Needlestick Safety and Prevention Act Directed OSHA to
























- Slides: 33

Needlestick Safety and Prevention Act Directed OSHA to revise BBP standard • Effective 4/18/01 – Compliance directive 11/27/01 • Requires use of engineering and work practice controls – Sharps safety devices included as engineering controls – Alternatives to needles are preferable • Document implementation in written plan – Review at least annually • Maintain detailed sharps injury log – Include device type & brand • Involve frontline workers

Bloodborne Pathogens Exposure Control Plan • Statement of employer • Implementation policy – HB surveillance (optional) • Designation of responsible – Post-exposure evaluation employees and follow-up – Housekeeping • Determination of employee – Labeling exposures • Mandated use of needles • Implementation and other sharps with – Standard (universal) integrated safety features precautions – Engineering controls • Safety Device Evaluation – Work practices controls Committee – Personal protective • Documentation of waivers equipment • Recordkeeping and – Training reporting – Hepatitis B immunizations

HBV Preexposure Prophylaxis • Recombinant DNA vaccine available since 1986 – From yeast cells – Subunit HBs. Ag • 1. 0 ml IM given at 0, 1 and 6 months – Accelerated 0, 1, 2 and 12 months • For HCWs, document immunity anti. HBS 2 -6 months post-vaccination • 6 doses maximum • Once immune, no boosters

Recordkeeping Rule • Effective 1/1/02 • New OSHA forms 300 log, 300 A summary and 301 incident report • Expanded general recordkeeping requirements • May use new log for recording contaminated sharps injuries if – Record all data required, including brand – Able to segregate sharps info from log for privacy concerns

Joint Commission on Accreditation of Healthcare Organizations Preventing Needlestick and Sharps Injuries Sentinel Event Alert, Issue 22, August 2001 • Cites and reviews NIOSH Alert and the Needlestick Safety and Prevention Act. • “In April 2002, JCAHO will begin assessing organizational compliance with the new provisions of the Needlestick Safety and Prevention Act. ”

“The prevention of occupational diseases is primarily the function of the industrial management, secondarily, the function of the plant physician. In an ideal industrial establishment the two work together; the physician is conversant with all the processes of manufacture and is therefore able to link up the disturbances of health he observes among the workers with the processes in which they are engaged. He cooperates with management in the effort to introduce safeguards. . He is, however, in a subordinate position and therefore the prime responsibility in the prevention of occupational disease lies with the management, which has the last word in regard to methods of work, substances used, and equipment for the prevention of disease. ” Alice Hamilton, MD & Harriet L. Hardy, MD Industrial Toxicology, 1949

Prevention of Work-related BBP Infection Haddon, 1970

Controlling Exposures In order of preference T R A I N G • • • Substitution Isolation or enclosure Ventilation (general/dilution & local exhaust) Work and hygiene practices Personal protective equipment (last line of defense)

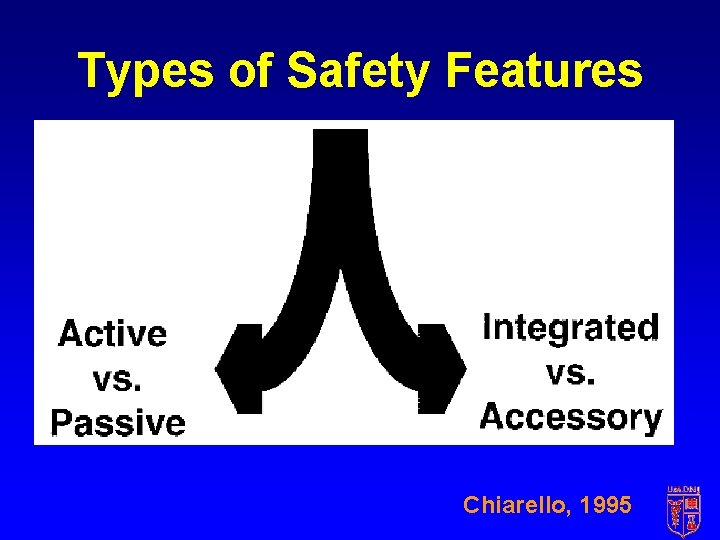
Types of Safety Features Chiarello, 1995

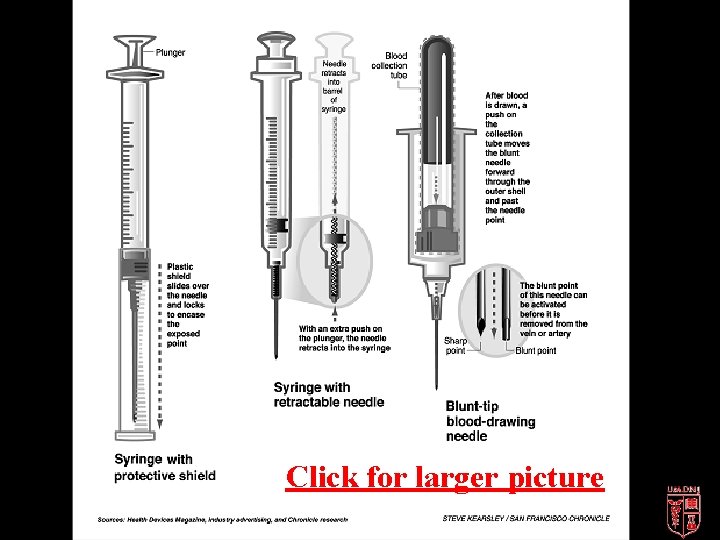
Design Features of a Safer Needle Device • Barrier between hands and needle after use • Allow or require worker’s hands to remain behind needle at all times • Integral part of device and not accessory • Be in effect before disassembly and remain in effect after disposal • Be simple, self-evident to operate and require little or no training FDA, 1992, 1995

Click for larger picture

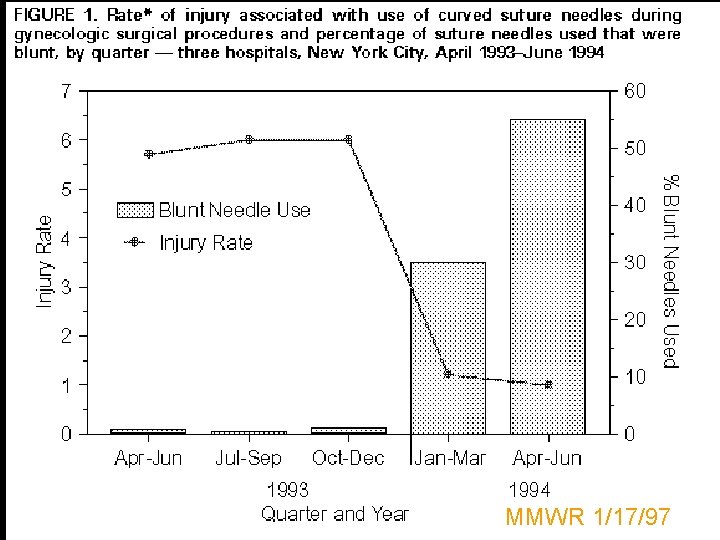
MMWR 1/17/97

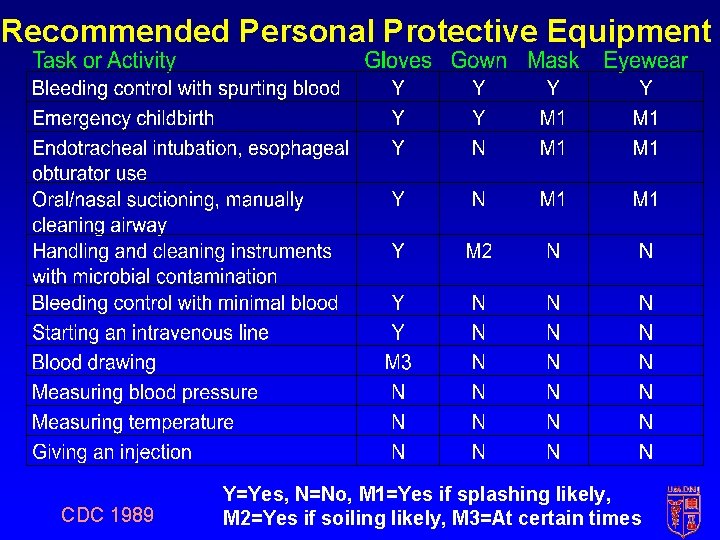
Recommended Personal Protective Equipment CDC 1989 Y=Yes, N=No, M 1=Yes if splashing likely, M 2=Yes if soiling likely, M 3=At certain times

Management of Occupational Blood Exposures • Provide immediate care to the exposure site – Wash wounds and skin with soap and water. – Flush mucous membranes with water. • Determine risk associated with exposure by – Type of fluid (e. g. , blood, visibly bloody fluid, other potentially infectious fluid or tissue, and concentrated virus) and – Type of exposure (i. e. , percutaneous injury, mucous membrane or nonintact skin exposure, and bites resulting in blood exposure).

Management of Occupational Blood Exposures • Evaluate exposure source – Assess risk of infection using available information. – Test known sources for HBs. Ag, anti-HCV, and HIV antibody (consider using rapid testing). – For unknown sources, assess risk of exposure to HBV, HCV, or HIV infection. – Do not test discarded needles or syringes for virus contamination.

Management of Occupational Blood Exposures • Evaluate the exposed person – HBV immune status – Tetanus prophylaxis – Baseline lab tests for HCV, HIV, chemistry profile, complete blood count, urinalysis, pregancy test PRN • Give PEP for exposures posing risk of infection transmission

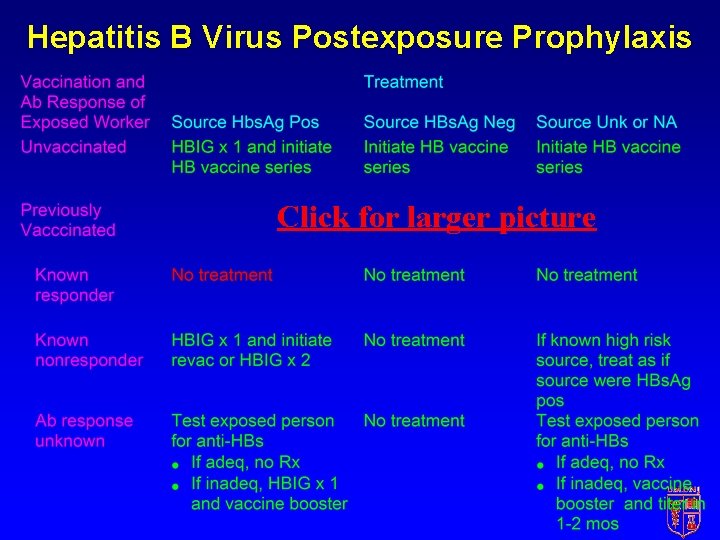
HBV Postexposure Prophylaxis Always indicated unless • Documented immune • Waiver signed

Hepatitis B Virus Postexposure Prophylaxis Click for larger picture

HCV Postexposure Prophylaxis

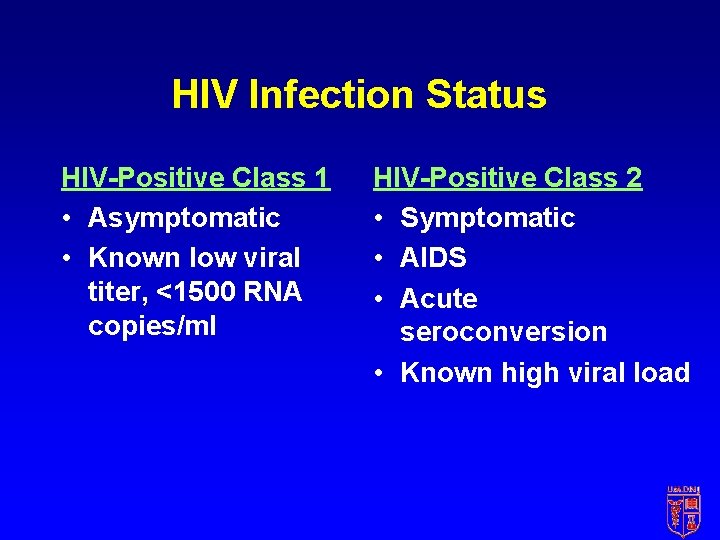
HIV Postexposure Prophylaxis Depends on • Type of exposure • Severity • Volume • Source HIV status Prophylactic treatment • May not be warranted • Basic regimen • Expanded regimen

HIV Infection Status HIV-Positive Class 1 • Asymptomatic • Known low viral titer, <1500 RNA copies/ml HIV-Positive Class 2 • Symptomatic • AIDS • Acute seroconversion • Known high viral load

Selected HIV PEP Regimens BASIC REGIMEN · Zidovudine (Retrovir™; ZDV; AZT) + Lamivudine (Epivir™; 3 TC); available as COMBIVIR™ - ZDV: 600 mg per day, in 2 or 3 divided doses - 3 TC: 150 mg twice daily EXPANDED REGIMEN Basic regimen plus · Indinavir (Crixivan™; IDV) - 800 mg every 8 hours, on an empty stomach

Recommended HIV Postexposure Prophylaxis for Percutaneous Injuries CDC. MMWR 2001.

Recommended HIV Postexposure Prophylaxis for Mucous Membrane and Non-intact Skin Exposures CDC. MMWR 2001.

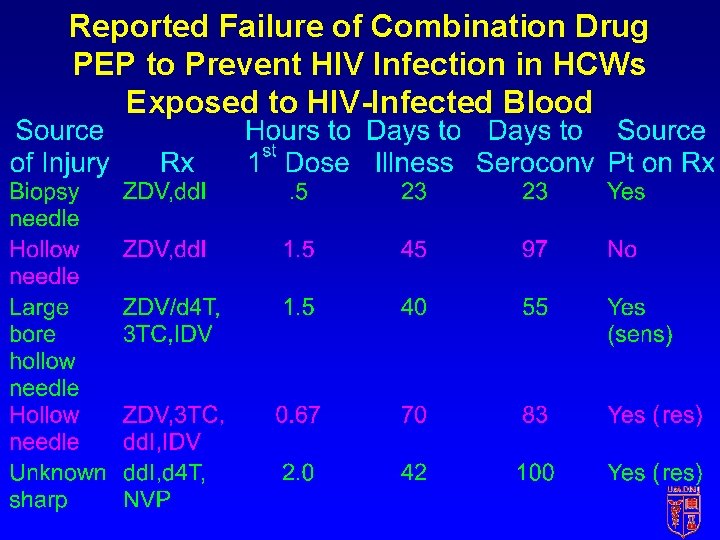
Reported Failure of Combination Drug PEP to Prevent HIV Infection in HCWs Exposed to HIV-Infected Blood

Management of Occupational Blood Exposures • Initiate HIV PEP as soon as possible, preferably within 2 hours of exposure • Offer pregnancy testing to all women of childbearing age not known to be pregnant • Seek expert consultation if viral resistance suspected • HIV PEP for 4 weeks if tolerated

Management of Occupational Blood Exposures • Provide counseling – Emotional effects – Risks of transmission – Medications, including adherence – Advise exposed persons to seek medical evaluation for any acute illness occurring during follow-up • Perform follow-up testing – Monitor for adverse effects – Seroconversion

Sample Protocol for Follow-up if on HIV PEP Medications

Primary Side Effects of Antiretroviral Agents

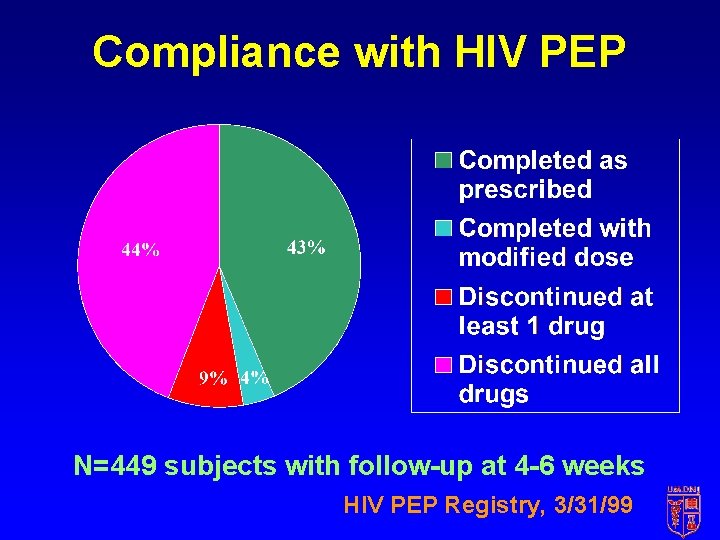
Compliance with HIV PEP N=449 subjects with follow-up at 4 -6 weeks HIV PEP Registry, 3/31/99

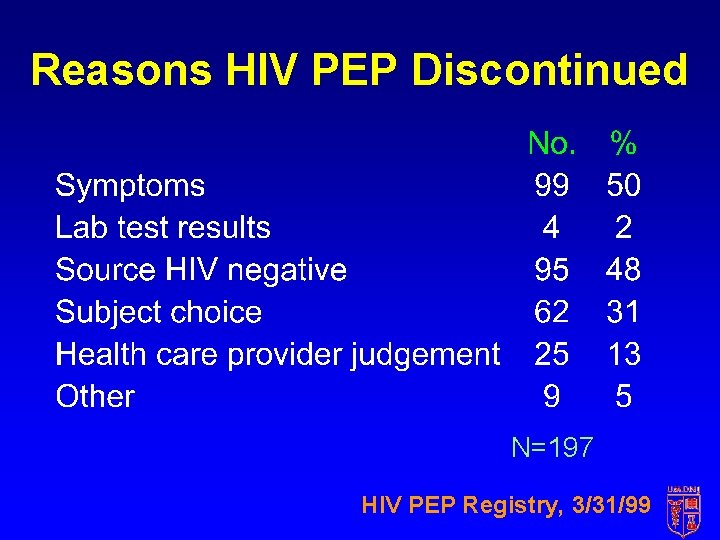
Reasons HIV PEP Discontinued N=197 HIV PEP Registry, 3/31/99

Management of Occupational Blood & Body Fluids Exposures Summary • Provide immediate care to the exposure site. • Determine risk associated with exposure. • Evaluate the exposed person. • Give PEP for exposures posing risk of infection transmission. • Provide counseling. • Perform follow-up testing.

Resources