Chapter 25 Voltammetry 1 Voltammetric instrumentation 1 1

- Slides: 12

Chapter 25 Voltammetry 1 Voltammetric instrumentation 1. 1 Three-electrode voltammetry - based on measurement of current I as a function of voltage Why 3 electrodes? - 2 electrodes OK with potentiometry (high impedance, very small I) In voltammetry, want to measure current I, and the larger I the better Potential drops when I is taken from electrode (IR drop) Must minimize current withdrawn from the reference electrode surface use 3 electrodes working, counter and reference electrodes

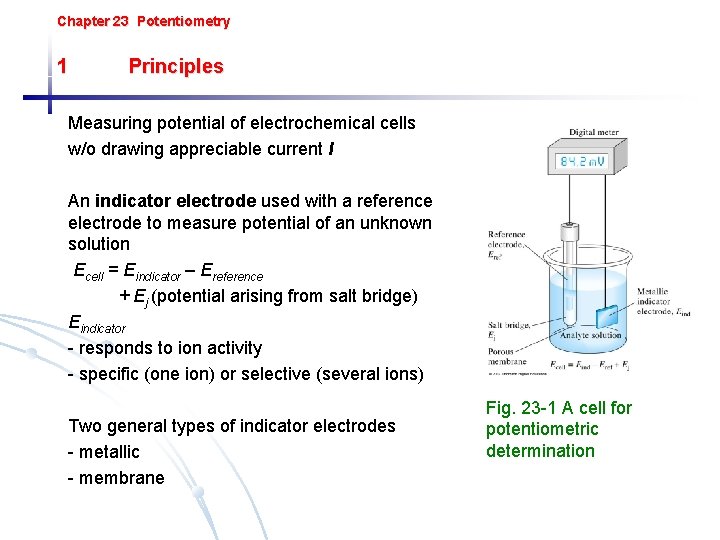
Chapter 23 Potentiometry 1 Principles Measuring potential of electrochemical cells w/o drawing appreciable current I An indicator electrode used with a reference electrode to measure potential of an unknown solution Ecell = Eindicator – Ereference + Ej (potential arising from salt bridge) Eindicator - responds to ion activity - specific (one ion) or selective (several ions) Two general types of indicator electrodes - metallic - membrane Fig. 23 -1 A cell for potentiometric determination

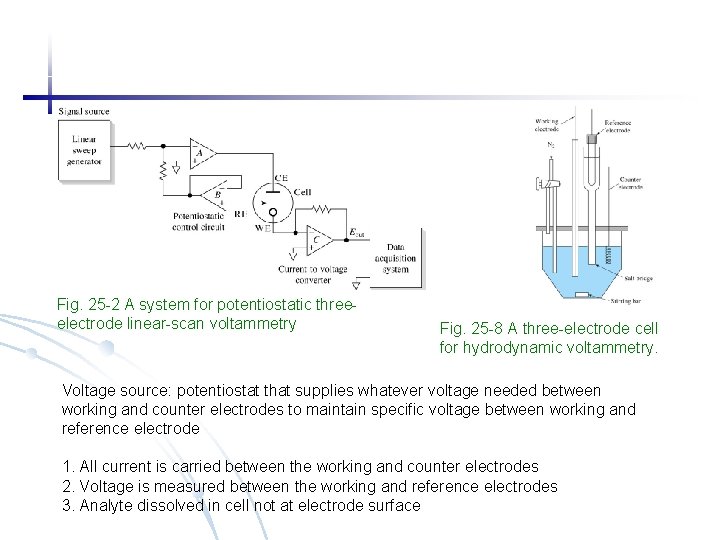
Fig. 25 -2 A system for potentiostatic threeelectrode linear-scan voltammetry Fig. 25 -8 A three-electrode cell for hydrodynamic voltammetry. Voltage source: potentiostat that supplies whatever voltage needed between working and counter electrodes to maintain specific voltage between working and reference electrode 1. All current is carried between the working and counter electrodes 2. Voltage is measured between the working and reference electrodes 3. Analyte dissolved in cell not at electrode surface

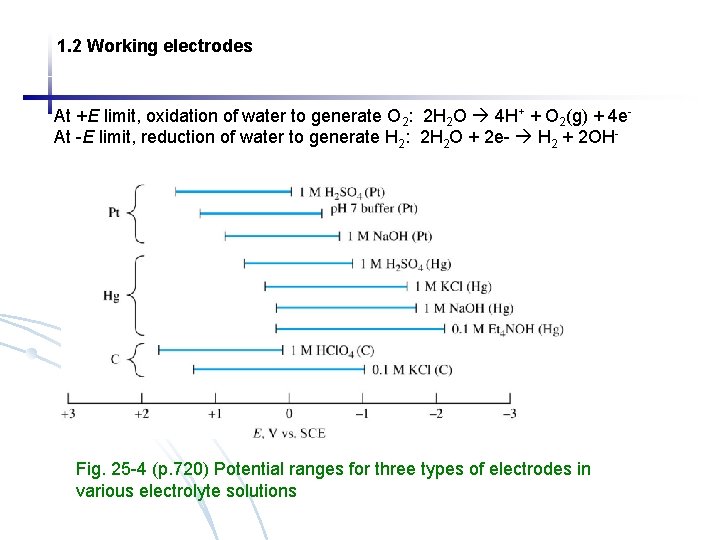
1. 2 Working electrodes At +E limit, oxidation of water to generate O 2: 2 H 2 O 4 H+ + O 2(g) + 4 e. At -E limit, reduction of water to generate H 2: 2 H 2 O + 2 e- H 2 + 2 OH- Fig. 25 -4 (p. 720) Potential ranges for three types of electrodes in various electrolyte solutions

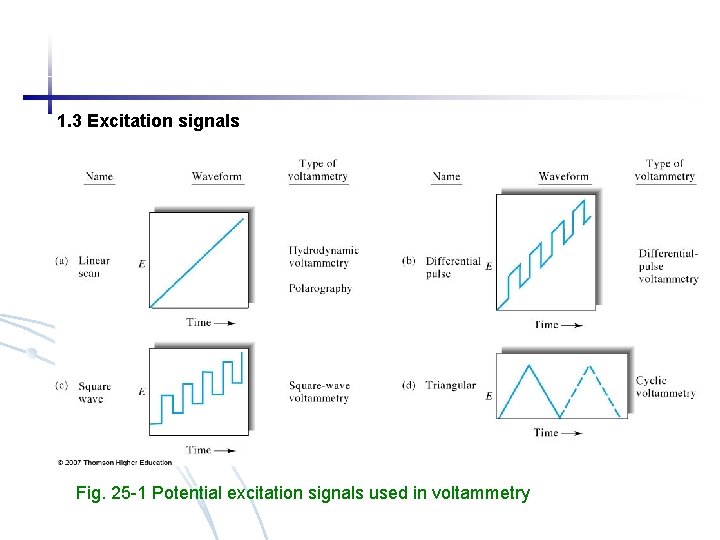
1. 3 Excitation signals Fig. 25 -1 Potential excitation signals used in voltammetry

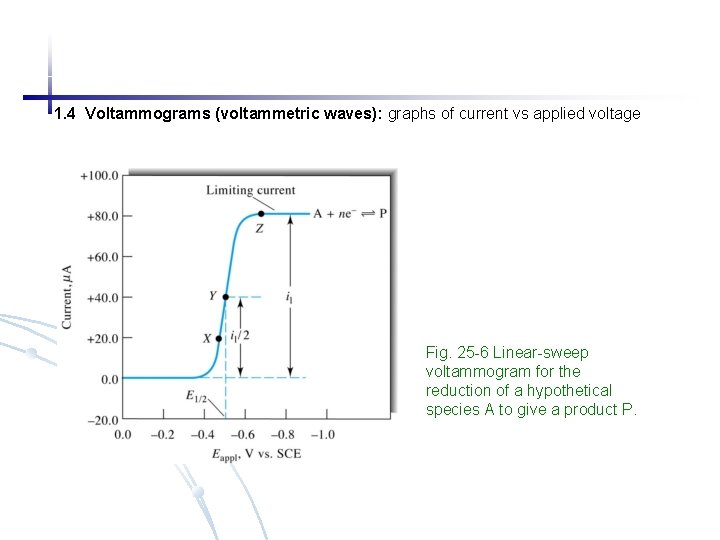
1. 4 Voltammograms (voltammetric waves): graphs of current vs applied voltage Fig. 25 -6 Linear-sweep voltammogram for the reduction of a hypothetical species A to give a product P.

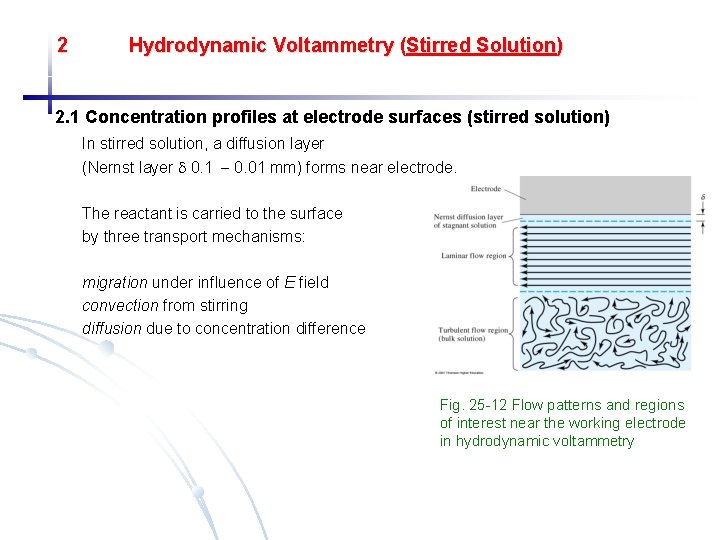
2 Hydrodynamic Voltammetry (Stirred Solution) 2. 1 Concentration profiles at electrode surfaces (stirred solution) In stirred solution, a diffusion layer (Nernst layer 0. 1 0. 01 mm) forms near electrode. The reactant is carried to the surface by three transport mechanisms: migration under influence of E field convection from stirring diffusion due to concentration difference Fig. 25 -12 Flow patterns and regions of interest near the working electrode in hydrodynamic voltammetry

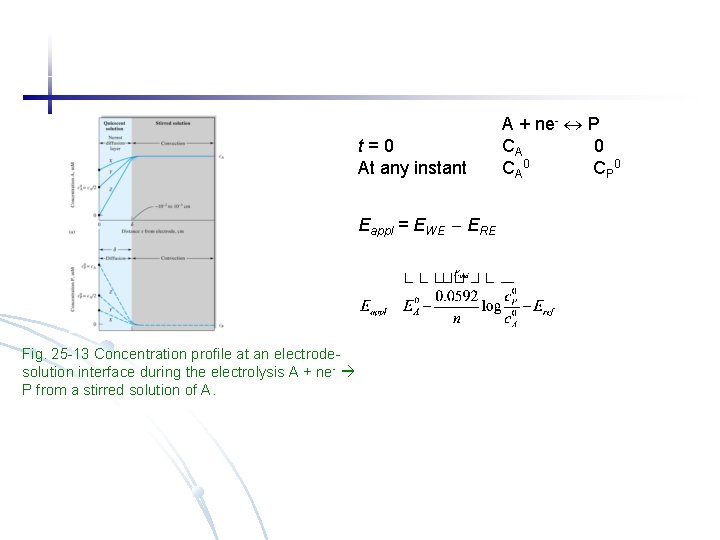
t=0 At any instant Eappl = EWE ERE Fig. 25 -13 Concentration profile at an electrodesolution interface during the electrolysis A + ne- P from a stirred solution of A. A + ne- P CA 0 CP 0

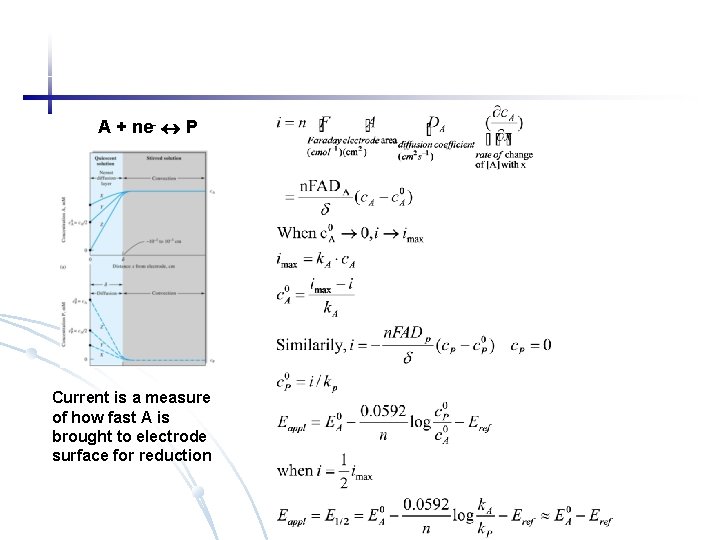
A + ne- P Current is a measure of how fast A is brought to electrode surface for reduction

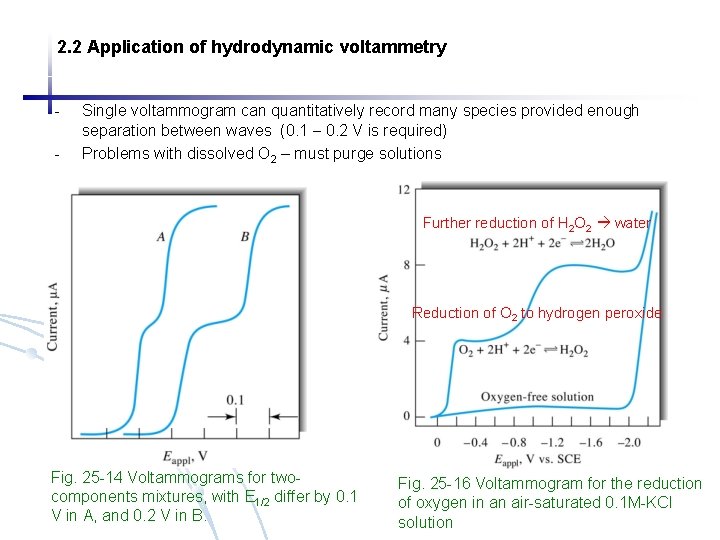
2. 2 Application of hydrodynamic voltammetry - Single voltammogram can quantitatively record many species provided enough separation between waves (0. 1 0. 2 V is required) Problems with dissolved O 2 – must purge solutions Further reduction of H 2 O 2 water Reduction of O 2 to hydrogen peroxide Fig. 25 -14 Voltammograms for twocomponents mixtures, with E 1/2 differ by 0. 1 V in A, and 0. 2 V in B. Fig. 25 -16 Voltammogram for the reduction of oxygen in an air-saturated 0. 1 M-KCl solution

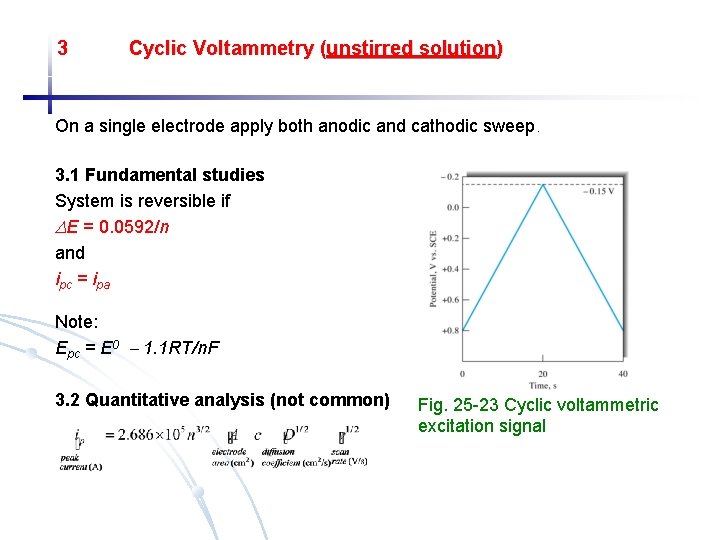
3 Cyclic Voltammetry (unstirred solution) On a single electrode apply both anodic and cathodic sweep. 3. 1 Fundamental studies System is reversible if E = 0. 0592/n and ipc = ipa Note: Epc = E 0 1. 1 RT/n. F 3. 2 Quantitative analysis (not common) Fig. 25 -23 Cyclic voltammetric excitation signal

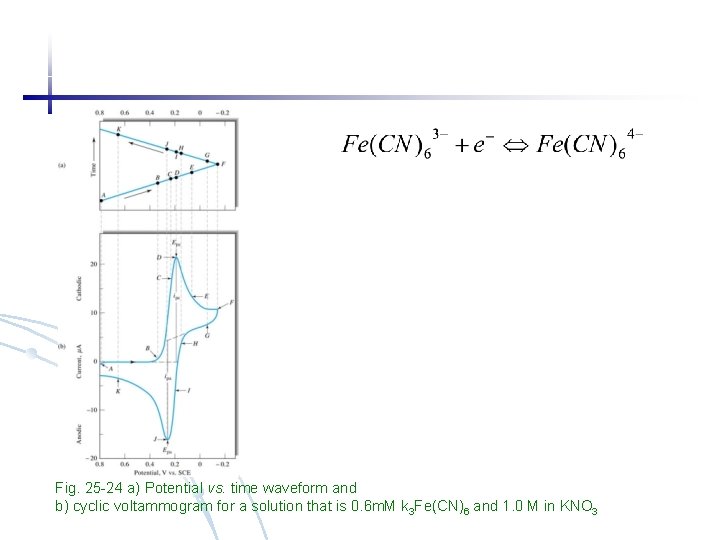
Fig. 25 -24 a) Potential vs. time waveform and b) cyclic voltammogram for a solution that is 0. 6 m. M k 3 Fe(CN)6 and 1. 0 M in KNO 3