M 4 Fuels Energy II Fuels Energy 2004














- Slides: 40

M 4 Fuels & Energy II: Fuels & Energy © 2004 -09 Dorje Gurung

Learning Objectives • Key Concepts: – Fossil fuel, petroleum (crude oil), hydrocarbon, distillation, fractional distillation, viscosity, combustion – Natural gas (methane), fossil fuel, petroleum (crude oil), hydrocarbon, distillation, fractional distillation, fraction, petrol, paraffin, diesel, lubricating fraction, bitumen – biomass, nuclear energy, solar energy, geothermal energy, tidal energy, wind energy, wave energy, hydroelectricity, biomass • Skills – Name the fossil fuels coal, natural gas and petroleum – Describe petroleum as a mixture of hydrocarbons – State that distillation can be used to separate components of liquid mixture M 4 Fuels & Energy: Fuels & Energy Slide 2 of 40

Learning Objectives – Be able to describe simple viscosity test for liquids and analyze the result – Name other sources of energy apart from those already named – Describe petroleum’s separation into useful fractions by fractional distillation – Be able to name the uses of some named fractions – Describe the difference in properties such as number of carbon, boiling point, viscosity and color of different fractions – Be able to describe some of the environmental problems associated with using fossil fuels – M 4 Fuels & Energy: Fuels & Energy Slide 3 of 40

What is a fuel? • A fuel is a substance or chemical that reacts with air releasing heat / thermal energy (or another kind of useful energy). Burning (reaction of a fuel with oxygen) is called “combustion”. We burn (combust) many types of fuels: Wood Coal paper Charcoal (for barbeques) Natural gas Petrol (methane, CH 4) (from crude oil) The combustion of the fuels provide the energy necessary for a host of different activities such as cooking, heating, operating automobiles and running factories. M 4 Fuels & Energy: Fuels & Energy Slide 4 of 40

Natural Fuels • The three fuels that are used most widely are coal, natural gas and petroleum. They are referred to as fossil fuels because they are remains of dead plants and animals. Coal from plants, and natural gas and petroleum from animals. M 4 Fuels & Energy: Fuels & Energy Slide 5 of 40

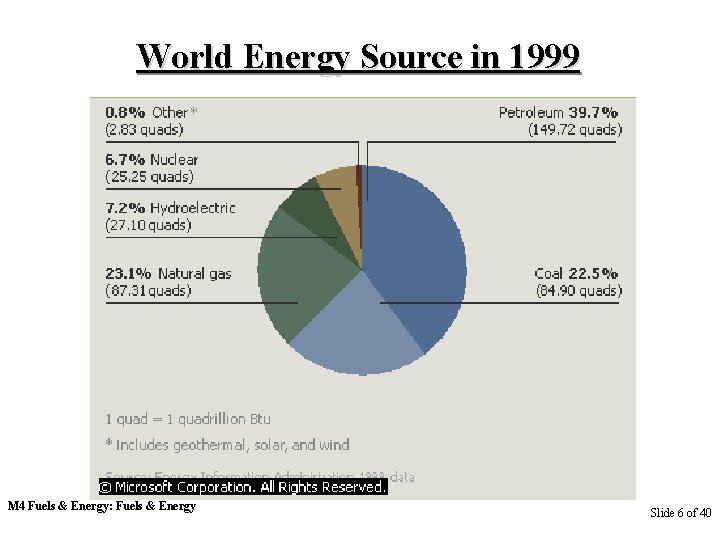
World Energy Source in 1999 M 4 Fuels & Energy: Fuels & Energy Slide 6 of 40

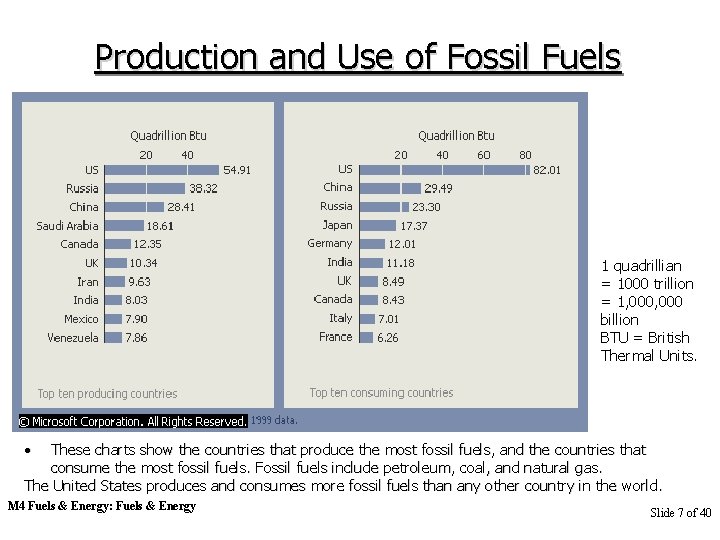
Production and Use of Fossil Fuels 1 quadrillian = 1000 trillion = 1, 000 billion BTU = British Thermal Units. • These charts show the countries that produce the most fossil fuels, and the countries that consume the most fossil fuels. Fossil fuels include petroleum, coal, and natural gas. The United States produces and consumes more fossil fuels than any other country in the world. M 4 Fuels & Energy: Fuels & Energy Slide 7 of 40

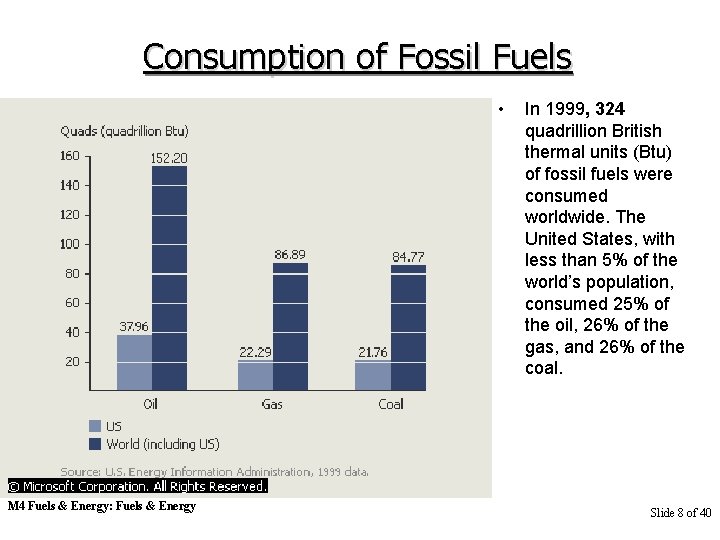
Consumption of Fossil Fuels • M 4 Fuels & Energy: Fuels & Energy In 1999, 324 quadrillion British thermal units (Btu) of fossil fuels were consumed worldwide. The United States, with less than 5% of the world’s population, consumed 25% of the oil, 26% of the gas, and 26% of the coal. Slide 8 of 40

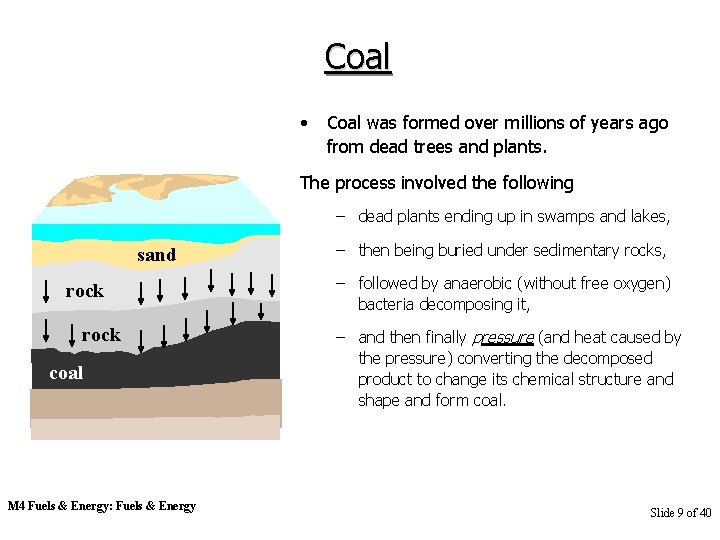
Coal • Coal was formed over millions of years ago from dead trees and plants. The process involved the following – dead plants ending up in swamps and lakes, sand rock coal M 4 Fuels & Energy: Fuels & Energy – then being buried under sedimentary rocks, – followed by anaerobic (without free oxygen) bacteria decomposing it, – and then finally pressure (and heat caused by the pressure) converting the decomposed product to change its chemical structure and shape and form coal. Slide 9 of 40

And now… sand We dig the coal out a coal “seam” using machines. The whole place is called a coalmine. A coalmine can be very deep inside the earth – kilometres down. rock M 4 Fuels & Energy: Fuels & Energy Slide 10 of 40

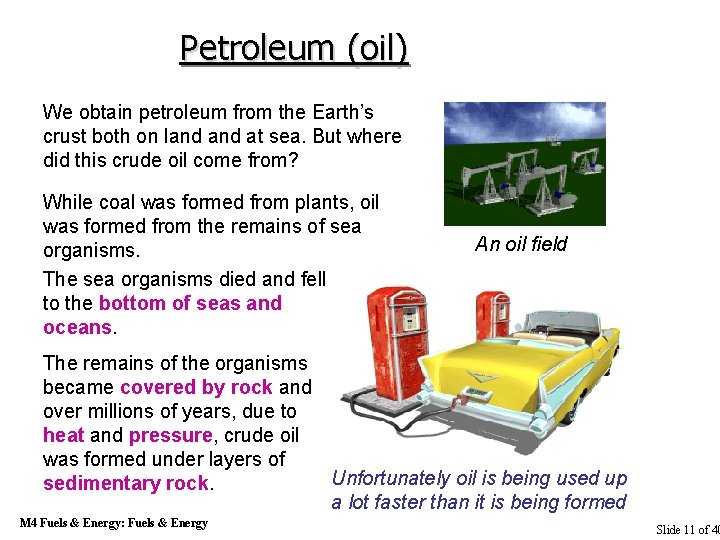
Petroleum (oil) We obtain petroleum from the Earth’s crust both on land at sea. But where did this crude oil come from? While coal was formed from plants, oil was formed from the remains of sea organisms. The sea organisms died and fell to the bottom of seas and oceans. An oil field The remains of the organisms became covered by rock and over millions of years, due to heat and pressure, crude oil was formed under layers of Unfortunately oil is being used up sedimentary rock. a lot faster than it is being formed M 4 Fuels & Energy: Fuels & Energy Slide 11 of 40

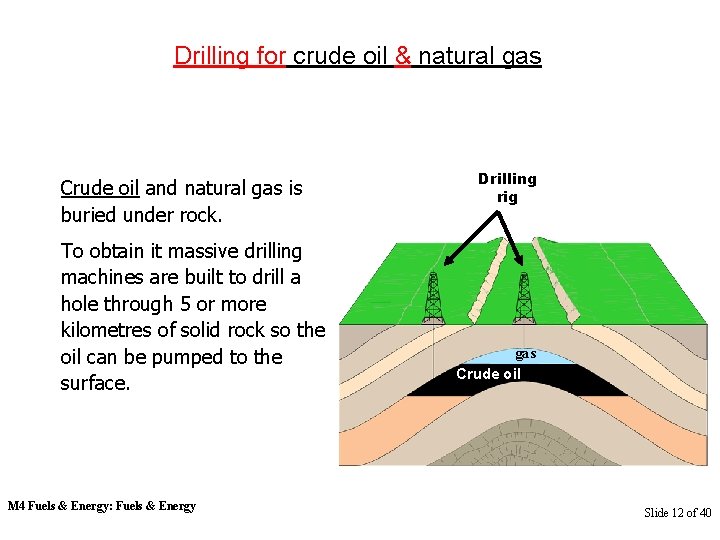
Drilling for crude oil & natural gas Crude oil and natural gas is buried under rock. To obtain it massive drilling machines are built to drill a hole through 5 or more kilometres of solid rock so the oil can be pumped to the surface. M 4 Fuels & Energy: Fuels & Energy Drilling rig gas Crude oil Slide 12 of 40

Off-shore Rig If the crude oil is buried beneath the land under the sea then a drilling platform is used to obtain the crude oil. What a beautiful sunset…thanks to all the pollutants!! M 4 Fuels & Energy: Fuels & Energy Slide 13 of 40

Why are they called FOSSIL FUELS again? coz they are the fossilized remains of dead plants M 4 Fuels & Energy: Fuels & Energy & dead sea animals Slide 14 of 40

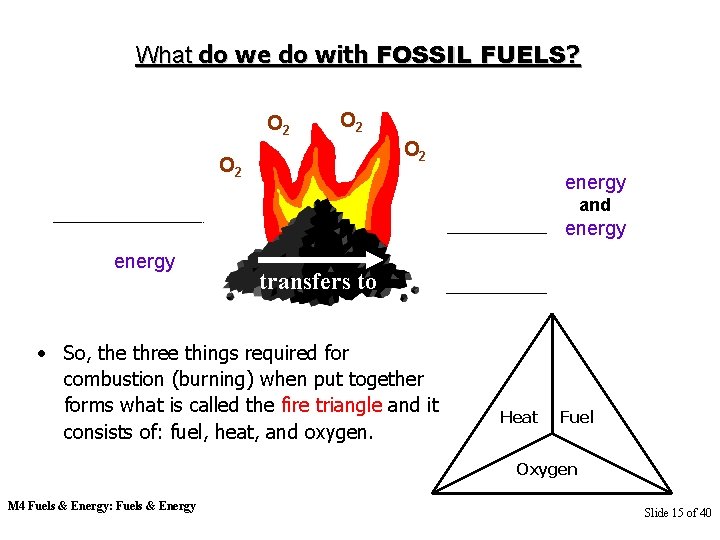
What do we do with FOSSIL FUELS? O 2 O 2 energy and energy transfers to • So, the three things required for combustion (burning) when put together forms what is called the fire triangle and it consists of: fuel, heat, and oxygen. Heat Fuel Oxygen M 4 Fuels & Energy: Fuels & Energy Slide 15 of 40

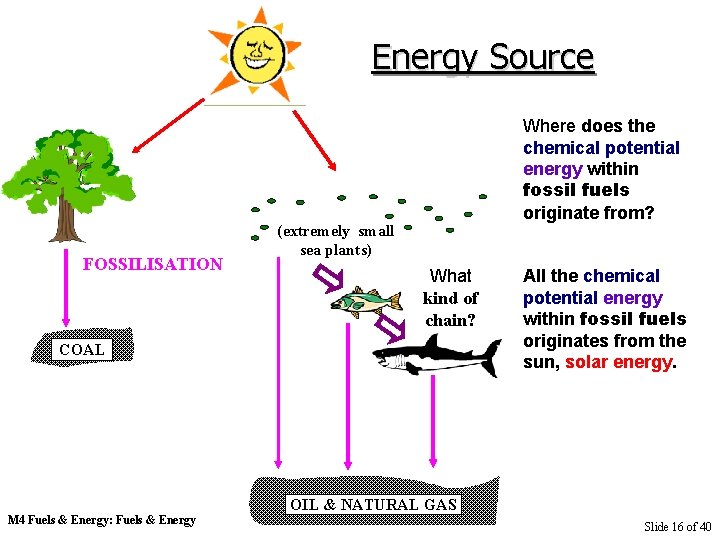
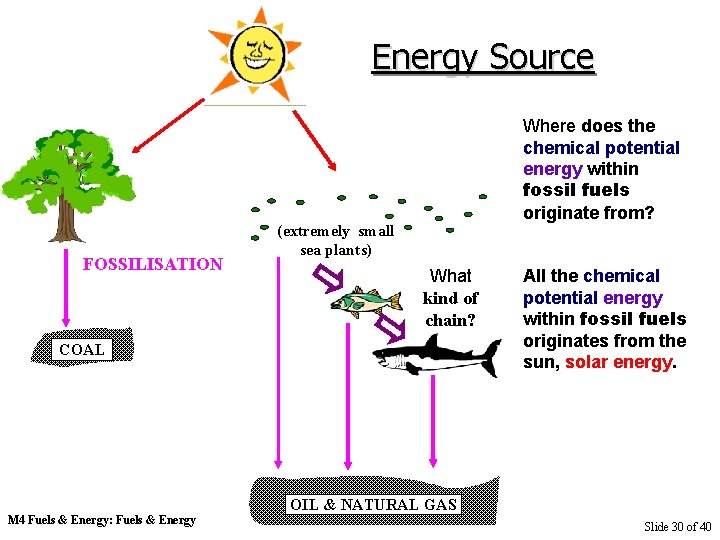
Energy Source FOSSILISATION Where does the chemical potential energy within fossil fuels originate from? (extremely small sea plants) What kind of chain? COAL M 4 Fuels & Energy: Fuels & Energy All the chemical potential energy within fossil fuels originates from the sun, solar energy. OIL & NATURAL GAS Slide 16 of 40

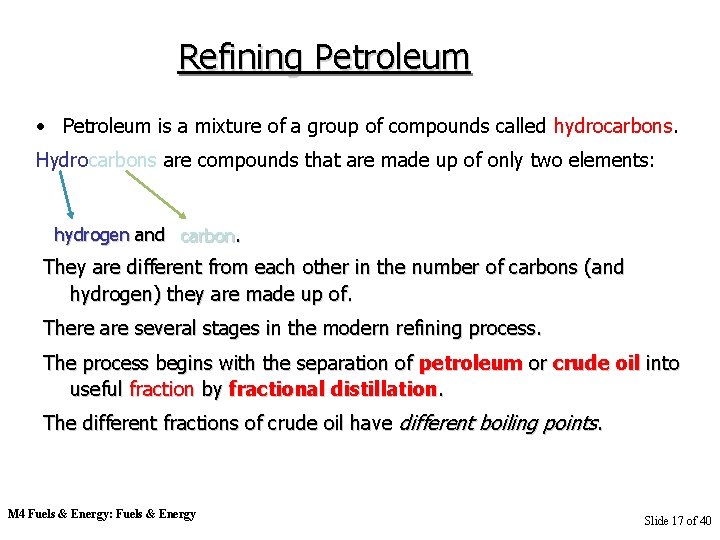
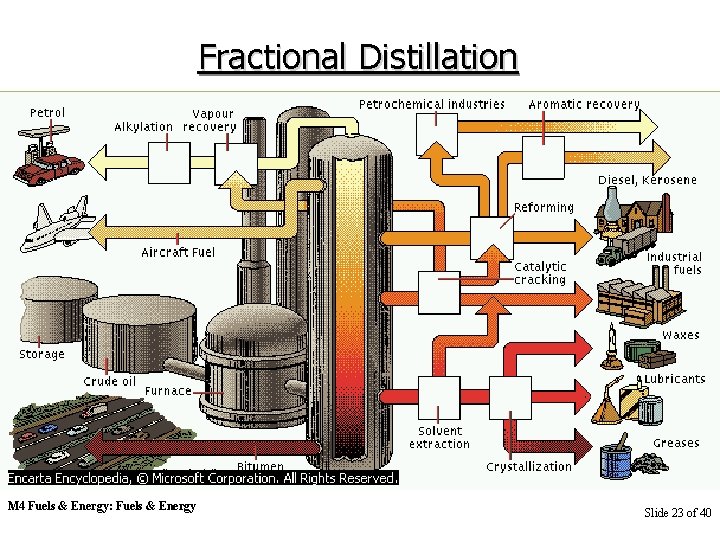
Refining Petroleum • Petroleum is a mixture of a group of compounds called hydrocarbons. Hydrocarbons are compounds that are made up of only two elements: hydrogen and carbon. They are different from each other in the number of carbons (and hydrogen) they are made up of. There are several stages in the modern refining process. The process begins with the separation of petroleum or crude oil into useful fraction by fractional distillation. The different fractions of crude oil have different boiling points. M 4 Fuels & Energy: Fuels & Energy Slide 17 of 40

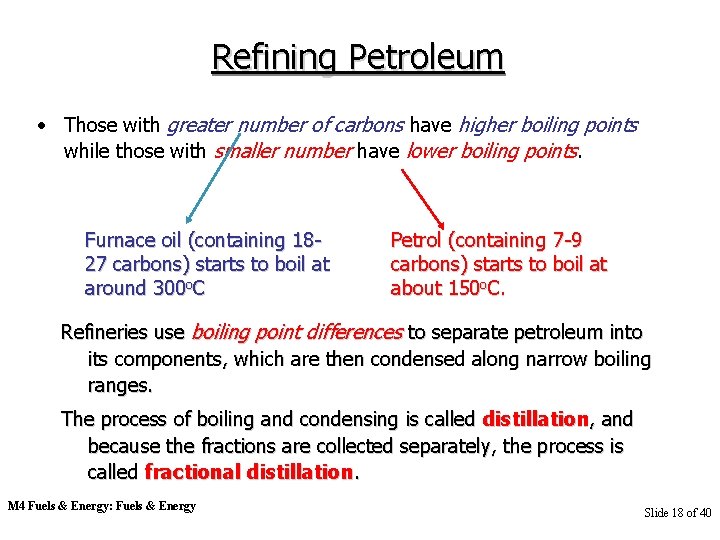
Refining Petroleum • Those with greater number of carbons have higher boiling points while those with smaller number have lower boiling points. Furnace oil (containing 1827 carbons) starts to boil at around 300 o. C Petrol (containing 7 -9 carbons) starts to boil at about 150 o. C. Refineries use boiling point differences to separate petroleum into its components, which are then condensed along narrow boiling ranges. The process of boiling and condensing is called distillation, and because the fractions are collected separately, the process is called fractional distillation. M 4 Fuels & Energy: Fuels & Energy Slide 18 of 40

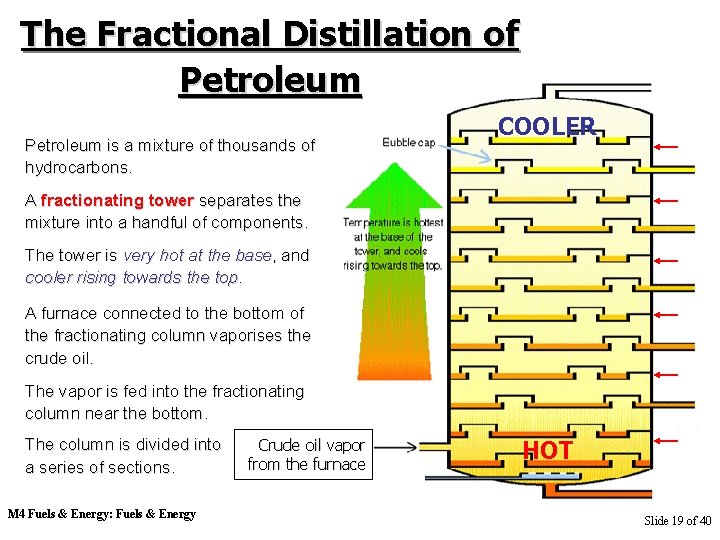
The Fractional Distillation of Petroleum is a mixture of thousands of hydrocarbons. COOLER A fractionating tower separates the mixture into a handful of components. The tower is very hot at the base, and cooler rising towards the top. A furnace connected to the bottom of the fractionating column vaporises the crude oil. The vapor is fed into the fractionating column near the bottom. The column is divided into a series of sections. M 4 Fuels & Energy: Fuels & Energy Crude oil vapor from the furnace HOT Slide 19 of 40

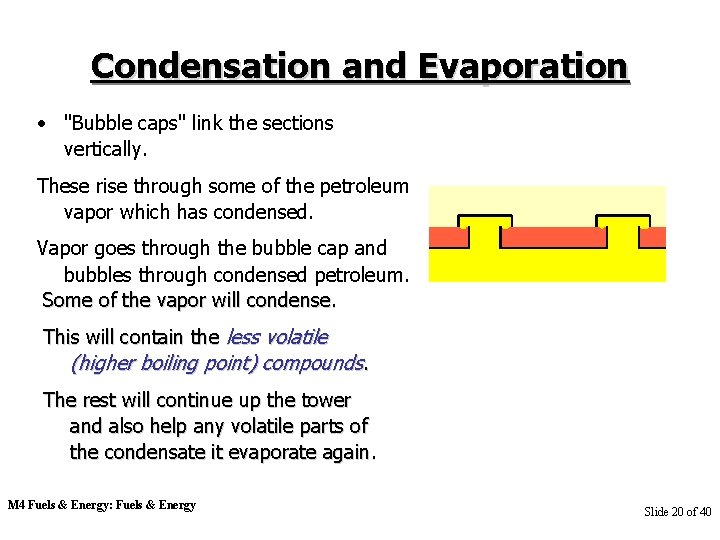
Condensation and Evaporation • "Bubble caps" link the sections vertically. These rise through some of the petroleum vapor which has condensed. Vapor goes through the bubble cap and bubbles through condensed petroleum. Some of the vapor will condense. This will contain the less volatile (higher boiling point) compounds. The rest will continue up the tower and also help any volatile parts of the condensate it evaporate again. M 4 Fuels & Energy: Fuels & Energy Slide 20 of 40

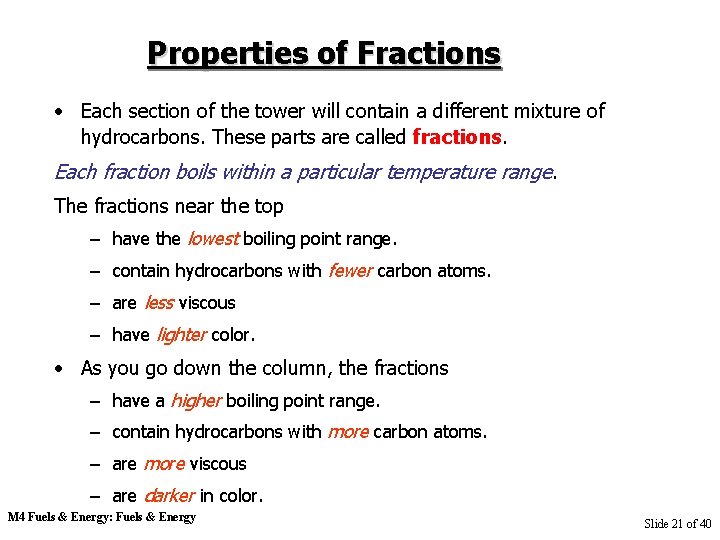
Properties of Fractions • Each section of the tower will contain a different mixture of hydrocarbons. These parts are called fractions. Each fraction boils within a particular temperature range. The fractions near the top – have the lowest boiling point range. – contain hydrocarbons with fewer carbon atoms. – are less viscous – have lighter color. • As you go down the column, the fractions – have a higher boiling point range. – contain hydrocarbons with more carbon atoms. – are more viscous – are darker in color. M 4 Fuels & Energy: Fuels & Energy Slide 21 of 40

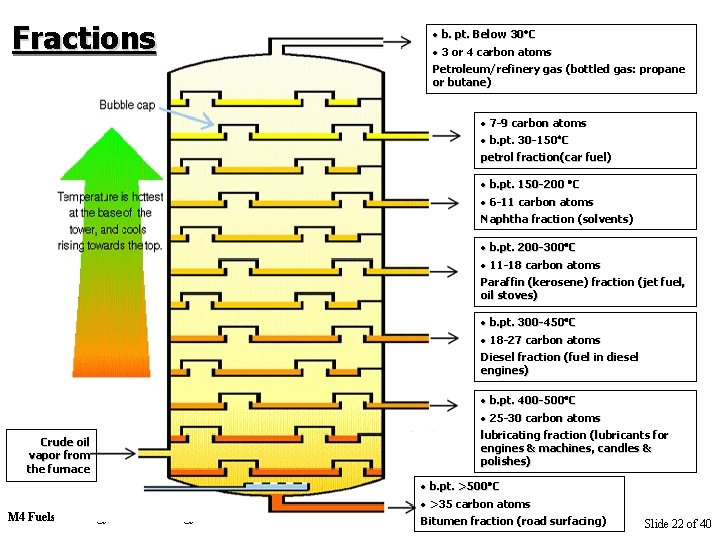
Fractions • b. pt. Below 30 30 C • 3 or 4 carbon atoms Petroleum/refinery gas (bottled gas: propane or butane) • 7 -9 carbon atoms • b. pt. 30 -150 C petrol fraction(car fuel) • b. pt. 150 -200 C • 6 -11 carbon atoms Naphtha fraction (solvents) • b. pt. 200 -300 C • 11 -18 carbon atoms Paraffin (kerosene) fraction (jet fuel, oil stoves) • b. pt. 300 -450 C • 18 -27 carbon atoms Diesel fraction (fuel in diesel engines) Crude oil vapor from the furnace M 4 Fuels & Energy: Fuels & Energy • b. pt. 400 -500 C • 25 -30 carbon atoms lubricating fraction (lubricants for engines & machines, candles & polishes) • b. pt. >500 C • >35 carbon atoms Bitumen fraction (road surfacing) Slide 22 of 40

Fractional Distillation M 4 Fuels & Energy: Fuels & Energy Slide 23 of 40

Practice Questions • 1. J 05/2/4. (e) (ii) Describe the process of fractional distillation which is used to separate the different fractions in petroleum. [2] (iii) State a use for the following petroleum fractions. petrol fraction lubricating fraction [2] M 4 Fuels & Energy: Fuels & Energy Slide 24 of 40

Practice Questions • 2. J 04/2/4 b. Which one of the following would be least likely to be obtained from the fractional distillation of petroleum? Put a ring around the correct answer. bitumen ethane M 4 Fuels & Energy: Fuels & Energy ethanol methane [1] Slide 25 of 40

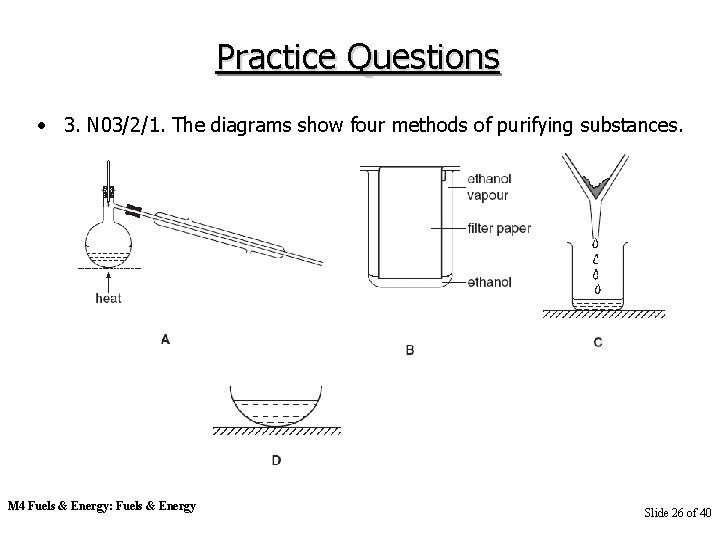
Practice Questions • 3. N 03/2/1. The diagrams show four methods of purifying substances. M 4 Fuels & Energy: Fuels & Energy Slide 26 of 40

Practice Questions • (a) Which of these methods, A, B, C or D, is best used for (i) separating the different colours in a sample of ink? (ii) separating two liquids with different boiling points? (iii) separating mud from water? (iv) making crystals of copper sulphate from copper sulphate solution? [4] • (b) State the name given to the method of separation shown in (i) diagram A, (ii) diagram B. M 4 Fuels & Energy: Fuels & Energy [2] Slide 27 of 40

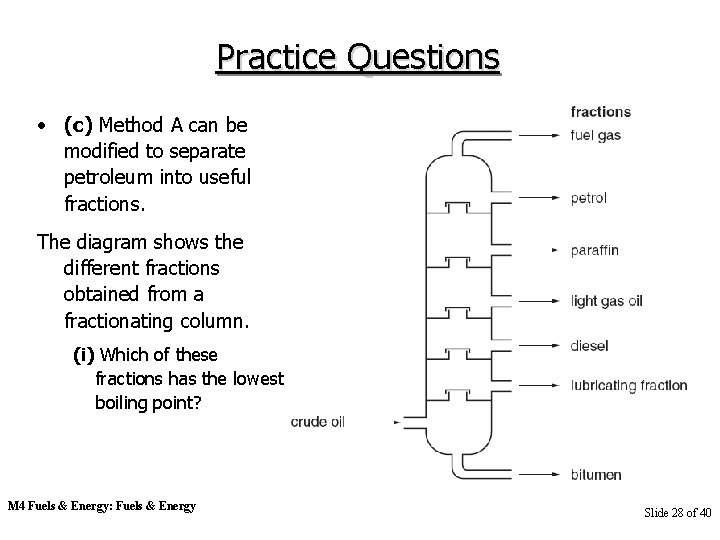
Practice Questions • (c) Method A can be modified to separate petroleum into useful fractions. The diagram shows the different fractions obtained from a fractionating column. (i) Which of these fractions has the lowest boiling point? M 4 Fuels & Energy: Fuels & Energy Slide 28 of 40

Practice Questions (ii) State one use for each of the following fractions. paraffin bitumen [3] • (d) Petroleum is a mixture of organic compounds. Which one of the following best describes the compounds found in petroleum? Put a ring around the correct answer. • acids [1] M 4 Fuels & Energy: Fuels & Energy alcohols carbohydrates hydrocarbons Slide 29 of 40

Energy Source FOSSILISATION Where does the chemical potential energy within fossil fuels originate from? (extremely small sea plants) What kind of chain? COAL M 4 Fuels & Energy: Fuels & Energy All the chemical potential energy within fossil fuels originates from the sun, solar energy. OIL & NATURAL GAS Slide 30 of 40

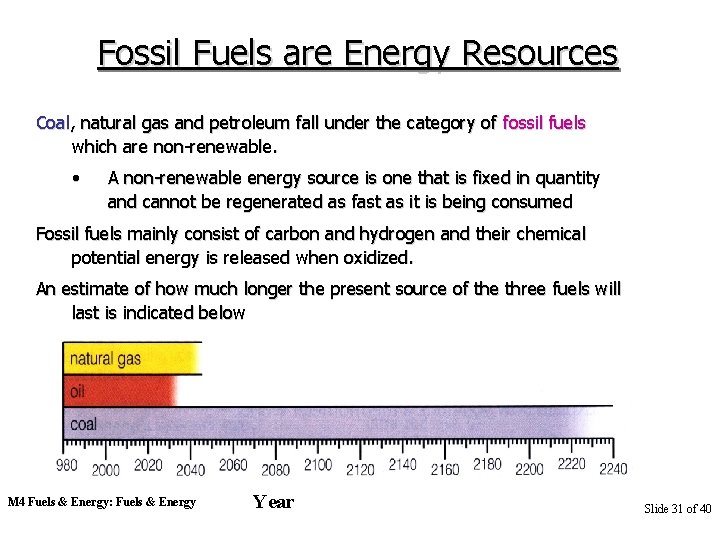
Fossil Fuels are Energy Resources Coal, natural gas and petroleum fall under the category of fossil fuels which are non-renewable. • A non-renewable energy source is one that is fixed in quantity and cannot be regenerated as fast as it is being consumed Fossil fuels mainly consist of carbon and hydrogen and their chemical potential energy is released when oxidized. An estimate of how much longer the present source of the three fuels will last is indicated below M 4 Fuels & Energy: Fuels & Energy Year Slide 31 of 40

Alternative: Nuclear fusion • The energy generated in the sun is from nuclear fusion. A type of reaction where small nuclei are combined to produce larger nuclei. In the process, exceedingly large amounts of energy is released. Another example of nuclear fusion is the hydrogen bomb which was used devastatingly in the second world war. However, controlled harnessing is still an on-going area of research. M 4 Fuels & Energy: Fuels & Energy Slide 32 of 40

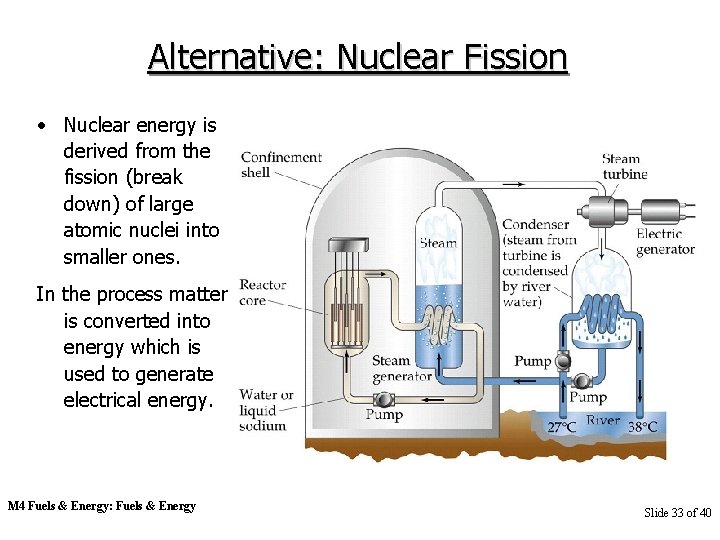
Alternative: Nuclear Fission • Nuclear energy is derived from the fission (break down) of large atomic nuclei into smaller ones. In the process matter is converted into energy which is used to generate electrical energy. M 4 Fuels & Energy: Fuels & Energy Slide 33 of 40

Renewable energy sources: Solar Energy • Harnessed by solar heating panels which capture and store the energy directly (such as heated water) or through photovoltaic cells. M 4 Fuels & Energy: Fuels & Energy Slide 34 of 40

Renewable energy sources: Wind Energy • Wind turns the turbine which turns the generator and produces electricity. M 4 Fuels & Energy: Fuels & Energy Slide 35 of 40

Renewable energy sources: Geothermal • M 4 Fuels & Energy: Fuels & Energy The utilization of heat stored in the interior of the earth generated by gravitational forces and natural radioactivity. Slide 36 of 40

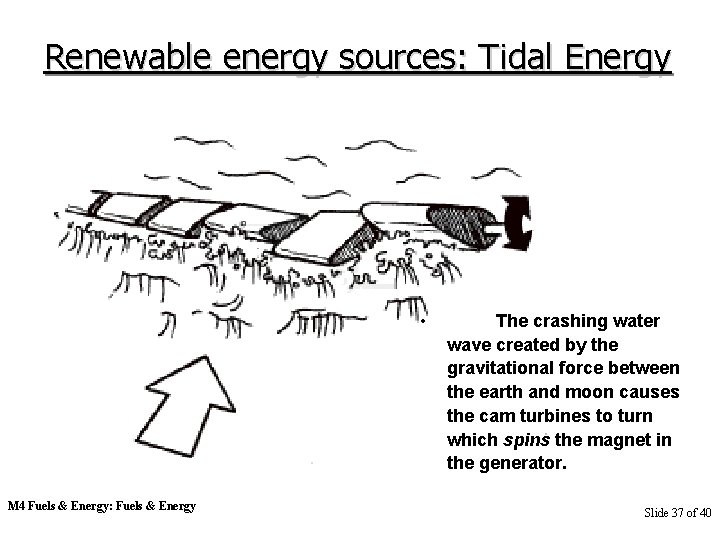
Renewable energy sources: Tidal Energy • M 4 Fuels & Energy: Fuels & Energy The crashing water wave created by the gravitational force between the earth and moon causes the cam turbines to turn which spins the magnet in the generator. Slide 37 of 40

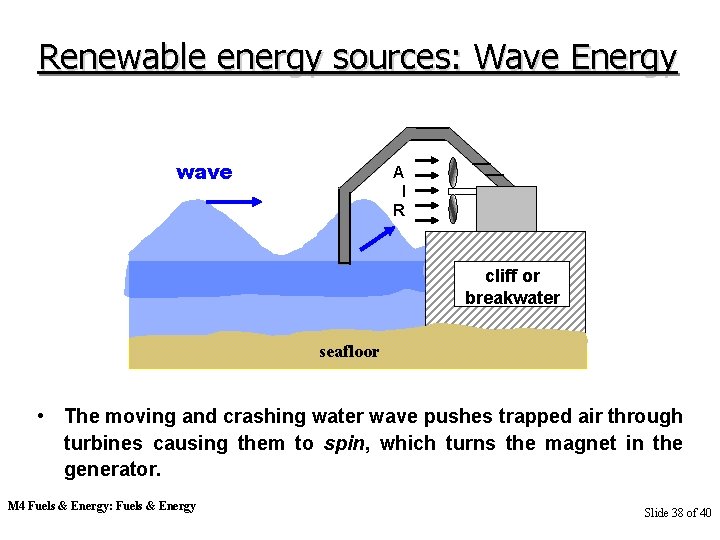
Renewable energy sources: Wave Energy wave A I R cliff or breakwater seafloor • The moving and crashing water wave pushes trapped air through turbines causing them to spin, which turns the magnet in the generator. M 4 Fuels & Energy: Fuels & Energy Slide 38 of 40

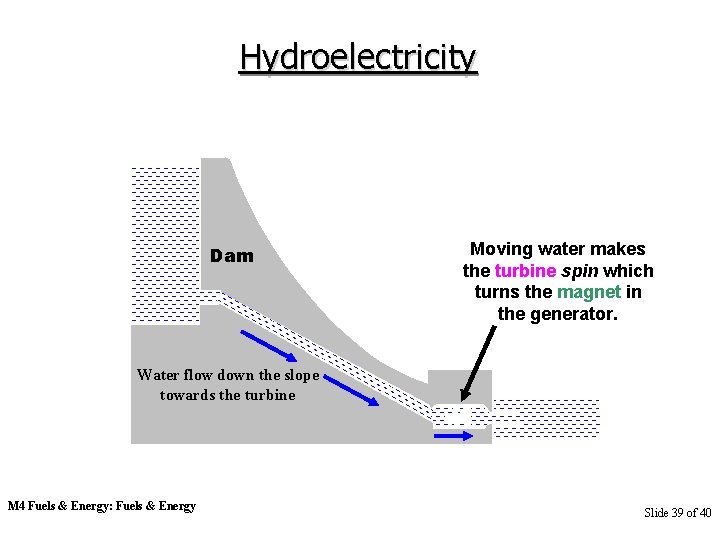
Hydroelectricity Dam Moving water makes the turbine spin which turns the magnet in the generator. Water flow down the slope towards the turbine M 4 Fuels & Energy: Fuels & Energy Slide 39 of 40

Biomass • Fuel produced by plants through biological process. The energy from the sun is converted to chemical potential energy in glucose when carbon dioxide and water are combined. The burning of plant material for fuel (such as wood) reverses the process releasing energy which is still used in many third world countries for mainly cooking and other purposes. Plant products can be changed further by fermentation to produce ethanol, an alternative fuel. In certain part of Latin America, notably Brazil it is replacing petrol. Though a renewable source, the rate of production cannot keep up with the demand of the modern society. M 4 Fuels & Energy: Fuels & Energy Slide 40 of 40
 Section 1 energy resources and fossil fuels answer key
Section 1 energy resources and fossil fuels answer key Wind power pros and cons
Wind power pros and cons Classification of fuels
Classification of fuels Advantage of fossil fuels
Advantage of fossil fuels Advantages of using oil
Advantages of using oil What are the environmental impacts of fossil fuels
What are the environmental impacts of fossil fuels Similarities between biofuels and fossil fuels
Similarities between biofuels and fossil fuels Advantages and disadvantages of fossil fuels
Advantages and disadvantages of fossil fuels Fossil fuels summary
Fossil fuels summary The origin of oil student worksheet answers
The origin of oil student worksheet answers Support nuclear energy
Support nuclear energy Types of natural resources
Types of natural resources Fossil fuels include
Fossil fuels include Fossil fuels formula
Fossil fuels formula Fossil fuels
Fossil fuels Resource that can be replaced
Resource that can be replaced Minerals and fuels
Minerals and fuels Advantages and disadvantages of nonrenewable energy
Advantages and disadvantages of nonrenewable energy Minerals and fuels
Minerals and fuels Fuels
Fuels What are fissil fuels
What are fissil fuels Igqi
Igqi Benefits of using fossil fuels
Benefits of using fossil fuels Energy transformation example
Energy transformation example Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Exemple macrocible
Exemple macrocible Pearson education 2004
Pearson education 2004 Richard murray caltech
Richard murray caltech Tabel rh
Tabel rh T. trimpe 2004 http //sciencespot.net/
T. trimpe 2004 http //sciencespot.net/ Dgc 2004
Dgc 2004 2004 dress code
2004 dress code Deped order on grievance machinery
Deped order on grievance machinery Sworn statement for esales registration
Sworn statement for esales registration Legge urbanistica regione campania 16/2004
Legge urbanistica regione campania 16/2004 Permenkes 81 tahun 2004
Permenkes 81 tahun 2004 Substansi struktur kurikulum 2004
Substansi struktur kurikulum 2004 2004 ford ranger 2.3 firing order
2004 ford ranger 2.3 firing order Age discrimination act 2004
Age discrimination act 2004 Ley idea 2004
Ley idea 2004






























































