Linear and Cyclic Scan Voltammetry in quiet solutions

- Slides: 25

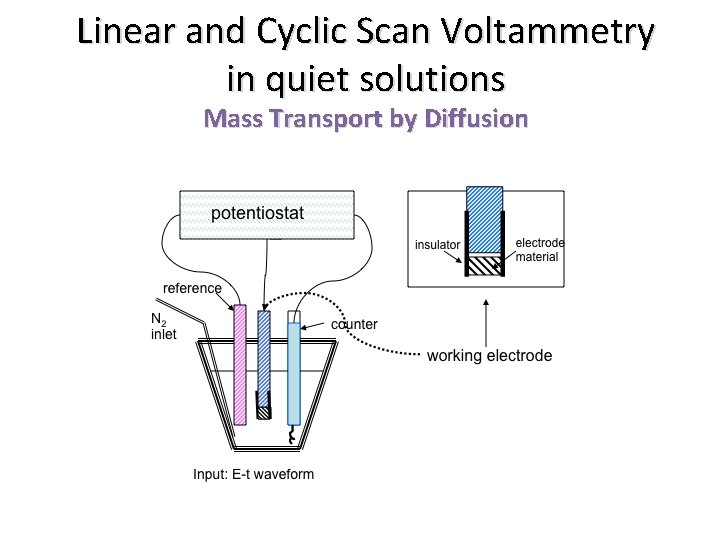
Linear and Cyclic Scan Voltammetry in quiet solutions Mass Transport by Diffusion F 2015

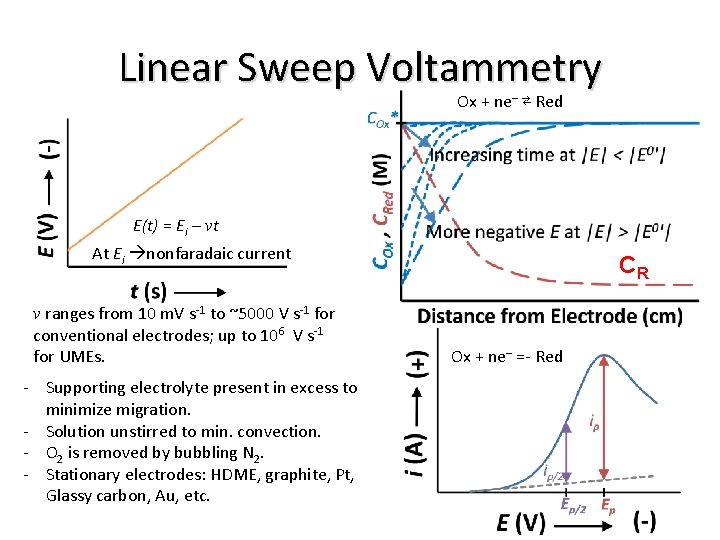
Linear Sweep Voltammetry Ox + ne– ⇄ Red E(t) = Ei – vt At Ei nonfaradaic current v ranges from 10 m. V s-1 to ~5000 V s-1 for conventional electrodes; up to 106 V s-1 for UMEs. - Supporting electrolyte present in excess to minimize migration. - Solution unstirred to min. convection. - O 2 is removed by bubbling N 2. - Stationary electrodes: HDME, graphite, Pt, Glassy carbon, Au, etc. CR Ox + ne– =- Red

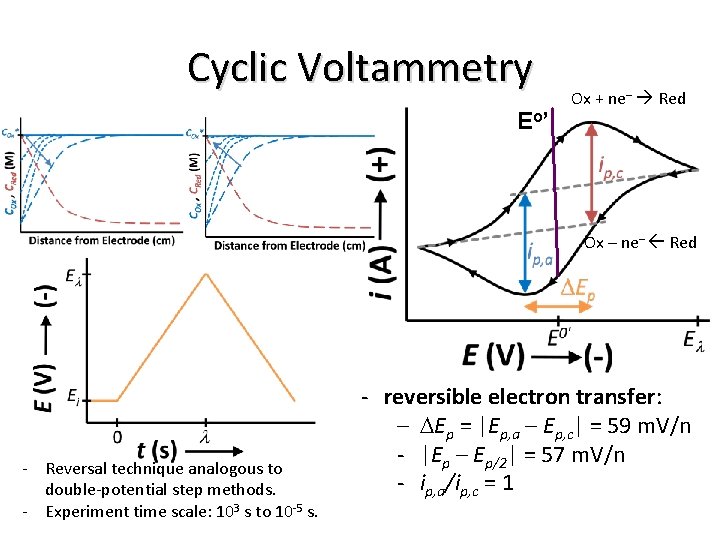
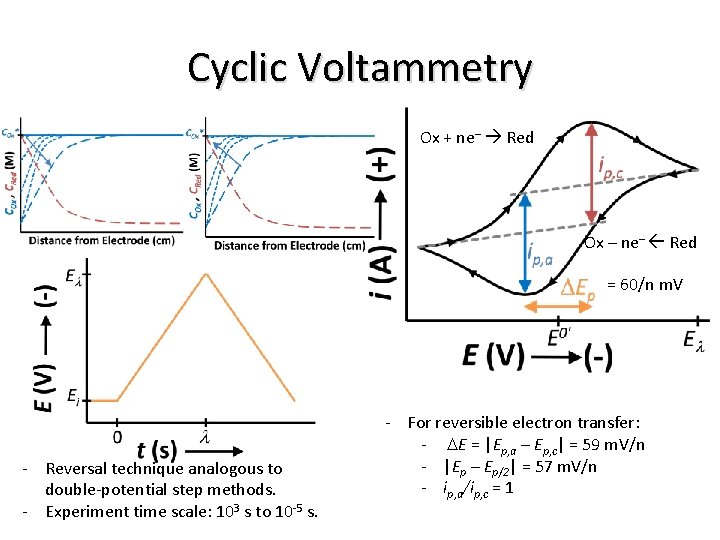
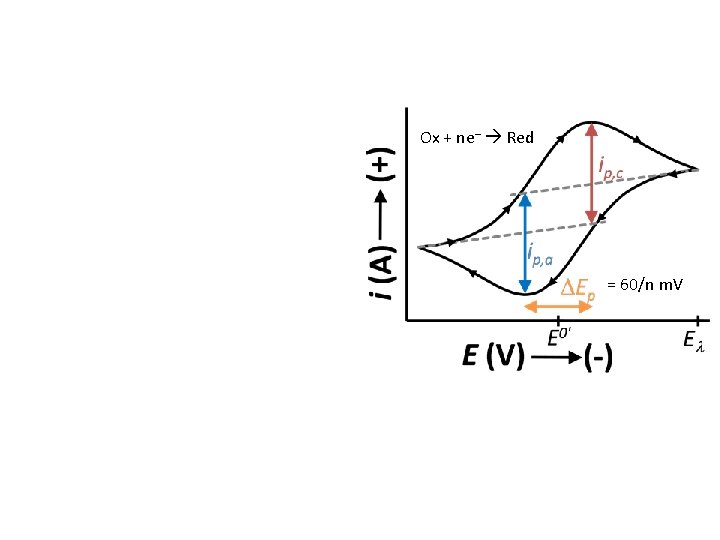
Cyclic Voltammetry E o’ Ox + ne– Red Ox – ne– Red - Reversal technique analogous to double-potential step methods. - Experiment time scale: 103 s to 10 -5 s. - reversible electron transfer: - DEp = |Ep, a – Ep, c| = 59 m. V/n - |Ep – Ep/2| = 57 m. V/n - ip, a/ip, c = 1

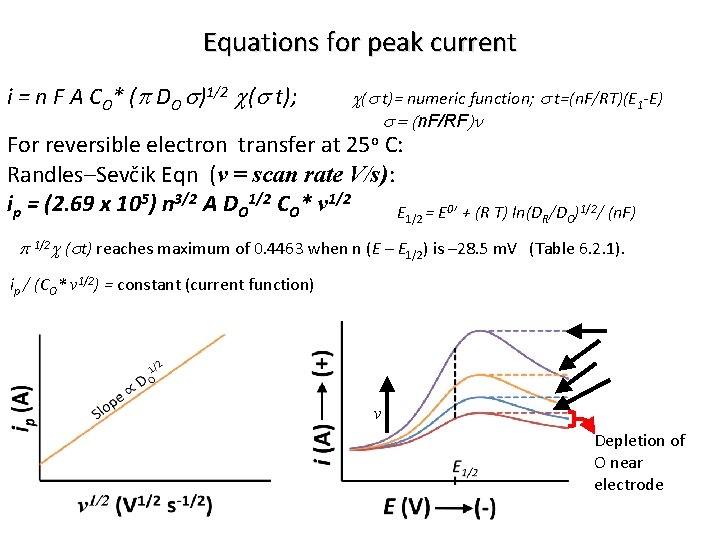
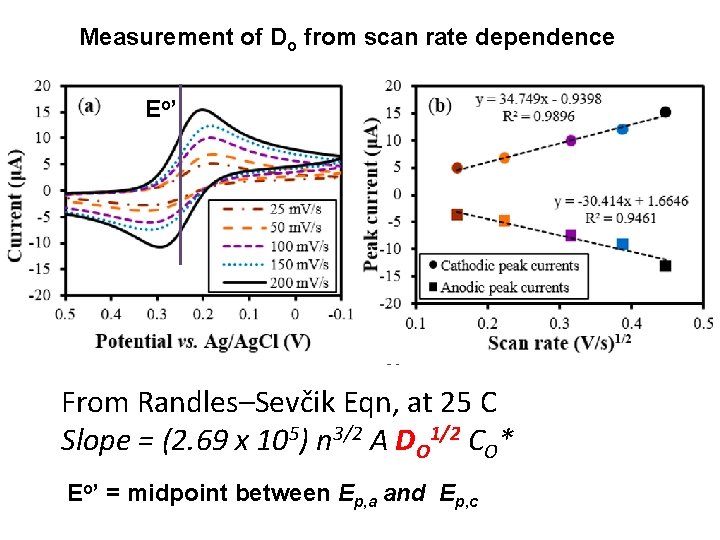
Equations for peak current i = n F A CO* (p DO s)1/2 c(s t); c(s t)= numeric function; s t=(n. F/RT)(E 1 -E) s = (n. F/RF)n For reversible electron transfer at 25 o C: Randles–Sevčik Eqn (v = scan rate V/s): ip = (2. 69 x 105) n 3/2 A DO 1/2 CO* v 1/2 E 1/2 = E 0’ + (R T) ln(DR/DO)1/2/ (n. F) p 1/2 c (st) reaches maximum of 0. 4463 when n (E – E 1/2) is – 28. 5 m. V (Table 6. 2. 1). ip / (CO* v 1/2) = constant (current function) v Depletion of O near electrode

Measurement of Do from scan rate dependence E o’ From Randles–Sevčik Eqn, at 25 C Slope = (2. 69 x 105) n 3/2 A DO 1/2 CO* Eo’ = midpoint between Ep, a and Ep, c

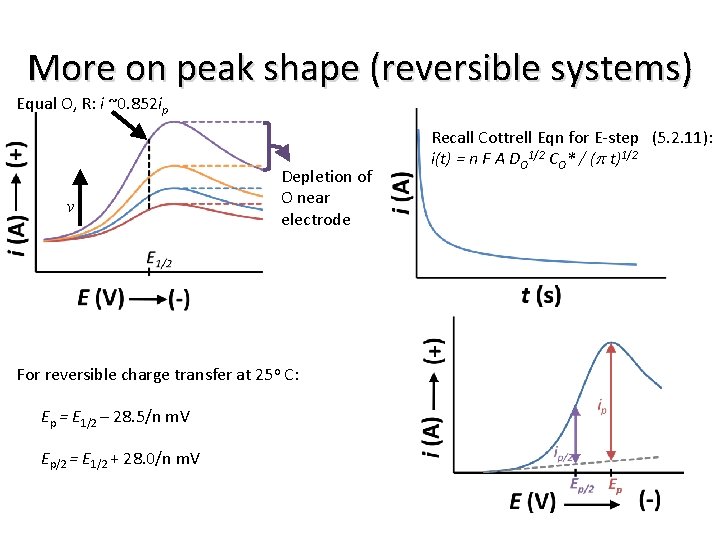
More on peak shape (reversible systems) Equal O, R: i ~0. 852 ip v Depletion of O near electrode For reversible charge transfer at 25 o C: Ep = E 1/2 – 28. 5/n m. V Ep/2 = E 1/2 + 28. 0/n m. V Recall Cottrell Eqn for E-step (5. 2. 11): i(t) = n F A DO 1/2 CO* / (p t)1/2

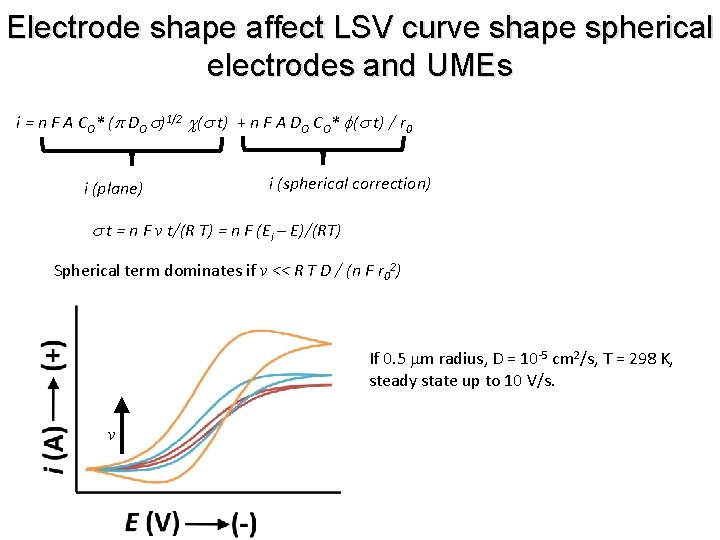
Electrode shape affect LSV curve shape spherical electrodes and UMEs i = n F A CO* (p DO s)1/2 c(s t) + n F A DO CO* f(s t) / r 0 i (plane) i (spherical correction) s t = n F v t/(R T) = n F (Ei – E)/(RT) Spherical term dominates if v << R T D / (n F r 02) If 0. 5 mm radius, D = 10 -5 cm 2/s, T = 298 K, steady state up to 10 V/s. v

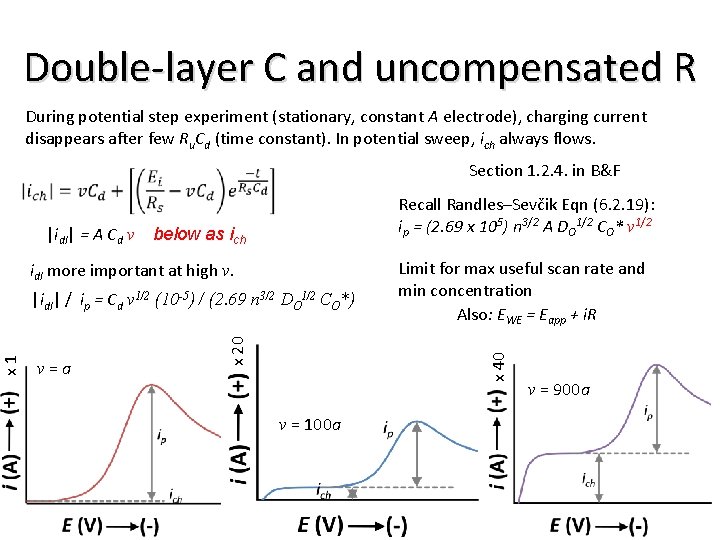
During potential step experiment (stationary, constant A electrode), charging current disappears after few Ru. Cd (time constant). In potential sweep, ich always flows. Section 1. 2. 4. in B&F |idl| = A Cd v Recall Randles–Sevčik Eqn (6. 2. 19): ip = (2. 69 x 105) n 3/2 A DO 1/2 CO* v 1/2 below as ich idl more important at high v. |idl| / ip = Cd v 1/2 (10 -5) / (2. 69 n 3/2 DO 1/2 CO*) Limit for max useful scan rate and min concentration Also: EWE = Eapp + i. R x 40 v=a x 20 x 1 Double-layer C and uncompensated R v = 100 a v = 900 a

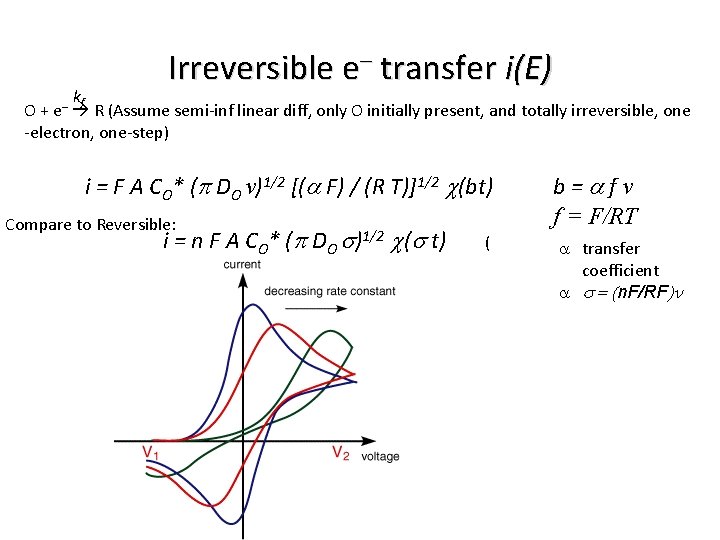
Irreversible e– transfer i(E) kf O + R (Assume semi-inf linear diff, only O initially present, and totally irreversible, one -electron, one-step) e– i = F A CO* (p DO v)1/2 [(a F) / (R T)]1/2 c(bt) Compare to Reversible: i = n F A CO* (p DO s)1/2 c(s t) ( b=afv f = F/RT a transfer coefficient a s = (n. F/RF)n

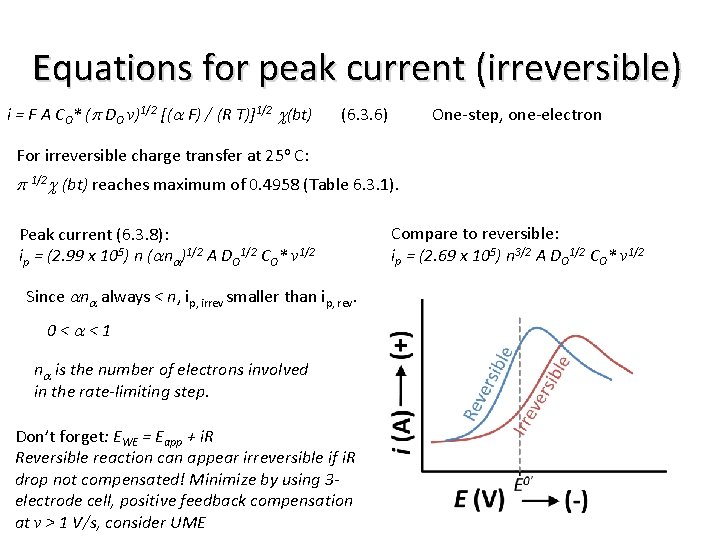
Equations for peak current (irreversible) i = F A CO* (p DO v)1/2 [(a F) / (R T)]1/2 c(bt) (6. 3. 6) One-step, one-electron For irreversible charge transfer at 25 o C: p 1/2 c (bt) reaches maximum of 0. 4958 (Table 6. 3. 1). Peak current (6. 3. 8): ip = (2. 99 x 105) n (ana)1/2 A DO 1/2 CO* v 1/2 Since ana always < n, ip, irrev smaller than ip, rev. 0<a<1 na is the number of electrons involved in the rate-limiting step. Don’t forget: EWE = Eapp + i. R Reversible reaction can appear irreversible if i. R drop not compensated! Minimize by using 3 electrode cell, positive feedback compensation at v > 1 V/s, consider UME Compare to reversible: ip = (2. 69 x 105) n 3/2 A DO 1/2 CO* v 1/2

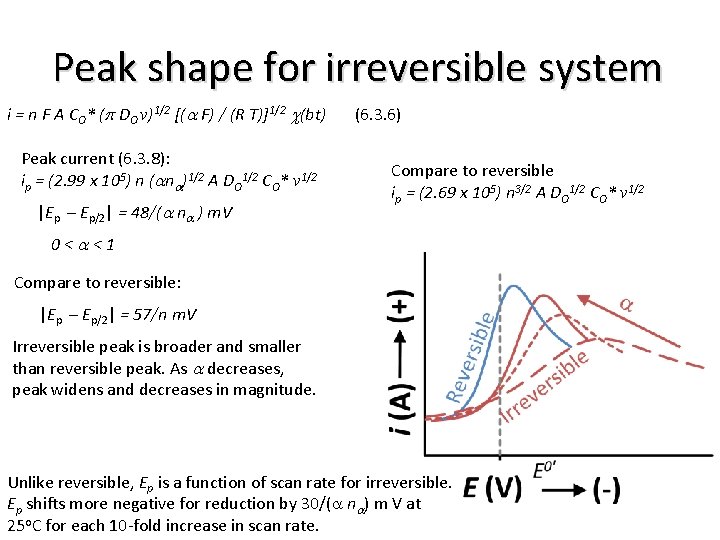
Peak shape for irreversible system i = n F A CO* (p DO v)1/2 [(a F) / (R T)]1/2 c(bt) Peak current (6. 3. 8): ip = (2. 99 x 105) n (ana)1/2 A DO 1/2 CO* v 1/2 |Ep – Ep/2| = 48/(a na ) m. V (6. 3. 6) Compare to reversible ip = (2. 69 x 105) n 3/2 A DO 1/2 CO* v 1/2 0<a<1 Compare to reversible: |Ep – Ep/2| = 57/n m. V Irreversible peak is broader and smaller than reversible peak. As a decreases, peak widens and decreases in magnitude. Unlike reversible, Ep is a function of scan rate for irreversible. Ep shifts more negative for reduction by 30/(a na) m V at 25 o. C for each 10 -fold increase in scan rate.

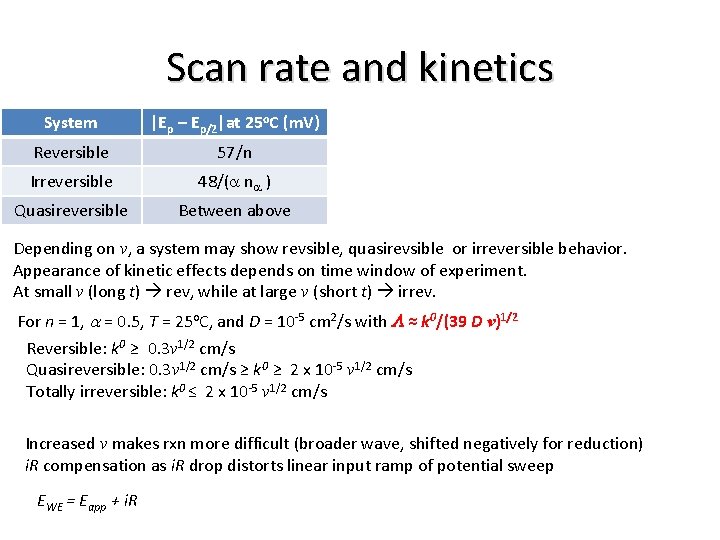
Scan rate and kinetics System |Ep – Ep/2|at 25 o. C (m. V) Reversible 57/n Irreversible 48/(a na ) Quasireversible Between above Depending on v, a system may show revsible, quasirevsible or irreversible behavior. Appearance of kinetic effects depends on time window of experiment. At small v (long t) rev, while at large v (short t) irrev. For n = 1, a = 0. 5, T = 25 o. C, and D = 10 -5 cm 2/s with L ≈ k 0/(39 D v)1/2 Reversible: k 0 ≥ 0. 3 v 1/2 cm/s Quasireversible: 0. 3 v 1/2 cm/s ≥ k 0 ≥ 2 x 10 -5 v 1/2 cm/s Totally irreversible: k 0 ≤ 2 x 10 -5 v 1/2 cm/s Increased v makes rxn more difficult (broader wave, shifted negatively for reduction) i. R compensation as i. R drop distorts linear input ramp of potential sweep EWE = Eapp + i. R

Cyclic Voltammetry Ox + ne– Red Ox – ne– Red = 60/n m. V - Reversal technique analogous to double-potential step methods. - Experiment time scale: 103 s to 10 -5 s. - For reversible electron transfer: - DE = |Ep, a – Ep, c| = 59 m. V/n - |Ep – Ep/2| = 57 m. V/n - ip, a/ip, c = 1

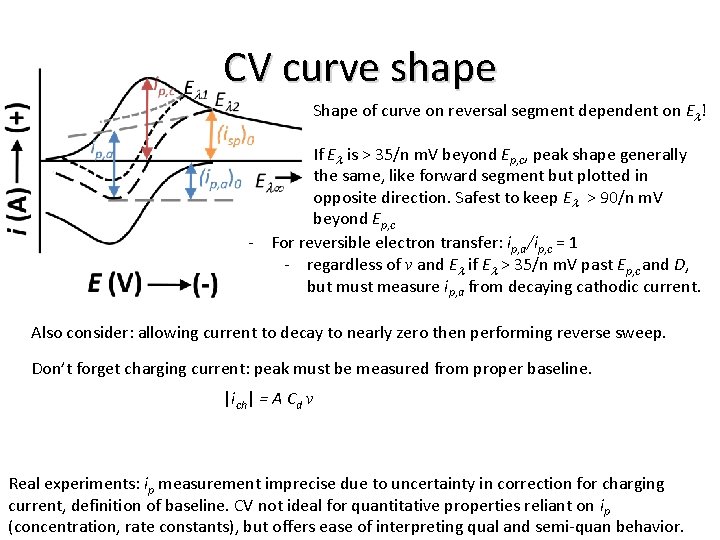
CV curve shape Shape of curve on reversal segment dependent on El! If El is > 35/n m. V beyond Ep, c, peak shape generally the same, like forward segment but plotted in opposite direction. Safest to keep El > 90/n m. V beyond Ep, c - For reversible electron transfer: ip, a/ip, c = 1 - regardless of v and El if El > 35/n m. V past Ep, c and D, but must measure ip, a from decaying cathodic current. Also consider: allowing current to decay to nearly zero then performing reverse sweep. Don’t forget charging current: peak must be measured from proper baseline. |ich| = A Cd v Real experiments: ip measurement imprecise due to uncertainty in correction for charging current, definition of baseline. CV not ideal for quantitative properties reliant on ip (concentration, rate constants), but offers ease of interpreting qual and semi-quan behavior.

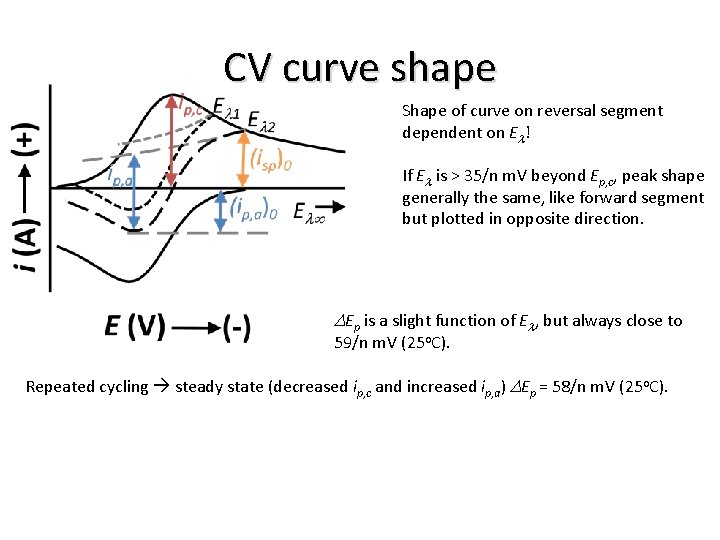
CV curve shape Shape of curve on reversal segment dependent on El! If El is > 35/n m. V beyond Ep, c, peak shape generally the same, like forward segment but plotted in opposite direction. DEp is a slight function of El, but always close to 59/n m. V (25 o. C). Repeated cycling steady state (decreased ip, c and increased ip, a) DEp = 58/n m. V (25 o. C).

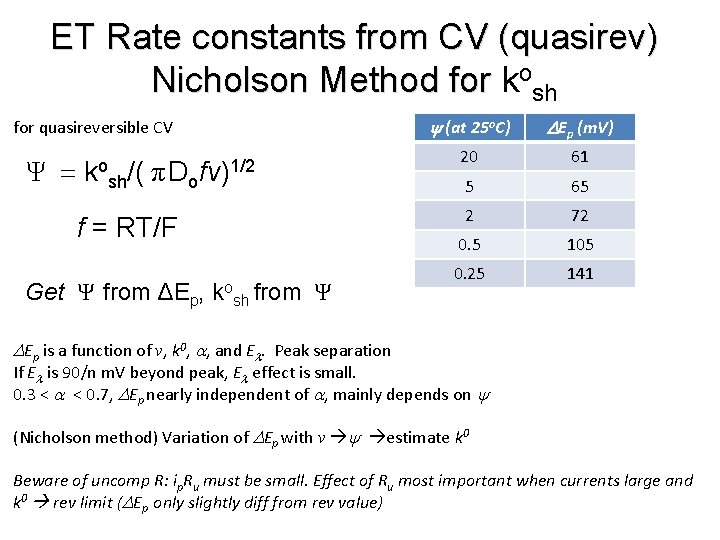
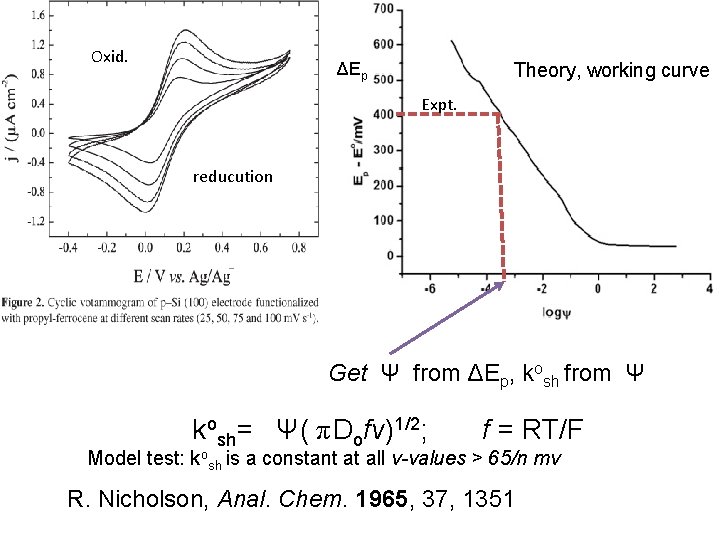
ET Rate constants from CV (quasirev) Nicholson Method for kosh for quasireversible CV Ψ = ko sh/( πDo fν)1/2 f = RT/F Get Ψ from ΔEp, kosh from Ψ y (at 25 o. C) DEp (m. V) 20 61 5 65 2 72 0. 5 105 0. 25 141 DEp is a function of v, k 0, a, and El. Peak separation If El is 90/n m. V beyond peak, El effect is small. 0. 3 < a < 0. 7, DEp nearly independent of a, mainly depends on y (Nicholson method) Variation of DEp with v y estimate k 0 Beware of uncomp R: ip. Ru must be small. Effect of Ru most important when currents large and k 0 rev limit (DEp only slightly diff from rev value)

Oxid. ΔEp Theory, working curve Expt. reducution Get Ψ from ΔEp, kosh from Ψ kosh= Ψ( πDofν)1/2; f = RT/F Model test: kosh is a constant at all ν-values > 65/n mv R. Nicholson, Anal. Chem. 1965, 37, 1351

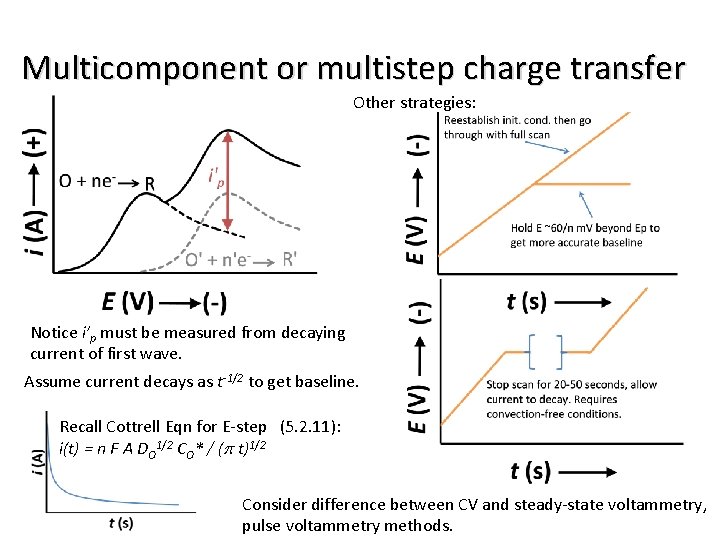
Multicomponent or multistep charge transfer Other strategies: Notice i’p must be measured from decaying current of first wave. Assume current decays as t-1/2 to get baseline. Recall Cottrell Eqn for E-step (5. 2. 11): i(t) = n F A DO 1/2 CO* / (p t)1/2 Consider difference between CV and steady-state voltammetry, pulse voltammetry methods.

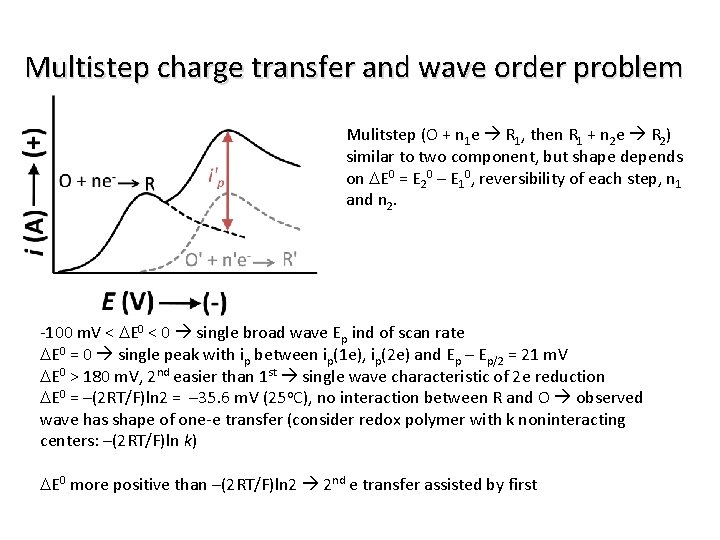
Multistep charge transfer and wave order problem Mulitstep (O + n 1 e R 1, then R 1 + n 2 e R 2) similar to two component, but shape depends on DE 0 = E 20 – E 10, reversibility of each step, n 1 and n 2. -100 m. V < DE 0 < 0 single broad wave Ep ind of scan rate DE 0 = 0 single peak with ip between ip(1 e), ip(2 e) and Ep – Ep/2 = 21 m. V DE 0 > 180 m. V, 2 nd easier than 1 st single wave characteristic of 2 e reduction DE 0 = –(2 RT/F)ln 2 = – 35. 6 m. V (25 o. C), no interaction between R and O observed wave has shape of one-e transfer (consider redox polymer with k noninteracting centers: –(2 RT/F)ln k) DE 0 more positive than –(2 RT/F)ln 2 2 nd e transfer assisted by first

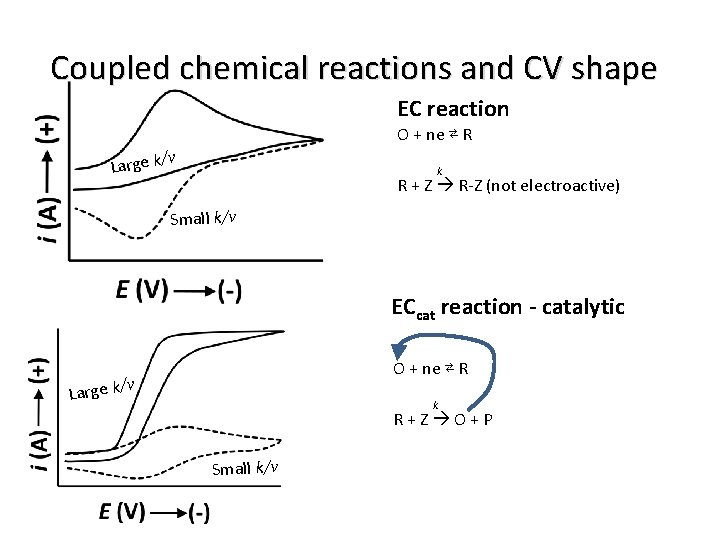
Coupled chemical reactions and CV shape EC reaction O + ne ⇄ R /v Large k k R + Z R-Z (not electroactive) Small k/v ECcat reaction - catalytic O + ne ⇄ R /v Large k k R+Z O+P Small k/v

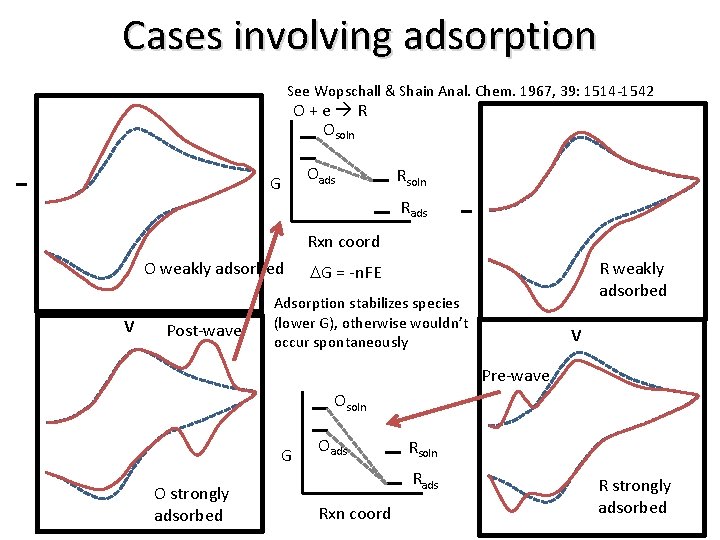
Cases involving adsorption See Wopschall & Shain Anal. Chem. 1967, 39: 1514 -1542 O+e R Osoln I Rsoln Rads I Oads G Rxn coord O weakly adsorbed V Post-wave R weakly adsorbed DG = -n. FE Adsorption stabilizes species (lower G), otherwise wouldn’t occur spontaneously V Pre-wave Osoln G O strongly adsorbed Oads Rsoln Rads Rxn coord R strongly adsorbed

Linear sweep methods • CV shapes can depend on electrode size, v, k 0 sh, a, El • Good for qualitative or semi-quantitative description of system based on ip, DEp, recognizable shape, • Experimental data - simulation models, obtain k 0 sh • ip measurement complicated by baseline correction – ich, i. R drop, reversal baseline – mathematical corrections, additional experiments • High scan rates: quasireversible , large i. R effect • Detection limit ~10 -6 to 5 x 10 -7 M; not very sensitive

Ox + ne– Red = 60/n m. V

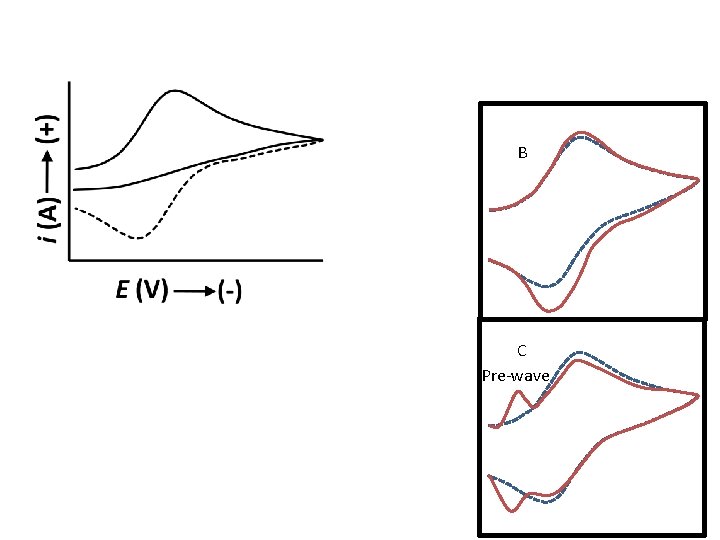
B C Pre-wave
