Cyclic Voltammetry An electrochemical technique which measures the

- Slides: 16

Cyclic Voltammetry - An electrochemical technique which measures the current that develops in an electrochemical cell under applied voltage - CV is performed by cycling the potential of a working electrode and measuring the resulting current - Involves linear scanning of potential of a stationary electrode using a triangular waveform - The most widely used technique for quantitative analysis of redox reactions Provides information on - thermodynamics of redox processes - the kinetics of heterogeneous electron transfer reactions - the kinetics of coupled reactions SAM, PCI Unit-3 1

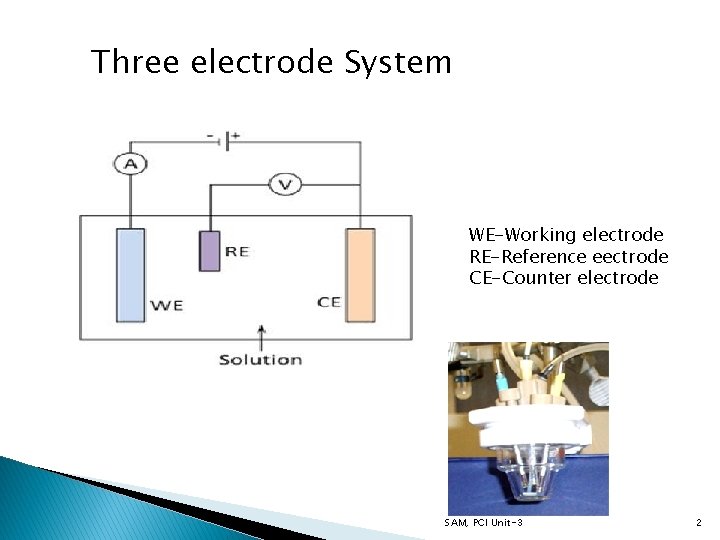
Three electrode System WE-Working electrode RE-Reference eectrode CE-Counter electrode SAM, PCI Unit-3 2

Working Electrode Ø Electrode on which the reaction of interest occurs Ø Common electrodes made of inert materials like Ag, Au, Pt, glassy carbon, etc. , Ø Size and shape of electrode varies based on applications Reference Electrode • Has a stable and well known electrode potential and used as a point of reference in electrochemical cell for the potential control and measurement • Current flow through RE kept close to zero which is achieved by using counter electrode • To close the circuit in the cell together with a very high input impedance (>100 Gohm) • Silver-silver chloride and calomel are commonly used Counter Electrode (Auxillary electrode) ü Used to close the circuit in electrochemical cell ü Usually made of inert material such as Pt, Au, graphite, etc. , ü Does not take part in electrochemical reaction SAM, PCI Unit-3 3

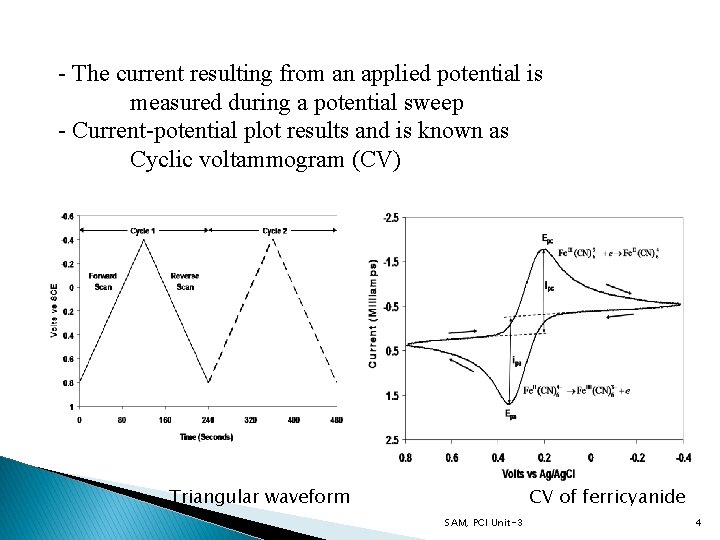
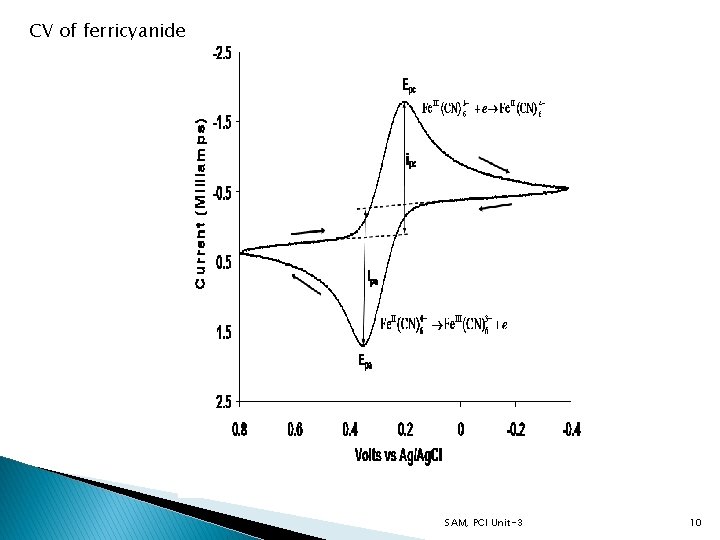
- The current resulting from an applied potential is measured during a potential sweep - Current-potential plot results and is known as Cyclic voltammogram (CV) Triangular waveform CV of ferricyanide SAM, PCI Unit-3 4

Working - Assume only Oxidant is present initially - A negative potential sweep results in the reduction of Oxidant to Reductant (starting from a value where no reduction of O initially occurs) - As potential approaches Eo for the redox process, a cathodic current is observed until a peak is reached - The direction of potential sweep is reversed after going beyond the region where reduction is observed - This region is at least 90/n m. V beyond the peak SAM, PCI Unit-3 5

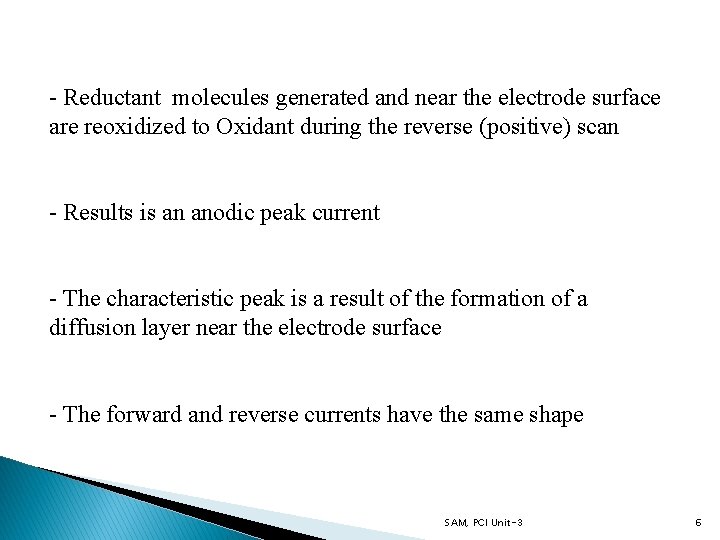
- Reductant molecules generated and near the electrode surface are reoxidized to Oxidant during the reverse (positive) scan - Results is an anodic peak current - The characteristic peak is a result of the formation of a diffusion layer near the electrode surface - The forward and reverse currents have the same shape SAM, PCI Unit-3 6

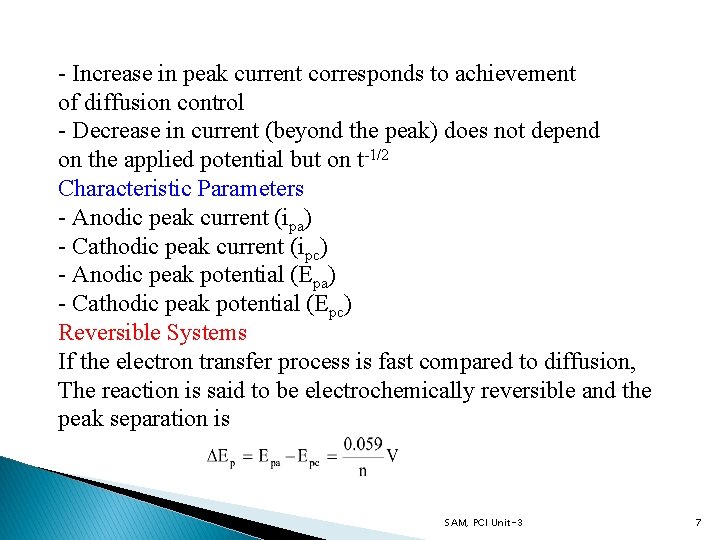
- Increase in peak current corresponds to achievement of diffusion control - Decrease in current (beyond the peak) does not depend on the applied potential but on t-1/2 Characteristic Parameters - Anodic peak current (ipa) - Cathodic peak current (ipc) - Anodic peak potential (Epa) - Cathodic peak potential (Epc) Reversible Systems If the electron transfer process is fast compared to diffusion, The reaction is said to be electrochemically reversible and the peak separation is SAM, PCI Unit-3 7

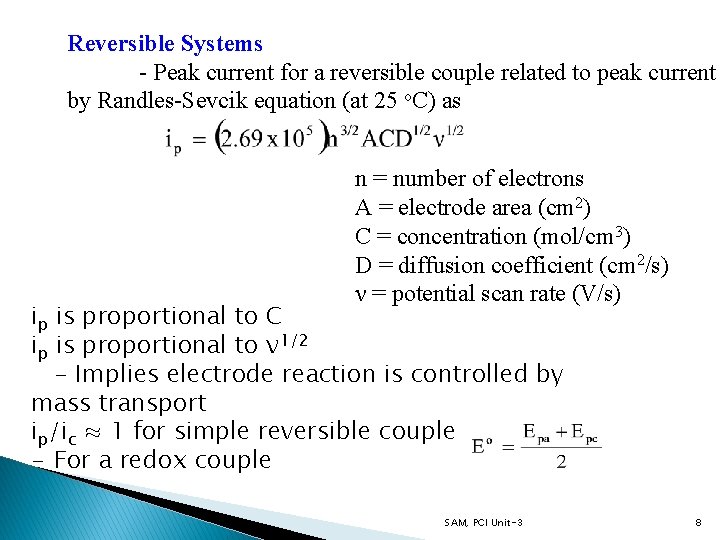
Reversible Systems - Peak current for a reversible couple related to peak current by Randles-Sevcik equation (at 25 o. C) as n = number of electrons A = electrode area (cm 2) C = concentration (mol/cm 3) D = diffusion coefficient (cm 2/s) ν = potential scan rate (V/s) ip is proportional to C ip is proportional to ν 1/2 - Implies electrode reaction is controlled by mass transport ip/ic ≈ 1 for simple reversible couple - For a redox couple SAM, PCI Unit-3 8

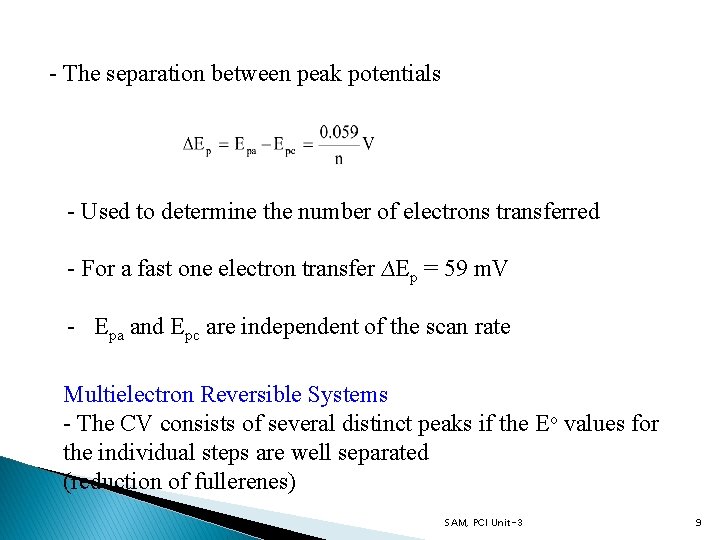
- The separation between peak potentials - Used to determine the number of electrons transferred - For a fast one electron transfer ∆Ep = 59 m. V - Epa and Epc are independent of the scan rate Multielectron Reversible Systems - The CV consists of several distinct peaks if the Eo values for the individual steps are well separated (reduction of fullerenes) SAM, PCI Unit-3 9

CV of ferricyanide SAM, PCI Unit-3 10

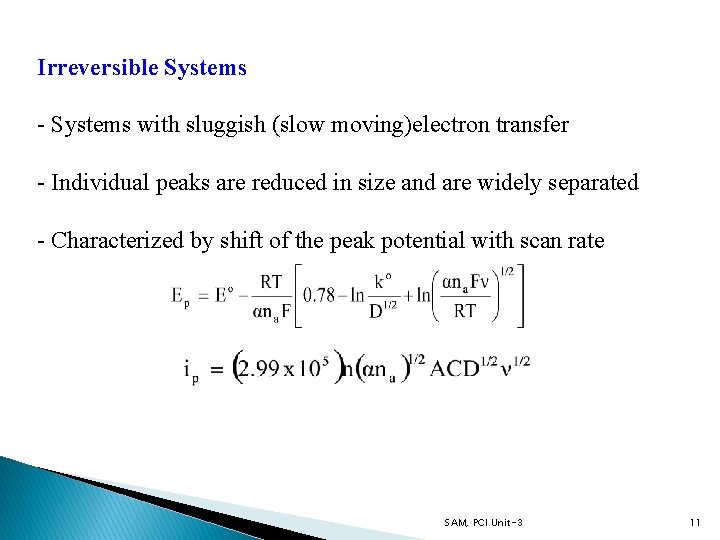
Irreversible Systems - Systems with sluggish (slow moving)electron transfer - Individual peaks are reduced in size and are widely separated - Characterized by shift of the peak potential with scan rate SAM, PCI Unit-3 11

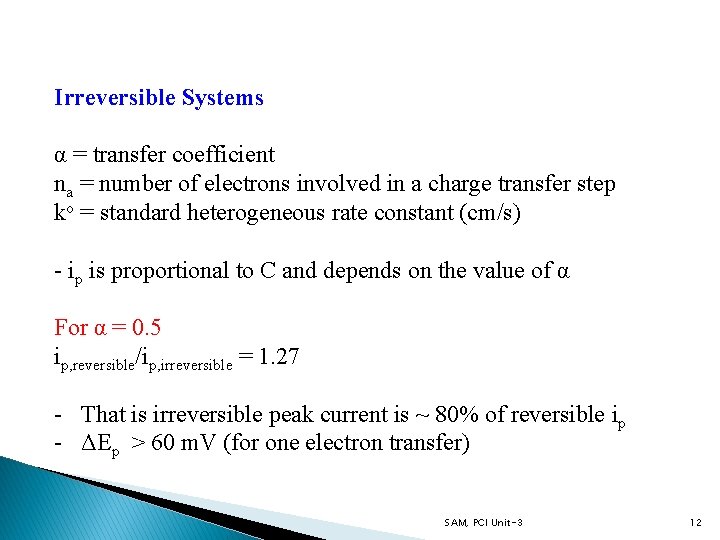
Irreversible Systems α = transfer coefficient na = number of electrons involved in a charge transfer step ko = standard heterogeneous rate constant (cm/s) - ip is proportional to C and depends on the value of α For α = 0. 5 ip, reversible/ip, irreversible = 1. 27 - That is irreversible peak current is ~ 80% of reversible ip - ΔEp > 60 m. V (for one electron transfer) SAM, PCI Unit-3 12

Quasi-reversible Systems - Current is controlled by both charge transfer and mass transport - Voltammograms are more drawn out - Exhibit larger separation in peak potentials compared to reversible systems - Shape depends on heterogeneous rate constant and scan rate - Exhibits irreversible behavior at very fast scan rates SAM, PCI Unit-3 13

SPECTROELECTROCHEMISTRY - Simultaneous measurement of spectral and electrochemical signals - Coupling of optical and electrochemical methods - Employs optically transparent electrode (OTE) that allows light to pass through the surface and adjacent solution Examples Indium tin oxide (ITO), platinum, gold, silver, nickel deposited on optically transparent glass or quartz substrate SAM, PCI Unit-3 14

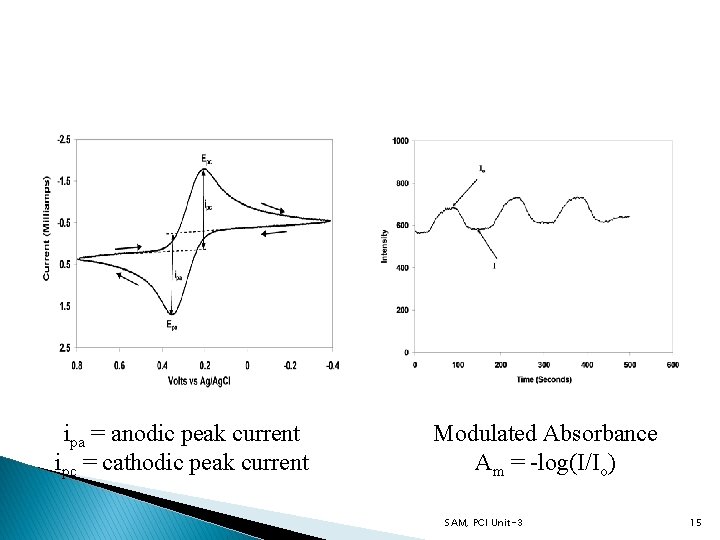
ipa = anodic peak current ipc = cathodic peak current Modulated Absorbance Am = -log(I/Io) SAM, PCI Unit-3 15

Applications - Useful for elucidation of reaction kinetics and mechanisms (for probing adsorption and desorption processes) - Thin layer SE methods for measuring Eo and n (Nernst equation) - Infrared SE methods for providing structural information - UV-Vis spectroscopic procedures - Vibration spectroscopic investigations - Luminescence reflectance and scattering studies SAM, PCI Unit-3 16