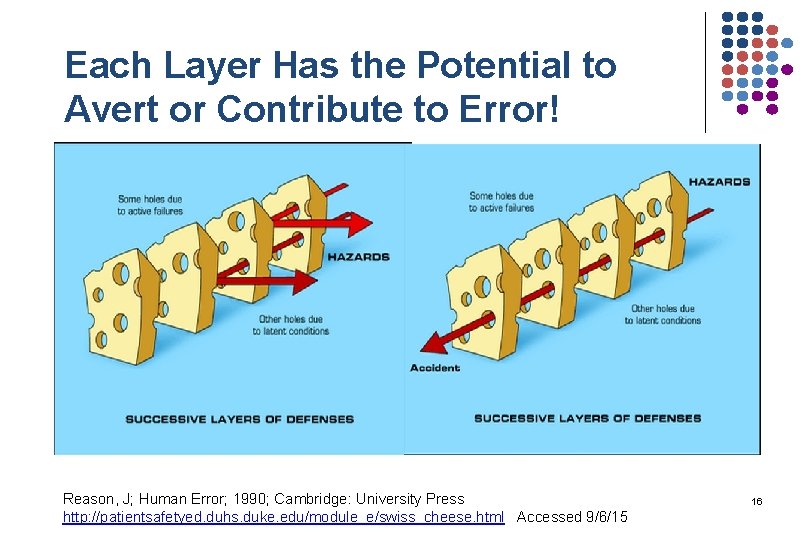
Each Layer Has the Potential to Avert or Contribute to Error! Reason, J; Human Error; 1990; Cambridge: University Press http: //patientsafetyed. duhs. duke. edu/module_e/swiss_cheese. html Accessed 9/6/15 16

Active or Latent Failure? Phlebotomist fails to identify the patient by 2 distinct identifiers and obtains an INR on the wrong patient. l Active (error occurred): mistake → skill-based error or slip (i. e. , oops); rule-based (i. e. , double identify good rule, but not applied; or risky business? Per-diem pharmacist filling in for an independent Rx in rural Maine notes the shelves are organized by trade name. Brintellix and Brilinta are stored next to each other on the shelf. l Latent (error waiting to occur): FDA advisory re: name confusion between vortioxetine and ticagrelor 7/30/15 17

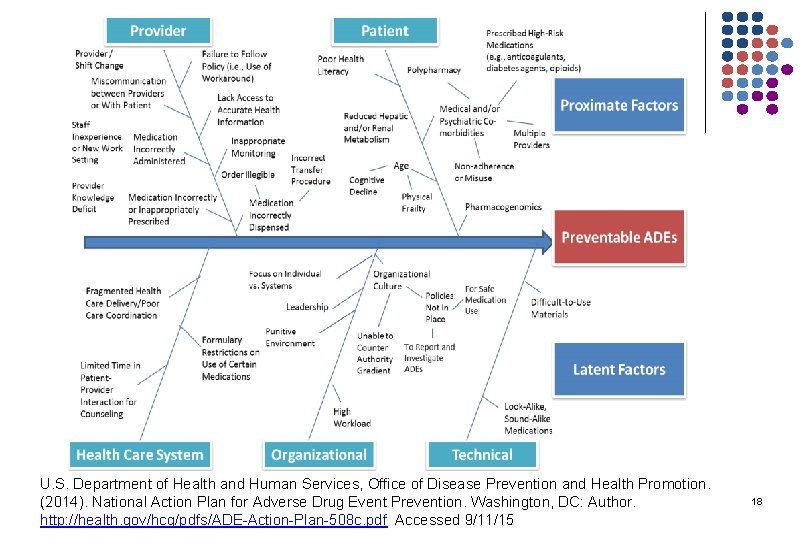
U. S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. (2014). National Action Plan for Adverse Drug Event Prevention. Washington, DC: Author. http: //health. gov/hcq/pdfs/ADE-Action-Plan-508 c. pdf Accessed 9/11/15 18

Following assessment of the patient, development and completion of a formal SOAP note, and determination of an appropriate dosing regimen, the pharmacist forgets to enter the order for vancomycin into the computer which goes unrecognized until the following day. This medication error would BEST be characterized as: A. An example or an active failure; action-based error B. An example of a latent failure; ineffective training C. An example of an active failure; rule-based error D. An example of a latent failure; uncertainty in role or responsibility ANSWER A: With the circumstances given, there is not suggestion of any deficiency in training, environment, or equipment etc which are often contributors to latent failure. Therefore, both B and D would be incorrect. So, since is an active error, one must characterize whethere were likely rules in place for this procedure or simply an 20 intended action that wasn’t carried out (i. e. , slip). The slip or lapse seems to fit this scenario best.

Error is the inevitable downside of having a brain! WHO 2010 21

Learning Objectives l l Distinguish between a preventable verses a nonpreventable adverse drug event. Describe the role that a latent failure (blunt end) may play in an active failure (sharp end) with respect to medication errors. Evaluate how the recent national initiatives focused on improving medication safety for high-risk patient populations might impact your practice. Summarize the potential impact of low health literacy on medication safety. 22

“Patients’ best source (and often ONLY source) of information regarding the medications they have been prescribed is on the prescription container label. ” “Lack of universal standards for labeling on dispensed prescription containers is a root cause for patient misunderstanding, nonadherence, and medication errors. ” USP 36: General Chapter <17>: Prescription Container Labeling; 23

WE NEED TO LOSE THE SPOON!! 24

White Paper & Policy Statement m. L Pediatrics; 135(4); April 2015 25

All Oral Liquid Dosing Devices Should Use METRIC dosing only! l National Alert Network (NAN) l Anticipated USP <17> update l Graduations “shall be legible and indelible, and the associated volume markings shall be in metric units and limited to a single measurement scale that corresponds with the dose instructions on the prescription container label” http: //www. ismp. org/NAN/files/NAN-20150630. pdf Accessed 9/7/15 26

WHAT’S IN A LABEL? (HUMAN/PATIENT-CENTERED DESIGN) 27

The Essential Elements of A Drug Facts Label! http: //www. fda. gov/Drugs/Resources. For. You/ucm 133411. htm 28

USP <17> Official Standard Universal Approach to Prescription Labeling l l Organize → reflect how patients seek out and understand; Minimize! May 1, 2013 Emphasize instructions Simplify language → clear, concise, familiar Explicit instruction (not implicit or implied) l l l Separate dose from timing 2 → not two Avoid numeracy (no HOURLY times) USP General Chapter <17>: Prescription Container Labeling http: //www. usp. org/uspnf/key-issues/usp-nf-general-chapter-prescription-container-labeling/download-usp-nfgeneral-chapter-prescription-container Accessed 9/1/2015 29

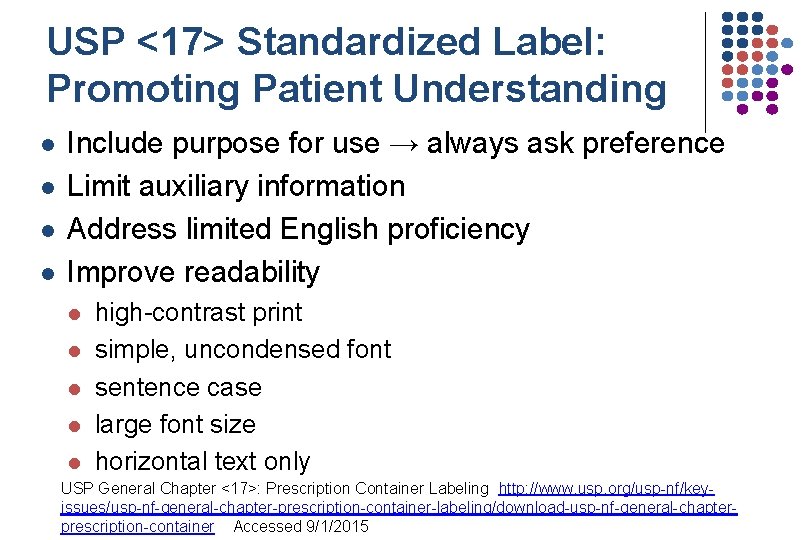
USP <17> Standardized Label: Promoting Patient Understanding l l Include purpose for use → always ask preference Limit auxiliary information Address limited English proficiency Improve readability l l l high-contrast print simple, uncondensed font sentence case large font size horizontal text only USP General Chapter <17>: Prescription Container Labeling http: //www. usp. org/usp-nf/keyissues/usp-nf-general-chapter-prescription-container-labeling/download-usp-nf-general-chapterprescription-container Accessed 9/1/2015

The Problem Take one tablet orally once every day. Take 1 tablet by mouth every morning. Take one tablet for cholesterol. Dozens of Different Ways to Say “Take 1 tablet a day. ” Take 1 tablet 1 time daily. Take ONE (1) tablet by mouth daily. Take one pill by mouth at bedtime. Take 1 tablet one time each day. Take one tablet by mouth once daily. Take one pill by mouth once each day. Bailey, SC et al; Comparison or Handwritten and Electronically Generated Prescription Drug Instructions; Ann Pharmacother; Jan 2009; 43(1): 151 -2 31

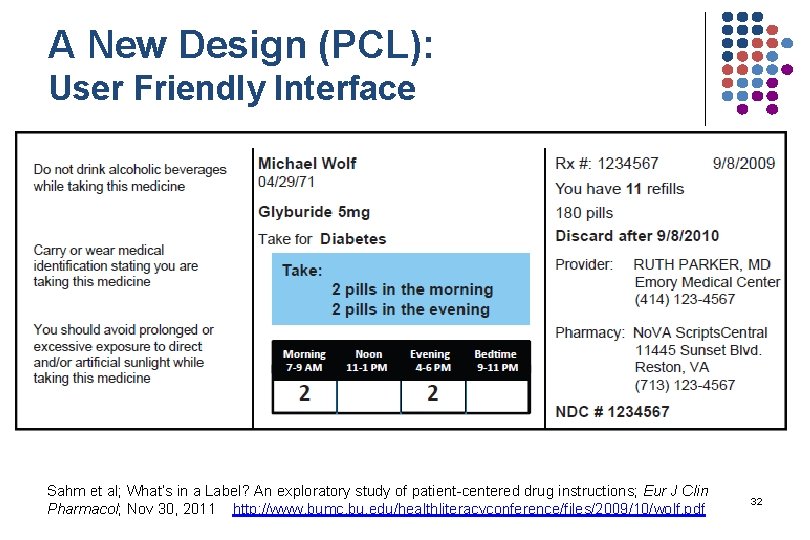
A New Design (PCL): User Friendly Interface Sahm et al; What’s in a Label? An exploratory study of patient-centered drug instructions; Eur J Clin Pharmacol; Nov 30, 2011 http: //www. bumc. bu. edu/healthliteracyconference/files/2009/10/wolf. pdf 32

THE PROBLEM WITH “APAP” 33

ISMP Unsafe Drug Name Abbreviations List Updated to Include: l APAP (added in 2012) l l Not recognized as acetaminophen No. AC (added in 2015) l No anticoagulant https: //www. ismp. org/tools/errorproneabbreviations. pdf 34

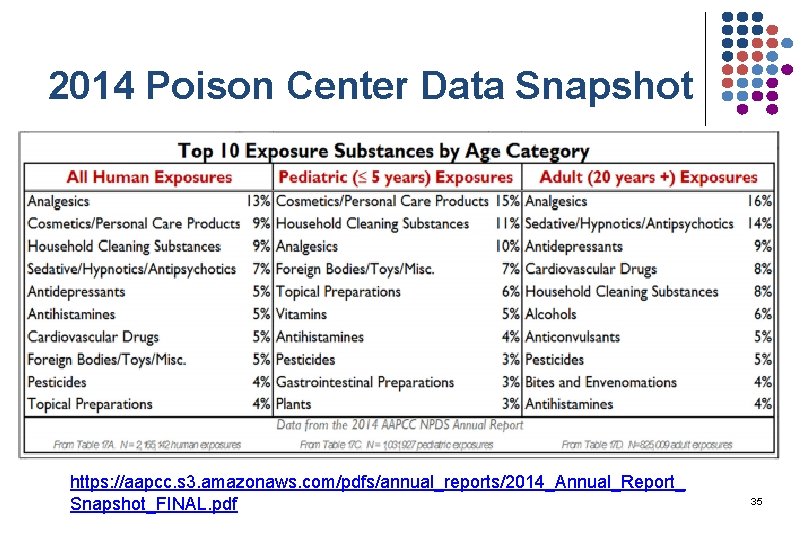
2014 Poison Center Data Snapshot https: //aapcc. s 3. amazonaws. com/pdfs/annual_reports/2014_Annual_Report_ Snapshot_FINAL. pdf 35

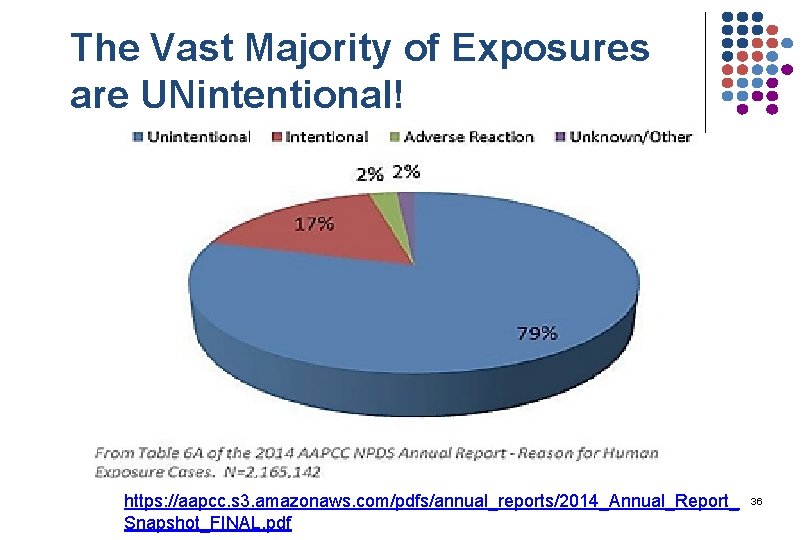
The Vast Majority of Exposures are UNintentional! https: //aapcc. s 3. amazonaws. com/pdfs/annual_reports/2014_Annual_Report_ Snapshot_FINAL. pdf 36

Minimizing Unintentional Acute Liver Failure From Acetaminophen l l l August 2011 → NCPDP White Paper re: acetaminophen: completely spell out all active ingredients January 2013 → Standard concomitant use + liver warning label on Rx in harmony w/ OTC l Prioritized to print in top 3 warning labels January 2014 → FDA mandated manufacturer compliance with 325 mg limit per tab/cap for all prescription products l Does NOT apply to OTC NCPDP Recommendations for Improved Prescription Container Labels for Medicines Containing Acetaminophen; January 2013 1. 1; http: //ncpdp. org/NCPDP/media/pdf/wp/NCPDPacetaminophen WPv 1. 1_31 jan 2013. pdf 37 Accessed 09/5/2015

Movement In the Right Direction re: OTC Acetaminophen! l August 2015, FDA issued guidance document for OTC oral liquid products containing acetaminophen l Does NOT establish legally enforceable responsibilities l Key recommendations labeled for use in < 12 y. o. : l Single-ingredient: “ 160 mg/5 m. L” or “ 160 mg per 5 m. L” l Images of child should be appropriately representative l Dosing directions only in m. L l Product package should include dosage delivery device l Dosage delivery device calibrated units expressed as m. L http: //www. fda. gov/downloads/Drugs/Guidance. Compliance. Regulatory. Information/Guida 38 nces/UCM 417568. pdf

Upcoming Challenges l l l 2014 U. S. Access Board Best Practices → patient -centered labeling for visually impaired Market 21 million blind and millions more with low vision Label → affixed to container, not folded, and not obscure original typed label; font > 18 http: //www. accessamed. com/downloads/Accessa. Med%20 White%20 Paper%202013. pdf 39

Learning Objectives l l Distinguish between a preventable verses a nonpreventable adverse drug event. Describe the role that a latent failure (blunt end) may play in an active failure (sharp end) with respect to medication errors. Evaluate how the recent national initiatives focused on improving medication safety for high-risk patient populations might impact your practice. Summarize the potential impact of low health literacy on medication safety. 40

Scope of Low Health Literacy l 90 million Americans may be at risk l l l 1 out of 5 reads at the 5 th grade level or below Average American reads at the 8 th to 9 th grade level Annual health care costs are 4 times higher with low literacy skills Only 25 -50% of all patients take medications as directed Medication misuse has resulted in > 1 million ADE’s annually in the U. S. http: //c. ymcdn. com/sites/www. npsf. org/resource/collection/9220 B 314 -9666 -40 DA-89 DA 9 F 46357530 F 1/Ask. Me 3_Stats_English. pdf 41

The Impact of Low Health Literacy l Served to raise awareness of, at the time, an underappreciated challenge l 2006 formed the Roundtable on Health Literacy l Lack of health literacy costs U. S. > $100 BILLION annually Neilson-Bohlman L, Panzer A, Kindig D; Health Literacy: A Prescription to End Confusion; National Academies Press; Washington, DC, 2004; https: //iom. nationalacademies. org/ Reports/2004/Health. Literacy-A-Prescription-to-End-Confusion. aspx Accessed 9/7/15 42

What does Health Literacy Mean? l “Degree to which people can obtain, process, and understand the basic health information and services they need to make appropriate health decisions. ” http: //www. healtheddesign. com/blog/2015/2/3/healthliteracy-and-medication-safety 43

Health Literacy Universal Precautions! l Structure your care as if EVERY patient/customer may have limited health literacy l It isn’t enough to simply hand individuals easier to read information Brega AG, et al; AHRQ Health Literacy Universal Precautions Toolkit, Second Edition. AHRQ Publication No. 15 -0023 -EF) Rockville, MD. 2015; http: //www. ahrq. gov/professionals/qualitypatient-safety/quality-resources/tools/literacy-toolkit/index. html 44

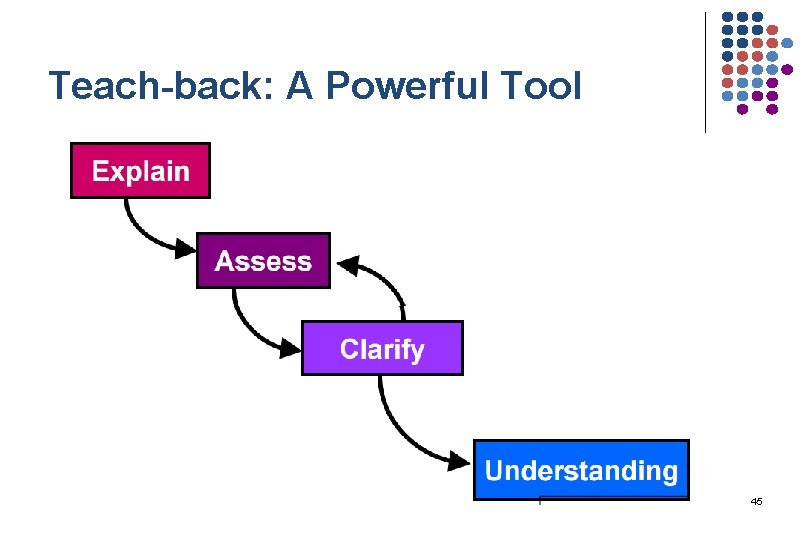
Teach-back: A Powerful Tool 45

Conclusion l Be proactive in identifying opportunities enhanced patient/medication safety, and thus quality of care l Pay close attention, but report when it fails → share what you’ve learned. l Practice health literacy universal precautions unless proven otherwise. 46

Medication Safety: An Ounce of Prevention 30 m. L 47

POST-ASSESSMENT QUESTIONS 48

Question # 1: Which of the following case vignettes BEST represents a preventable adverse drug event? A. B. C. D. A 68 y. o. male discharged 3 months earlier following a new stroke complicated by seizure. Patient was discharged on carbamazepine and now presents with acute mental status changes presumed to be second to serum Na+ 119 m. Eq/L. A 53 y. o. female is admitted to ICU with severe sepsis. Her husband shares the only past medical history is his wife suffered a MVA 6 years earlier requiring a splenectomy. A review of her hospital and clinic EHR is devoid of any record of vaccinations other than influenza. A 27 y. o. male intubated trauma patient placed on fentanyl and propofol for analgesia and sedation. On day 2 of ICU stay, serum triglycerides were 520 mg/d. L. The propofol is discontinued and a midazolam drip is started. A 64 y. o. male with no known allergies has advanced Stage 4 CKD. His progressive anemia is so severe that the decision is made to convert from oral to parenteral iron. He develops an anaphylactic reaction with the first test dose of In. Fed. 49

Question #2: Which of the following would be most likely to optimize patient safety by identifying sources of latent failures? A. Lack of independent double checks prior to dispensing B. Incomplete patient information such as comorbidities C. Excess or insignificant computer warnings or alerts D. Open supportive environment for discussing errors/near misses 50

Question #3: The National Council for Prescription Drug Products (NCPDP) recently published a patient safety white paper that provides best practice guidance to mitigate patient risk associated with dosing of liquid medications. According to this document, what is the preferred unit of measure for all oral liquid formulations? A. B. C. D. drops tsp m. L cc 51

POST ASSESSMENT KEY 52

Post Assessment Question #1: Answer B is correct. Of the options listed, this is the only example of an adverse drug event which was preventable. Note a preventable adverse drug event can involve an error of omission (i. e. , an untreated indication). According to the 2016 Adult Immunization schedule this patient should be vaccinated for encapsulated organisms which include: l Pneumococcal l 13 -valent pneumococcal conjugate vaccine (PCV 13); 23 -valent pneumococcal polysaccharide vaccine (PPSV 23) at least 8 weeks later. REPEAT 23 -valent pneumococcal polysaccharide (PPSV 23) in 5 years. Revaccinate 23 -valent pneumococcal polysaccharide (PPSV 23) at age 65 l Meningococcal l Serogroup A, C, W, and Y meningococcal vaccine (Men. ACWY); REPEAT Men. ACWY at least 8 week later. Revaccinate Men. ACWY every 5 years l Haemophilus influenza type B Hyponatremia, although a known side effect of carbamazepine, is not predictable and hence not preventable. Likewise, propofol is mixed in 1% lipid emulsion and is associated with hypertriglyceridemia. Anaphylactic reactions to parenteral iron products are very rare. There are some products which large-scale observational studies suggest may be associated with less frequency than dextran containing formulations (i. e. , iron sucrose). However, none of the above were prescribed, inappropriately, forseeable, or preventable. Answers A, C, and D are not correct. 53

Post Assessment Question #2: Answer D is correct. Answers A, B, and C are all examples of latent failures which can predispose active failure or the “sharp end”. Latent failures, also referred to as contributing factors, are weaknesses in structure that support medication processes. They are often subtle and “worked around” or tolerated until a slip or individual failure exposes the failure which often occur in combination (i. e. , swiss cheese). An open supportive environment allowing discussion of errors and near misses is one of the best ways to identify the latent failure and correct it.

Post Assessment Question # 3: Answer C is correct. In March, 2014, NCPDP published a white paper recommending best practice to decrease dosing errors associated with oral liquid medications. Among the recommendations is the standard unit of measure of m. L. In addition, it is recommended to AVOID cc, ml, or ML and the term milliliters spelled out. The Institute of Safe Medication Practice (ISMP) suggests a proposed change to United States Pharmacopeia (USP) General Chapter <17>: Prescription Container Labeling which will also endorse provision of a dosing device to accompany oral liquid products that are labeled in metric units ONLY! As such, answers A, B, and D are incorrect. 55
 Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention An ounce of prevention emergency
An ounce of prevention emergency An ounce of prevention is worth a pound of cure
An ounce of prevention is worth a pound of cure 2 oz of meat
2 oz of meat Ounce and pounds
Ounce and pounds 3 oz chicken breast
3 oz chicken breast 3 ounce water test
3 ounce water test Medication safety module
Medication safety module Closed loop medication administration safety initiative
Closed loop medication administration safety initiative Standard 4 medication safety
Standard 4 medication safety Van nuffelen marc
Van nuffelen marc Gwen graphs
Gwen graphs Mr ames is dragging a box
Mr ames is dragging a box Suburban sonnet gwen harwood
Suburban sonnet gwen harwood Gwen nuttall
Gwen nuttall Burgerparticipatie en veiligheidsgevoel
Burgerparticipatie en veiligheidsgevoel What came down the chimney in freak the mighty
What came down the chimney in freak the mighty Gwen clifford
Gwen clifford Transcount
Transcount Gwen harwood: selected poems
Gwen harwood: selected poems Gwen blumberg
Gwen blumberg Gwen hansen
Gwen hansen Injuries first aid
Injuries first aid Osha needlestick protocol
Osha needlestick protocol Fire hose reel signage standards
Fire hose reel signage standards Basic safety construction site safety orientation
Basic safety construction site safety orientation Safety care 2 person stability hold
Safety care 2 person stability hold Personal safety vs process safety
Personal safety vs process safety Basic safety construction site safety orientation
Basic safety construction site safety orientation Safety assessment for ind safety reporting
Safety assessment for ind safety reporting Safety depth formula in ecdis
Safety depth formula in ecdis Medication transfer form
Medication transfer form Purpose of medication
Purpose of medication Injectable medication administration pretest
Injectable medication administration pretest Biomedical therapy definition
Biomedical therapy definition Welvista medication list
Welvista medication list Pediatric dose calculation formula
Pediatric dose calculation formula Psychotropic medication
Psychotropic medication Defaulter vaccination schedule
Defaulter vaccination schedule Medication possession ratio calculation
Medication possession ratio calculation Best possible medication history form
Best possible medication history form 10 rights of medication
10 rights of medication Medication administration program massachusetts
Medication administration program massachusetts Drug dose calculation formula
Drug dose calculation formula Medication utilization evaluation
Medication utilization evaluation Classification of hypertension
Classification of hypertension Nahco3 medication factory
Nahco3 medication factory Medication passport
Medication passport Iv push calculation
Iv push calculation Pbs hospital medication chart
Pbs hospital medication chart Fluid chart nsw health
Fluid chart nsw health Traxoline medication
Traxoline medication Medication formula
Medication formula 7 rights of medication administration in order
7 rights of medication administration in order Chronic medication service
Chronic medication service