VOLTAMMETRY A Comparison of Voltammetry to Other Electrochemical





![Voltammograms for Mixtures of Reactants [Fe 3+]=1 x 10 -4 M [Fe 2+]=0. 5 Voltammograms for Mixtures of Reactants [Fe 3+]=1 x 10 -4 M [Fe 2+]=0. 5](https://slidetodoc.com/presentation_image/2defa11cd3522d747a37df75a13dd9bf/image-18.jpg)



- Slides: 25

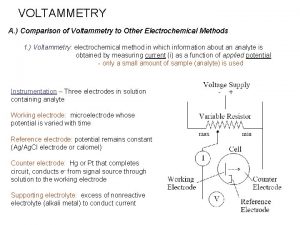
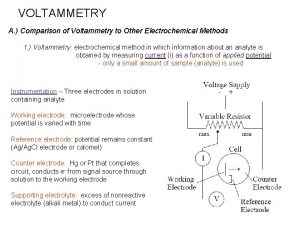
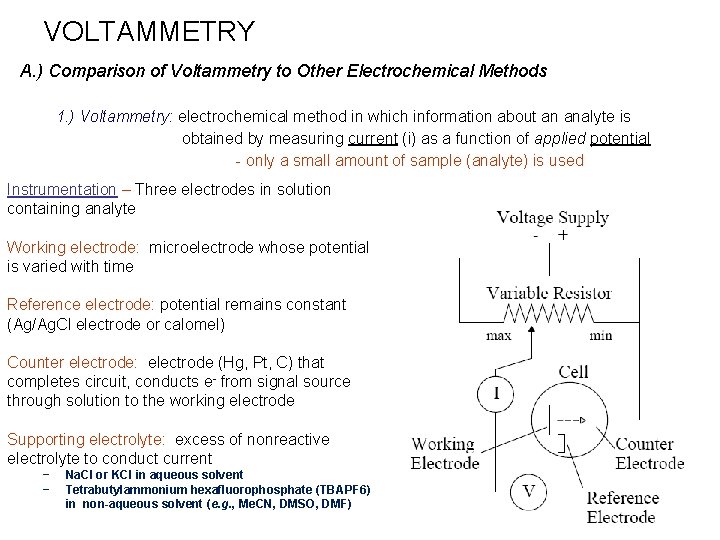
VOLTAMMETRY A. ) Comparison of Voltammetry to Other Electrochemical Methods 1. ) Voltammetry: electrochemical method in which information about an analyte is obtained by measuring current (i) as a function of applied potential - only a small amount of sample (analyte) is used Instrumentation – Three electrodes in solution containing analyte Working electrode: microelectrode whose potential is varied with time Reference electrode: potential remains constant (Ag/Ag. Cl electrode or calomel) Counter electrode: electrode (Hg, Pt, C) that completes circuit, conducts e- from signal source through solution to the working electrode Supporting electrolyte: excess of nonreactive electrolyte to conduct current − − Na. Cl or KCl in aqueous solvent Tetrabutylammonium hexafluorophosphate (TBAPF 6) in non-aqueous solvent (e. g. , Me. CN, DMSO, DMF)

VOLTAMMETRY Instrumentation – Three electrodes in solution containing analyte

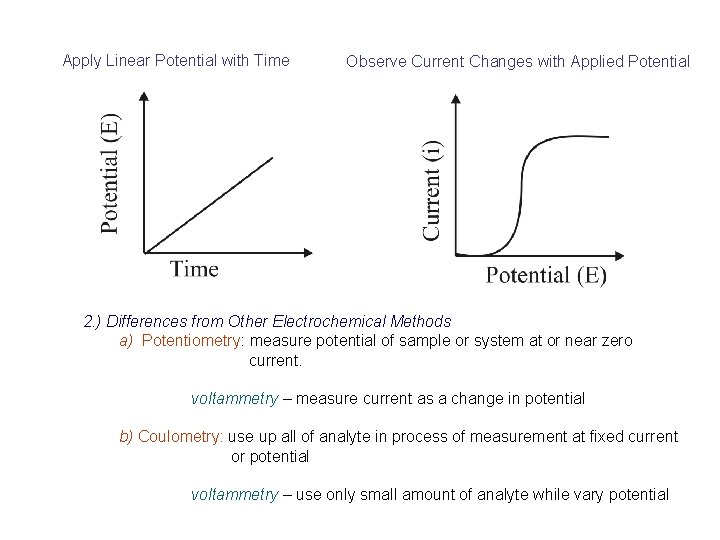
Apply Linear Potential with Time Observe Current Changes with Applied Potential 2. ) Differences from Other Electrochemical Methods a) Potentiometry: measure potential of sample or system at or near zero current. voltammetry – measure current as a change in potential b) Coulometry: use up all of analyte in process of measurement at fixed current or potential voltammetry – use only small amount of analyte while vary potential

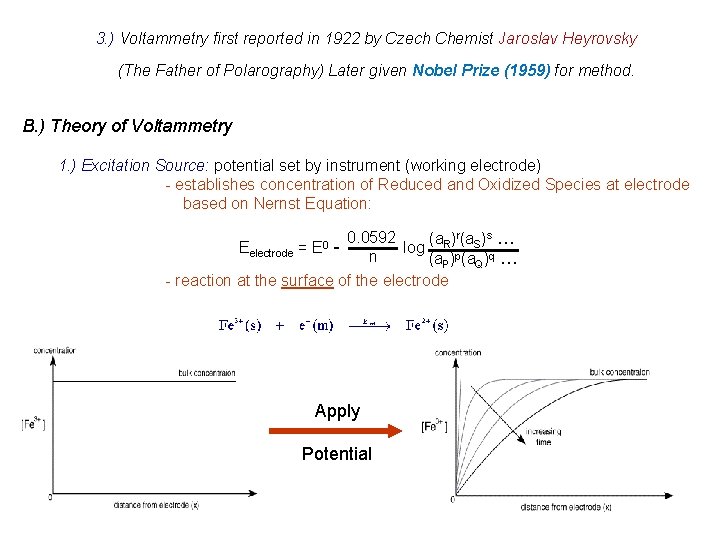
3. ) Voltammetry first reported in 1922 by Czech Chemist Jaroslav Heyrovsky (The Father of Polarography) Later given Nobel Prize (1959) for method. B. ) Theory of Voltammetry 1. ) Excitation Source: potential set by instrument (working electrode) - establishes concentration of Reduced and Oxidized Species at electrode based on Nernst Equation: 0. 0592 (a. R)r(a. S)s … Eelectrode = log n (a. P)p(a. Q)q … - reaction at the surface of the electrode E 0 Apply Potential

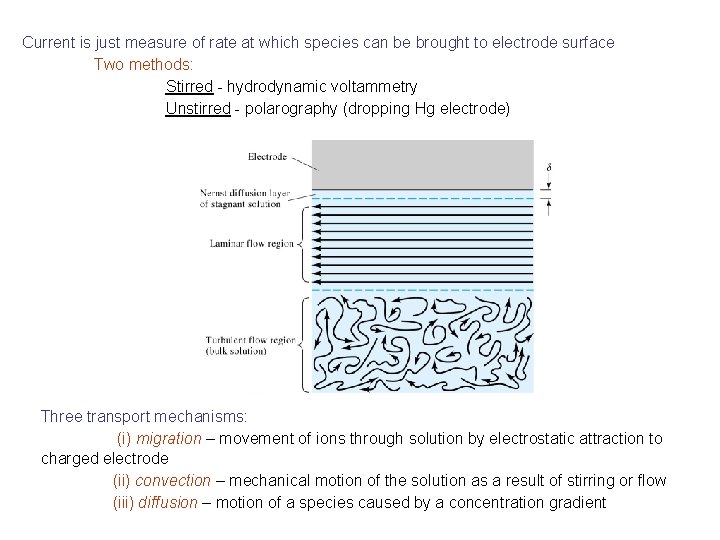
Current is just measure of rate at which species can be brought to electrode surface Two methods: Stirred - hydrodynamic voltammetry Unstirred - polarography (dropping Hg electrode) Three transport mechanisms: (i) migration – movement of ions through solution by electrostatic attraction to charged electrode (ii) convection – mechanical motion of the solution as a result of stirring or flow (iii) diffusion – motion of a species caused by a concentration gradient

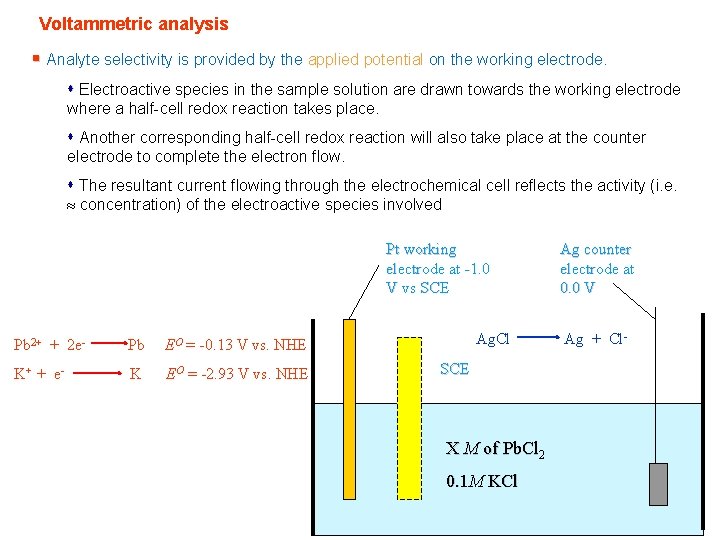
Voltammetric analysis § Analyte selectivity is provided by the applied potential on the working electrode. s Electroactive species in the sample solution are drawn towards the working electrode where a half-cell redox reaction takes place. s Another corresponding half-cell redox reaction will also take place at the counter electrode to complete the electron flow. s The resultant current flowing through the electrochemical cell reflects the activity (i. e. concentration) of the electroactive species involved Pt working electrode at -1. 0 V vs SCE Pb 2+ + 2 e- Pb EO = -0. 13 V vs. NHE K+ + e - K EO = -2. 93 V vs. NHE Ag. Cl SCE X M of Pb. Cl 2 0. 1 M KCl Ag counter electrode at 0. 0 V Ag + Cl-

Pb 2+ + 2 e- Concentration gradient created between the surrounding of the electrode and the bulk solution -1. 0 V vs SCE Pb K+ Pb 2+ K+ K+ Pb 2+ K+ Pb 2+ K+ K+ K+ Pb 2+ K+ Pb 2+ K+ K+ K+ 2+ Pb K+Pb 2+ migrate to the electrode via diffusion Pb 2+ K+ K+ Pb 2+ Layers of K+ build up around the electrode stop the migration of Pb 2+ via coulombic attraction

C) Types of Voltammetry 1. Polarography − first type of Voltammetry − controlled by diffusion, eliminates convection − uses dropping Hg electrode (DME) as working electrode; current varies as drop grows then falls off

a. Advantages of Hg Drop Electrode − High overpotential for reduction of H+ 2 H+ + 2 e- H 2 (g) (0 V vs NHE) • Allows use of Hg electrode at lower potentials than indicated from thermodynamic potentials • Example: Zn 2+ and Cd 2+ can be reduced in acidic solutions even though E 0 vs NHE = -0. 403 (Cd 2+/Cd) and -0. 763 (Zn 2+/Zn) − new electrode surface is continuously generated • Independent of past samples or absorbed impurities − reproducible currents quickly produced

b. Disadvantages of Hg Drop Electrode − Hg oxidation • Around +0. 25 V vs. SCE • Can not be used above a potential of +0. 25 V § Hg undergoes anodic dissolution ~ +0. 25 V vs. SCE and is oxidized to insoluble Hg 2 Cl 2 in presence of Cl- at zero V vs. SCE. § It cannot be used for anodic oxidation above +0. 25 V vs. SCE. − Non-Faradaic (charging/capacitance) current • limits the sensitivity to ~ 10 -5 M • residual current is > diffusion current at lower concentrations − cumbersome to use • tends to clog, causing malfunction − Hg disposal problems • mercury vapors are also very poisonous

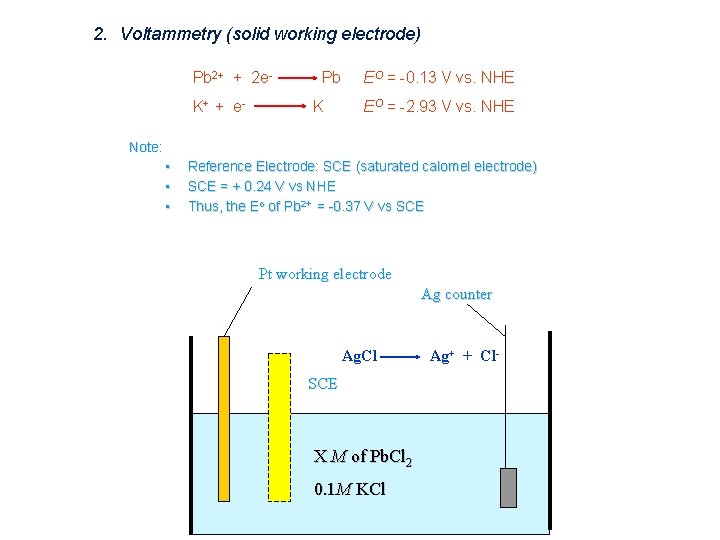
2. Voltammetry (solid working electrode) Pb 2+ + 2 e. K+ + e - Pb K EO = -0. 13 V vs. NHE EO = -2. 93 V vs. NHE Note: • • • Reference Electrode: SCE (saturated calomel electrode) SCE = + 0. 24 V vs NHE Thus, the Eo of Pb 2+ = -0. 37 V vs SCE Pt working electrode Ag counter Ag. Cl SCE X M of Pb. Cl 2 0. 1 M KCl Ag+ + Cl-

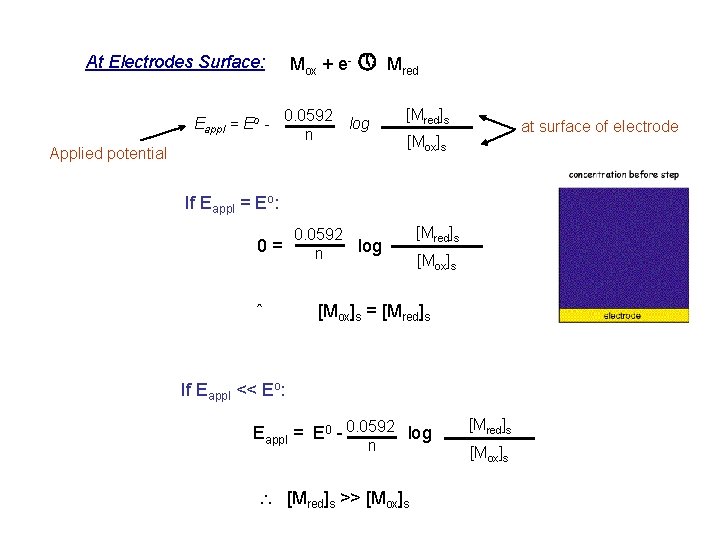
At Electrodes Surface: Mox + e- » Mred Eappl = Eo - 0. 0592 n log [Mred]s at surface of electrode [Mox]s Applied potential If Eappl = Eo: 0. 0592 0= log n ˆ [Mred]s [Mox]s = [Mred]s If Eappl << Eo: Eappl = E 0 - 0. 0592 log n [Mred]s >> [Mox]s [Mred]s [Mox]s

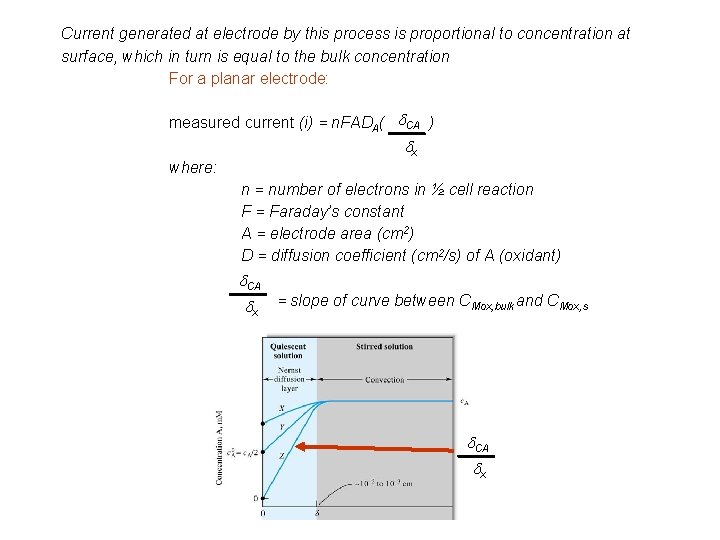
Current generated at electrode by this process is proportional to concentration at surface, which in turn is equal to the bulk concentration For a planar electrode: measured current (i) = n. FADA( d. CA ) dx where: n = number of electrons in ½ cell reaction F = Faraday’s constant A = electrode area (cm 2) D = diffusion coefficient (cm 2/s) of A (oxidant) d. CA dx = slope of curve between CMox, bulk and CMox, s d. CA dx

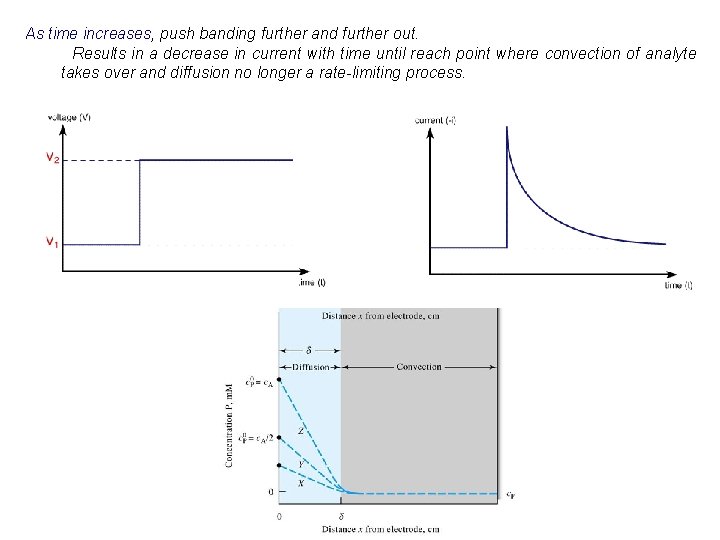
As time increases, push banding further and further out. Results in a decrease in current with time until reach point where convection of analyte takes over and diffusion no longer a rate-limiting process.

Thickness of Diffusion Layer (d): i = n. FADox (cox, bulk – cox, s) d - largest slope (highest current) will occur if: Eappl << Eo (cox, s. then i= where: k= 0) n. FADox d (cox, bulk – 0) n. FADox d so: i = kcox, bulk therefore: current is proportional to bulk concentration - also, as solution is stirred, d decreases and i increases

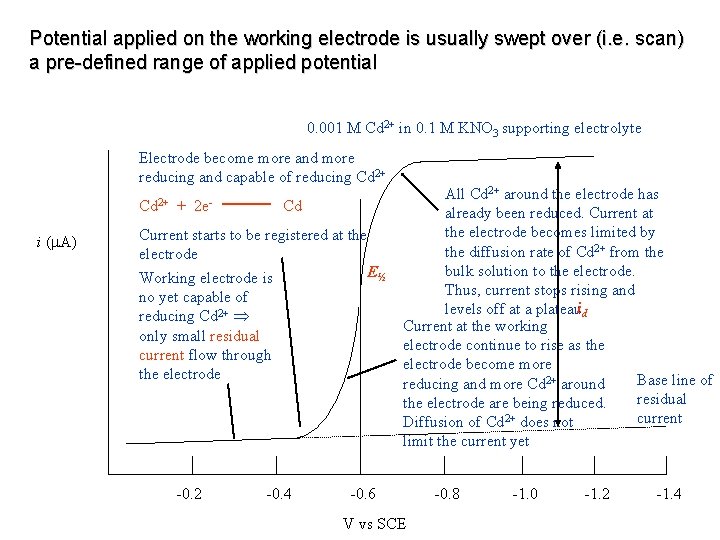
Potential applied on the working electrode is usually swept over (i. e. scan) a pre-defined range of applied potential 0. 001 M Cd 2+ in 0. 1 M KNO 3 supporting electrolyte Electrode become more and more reducing and capable of reducing Cd 2+ i ( A) + 2 e- Cd Current starts to be registered at the electrode E½ Working electrode is no yet capable of reducing Cd 2+ only small residual current flow through the electrode -0. 2 -0. 4 All Cd 2+ around the electrode has already been reduced. Current at the electrode becomes limited by the diffusion rate of Cd 2+ from the bulk solution to the electrode. Thus, current stops rising and levels off at a plateauid Current at the working electrode continue to rise as the electrode become more Base line of reducing and more Cd 2+ around residual the electrode are being reduced. current Diffusion of Cd 2+ does not limit the current yet -0. 6 V vs SCE -0. 8 -1. 0 -1. 2 -1. 4

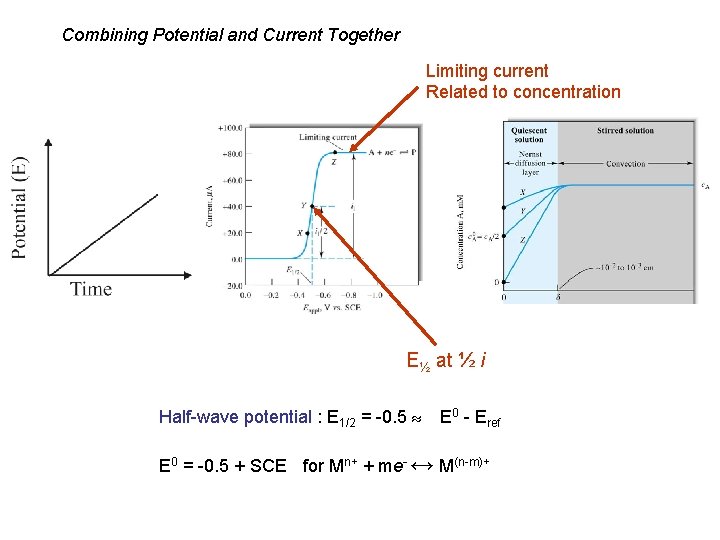
Combining Potential and Current Together Limiting current Related to concentration E½ at ½ i Half-wave potential : E 1/2 = -0. 5 E 0 - Eref E 0 = -0. 5 + SCE for Mn+ + me- ↔ M(n-m)+
![Voltammograms for Mixtures of Reactants Fe 31 x 10 4 M Fe 20 5 Voltammograms for Mixtures of Reactants [Fe 3+]=1 x 10 -4 M [Fe 2+]=0. 5](https://slidetodoc.com/presentation_image/2defa11cd3522d747a37df75a13dd9bf/image-18.jpg)
Voltammograms for Mixtures of Reactants [Fe 3+]=1 x 10 -4 M [Fe 2+]=0. 5 x 10 -4 M [Fe 3+]=0. 5 x 10 -4 M 0. 1 V 0. 2 V Two or more species are observed in voltammogram if difference in separate half-wave potentials are sufficient [Fe 2+]=1 x 10 -4 M Different concentrations result in different currents, but same potential

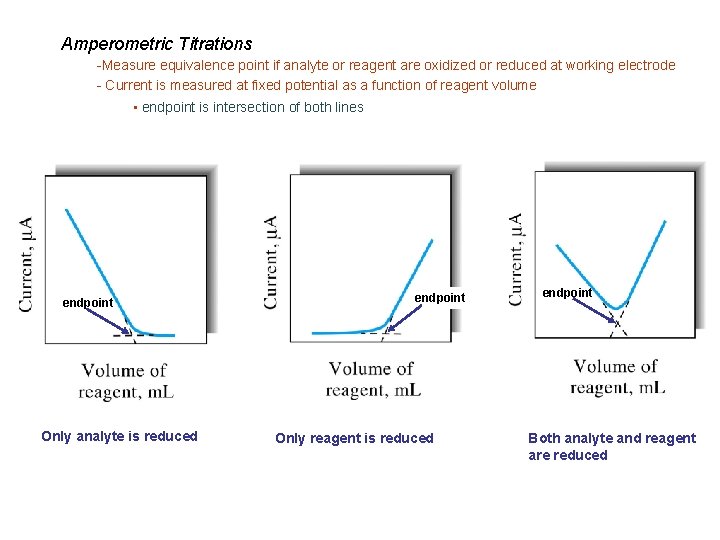
Amperometric Titrations -Measure equivalence point if analyte or reagent are oxidized or reduced at working electrode - Current is measured at fixed potential as a function of reagent volume • endpoint is intersection of both lines endpoint Only analyte is reduced endpoint Only reagent is reduced endpoint Both analyte and reagent are reduced

3. Anodic Stripping Voltammetry (ASV) - Analyte first deposited (reduced) onto the working electrode from a stirred solution -“deposit” analyte for a known period of time - analyte is redissolved or stripped (oxidized) from the electrode - analyte “preconcentrated” onto electrode, thus ASV yields lowest detection limit among all voltammetric techniques

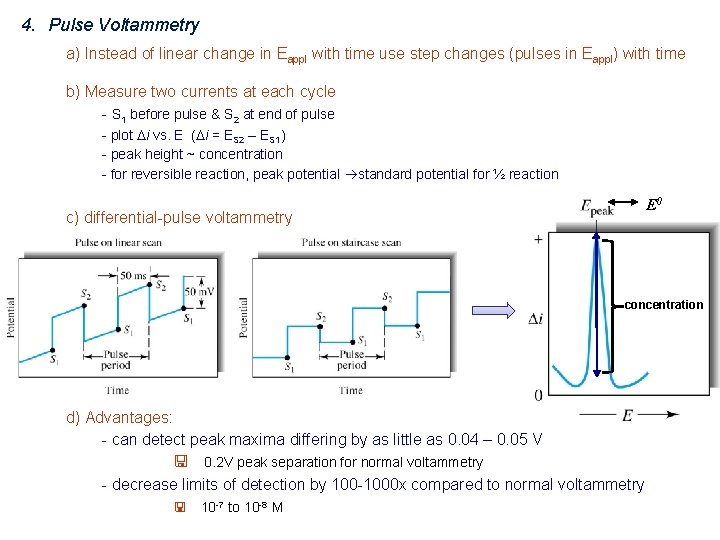
4. Pulse Voltammetry a) Instead of linear change in Eappl with time use step changes (pulses in Eappl) with time b) Measure two currents at each cycle - S 1 before pulse & S 2 at end of pulse - plot Di vs. E (Di = ES 2 – ES 1) - peak height ~ concentration - for reversible reaction, peak potential standard potential for ½ reaction E 0 c) differential-pulse voltammetry concentration d) Advantages: - can detect peak maxima differing by as little as 0. 04 – 0. 05 V < 0. 2 V peak separation for normal voltammetry - decrease limits of detection by 100 -1000 x compared to normal voltammetry < 10 -7 to 10 -8 M

5. Cyclic Voltammetry a. Method used to look at mechanisms of redox reactions in solution. b. Looks at i vs. E response of small, stationary electrode in unstirred solution using triangular waveform for excitation Cyclic voltammogram (solution phase redox species) gm en Se t 2 en gm Se t 1 Segment 2

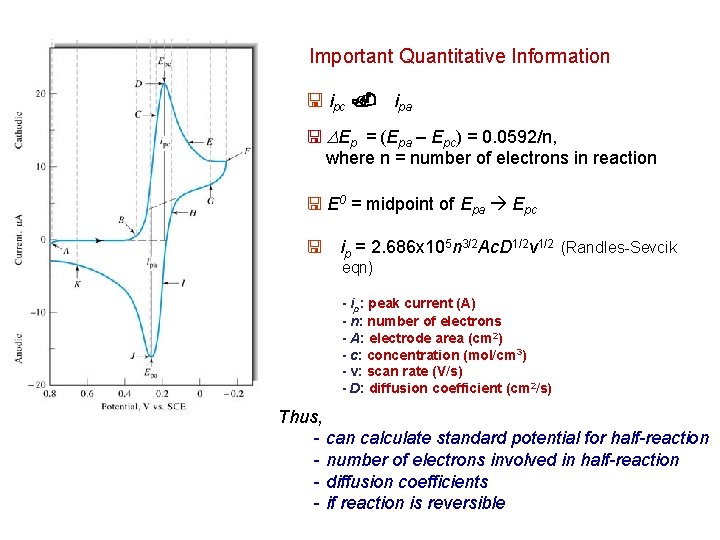
Mox + ne- Mred Fe 3+ + e- Fe 2+ - in forward scan, as E approaches E 0’ , current flow due to Mox + ne. Mred - governed by Nernst equation • concentrations made to meet Nernst equation at surface - eventually reach i max - solution not stirred, so d grows with time, leads to decrease in i max - in reverse scan - see less current as potential increases until reduction no longer occurs - then reverse reaction takes place (if reaction is reversible) - important parameters - Epc – cathodic peak potential - Epa – anodic peak potential - ipc – cathodic peak current - ipa – anodic peak current ipc ~ ipa (or 1 pc/1 pa ~ 1) Δ Ep = (Epa – Epc) = 0. 0592 V / n n = number of electrons involved in the reaction Formal reduction potential Eo’ (E 1/2) = = (Epa + Epc ) / 2

Important Quantitative Information < ipc. ipa < DEp = (Epa – Epc) = 0. 0592/n, where n = number of electrons in reaction < E 0 = midpoint of Epa Epc < ip = 2. 686 x 105 n 3/2 Ac. D 1/2 v 1/2 (Randles-Sevcik eqn) - ip: peak current (A) - n: number of electrons - A: electrode area (cm 2) - c: concentration (mol/cm 3) - v: scan rate (V/s) - D: diffusion coefficient (cm 2/s) Thus, - can calculate standard potential for half-reaction - number of electrons involved in half-reaction - diffusion coefficients - if reaction is reversible

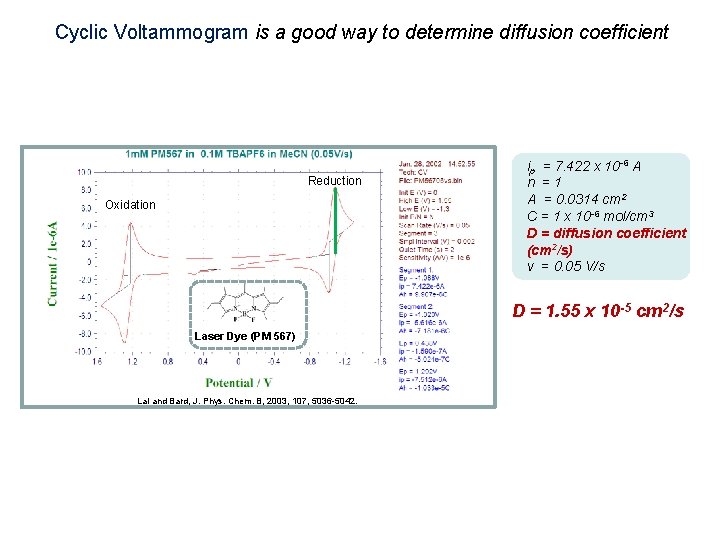
Cyclic Voltammogram is a good way to determine diffusion coefficient Reduction Oxidation ip = 7. 422 x 10 -6 A n =1 A = 0. 0314 cm 2 C = 1 x 10 -6 mol/cm 3 D = diffusion coefficient (cm 2/s) v = 0. 05 V/s D = 1. 55 x 10 -5 cm 2/s Laser Dye (PM 567) Lai and Bard, J. Phys. Chem. B, 2003, 107, 5036 -5042.
 Cv vs lsv
Cv vs lsv Stress corrosion
Stress corrosion Spontaneity of redox reactions
Spontaneity of redox reactions Electrochemical theory of corrosion
Electrochemical theory of corrosion Electrochemical impedance spectroscopy basics
Electrochemical impedance spectroscopy basics Electrochemical impulse
Electrochemical impulse Types of electrochemical sensors
Types of electrochemical sensors Electrochemistry is
Electrochemistry is The stage that creates an “electrochemical gradient”. *
The stage that creates an “electrochemical gradient”. * Electrochemical etch stop
Electrochemical etch stop Electrochemical gradient
Electrochemical gradient Electrochemical machining animation
Electrochemical machining animation Differences between nervous system and endocrine
Differences between nervous system and endocrine The electrochemical series table
The electrochemical series table Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy Cathode vs anode equation
Cathode vs anode equation How breathalyzer electrochemical
How breathalyzer electrochemical What are electrochemical series
What are electrochemical series Giner electrochemical systems
Giner electrochemical systems Electrochemical gradient
Electrochemical gradient Types of electrochemical corrosion
Types of electrochemical corrosion Khancademy
Khancademy Diffusion limited
Diffusion limited Melvin bae
Melvin bae Electrochemical deposition
Electrochemical deposition Electrochemical series order
Electrochemical series order