Chapter 25 Voltammetry 1 Voltammetric instrumentation 1 1
- Slides: 9

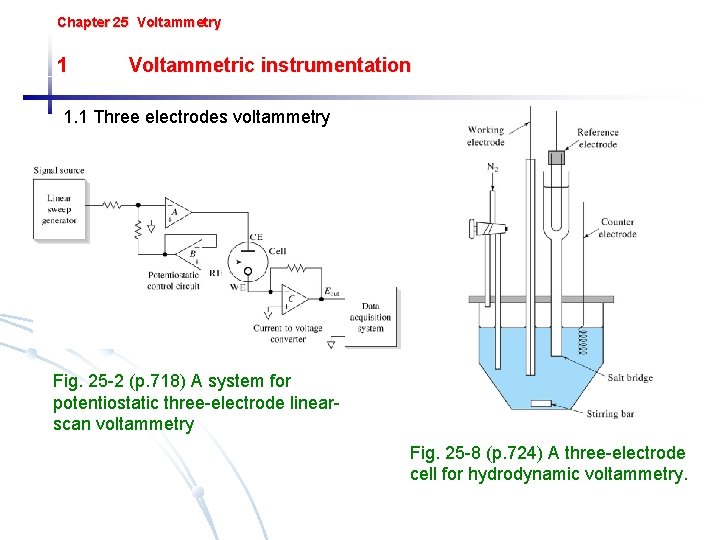
Chapter 25 Voltammetry 1 Voltammetric instrumentation 1. 1 Three electrodes voltammetry Fig. 25 -2 (p. 718) A system for potentiostatic three-electrode linearscan voltammetry Fig. 25 -8 (p. 724) A three-electrode cell for hydrodynamic voltammetry.

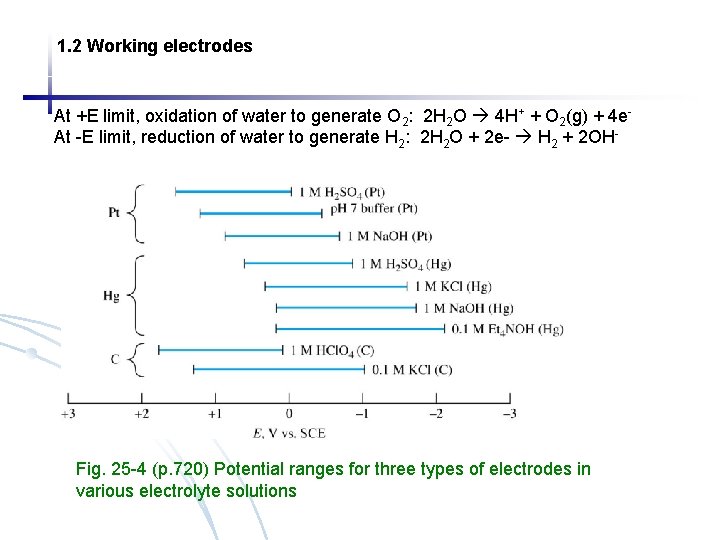
1. 2 Working electrodes At +E limit, oxidation of water to generate O 2: 2 H 2 O 4 H+ + O 2(g) + 4 e. At -E limit, reduction of water to generate H 2: 2 H 2 O + 2 e- H 2 + 2 OH- Fig. 25 -4 (p. 720) Potential ranges for three types of electrodes in various electrolyte solutions

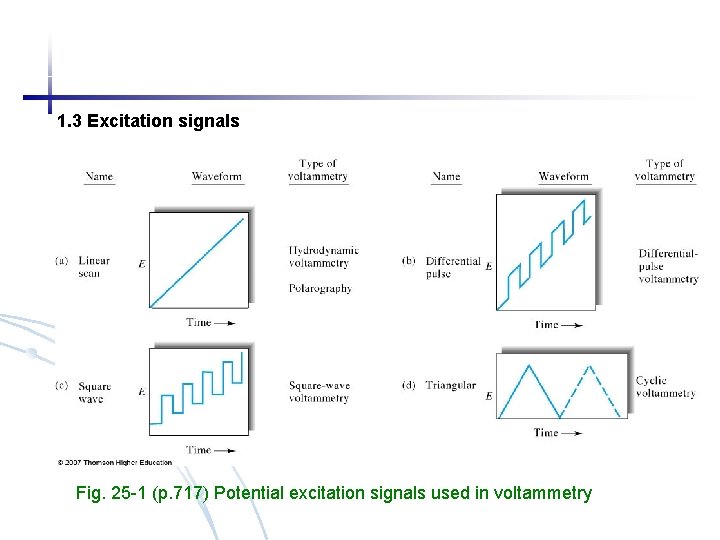
1. 3 Excitation signals Fig. 25 -1 (p. 717) Potential excitation signals used in voltammetry

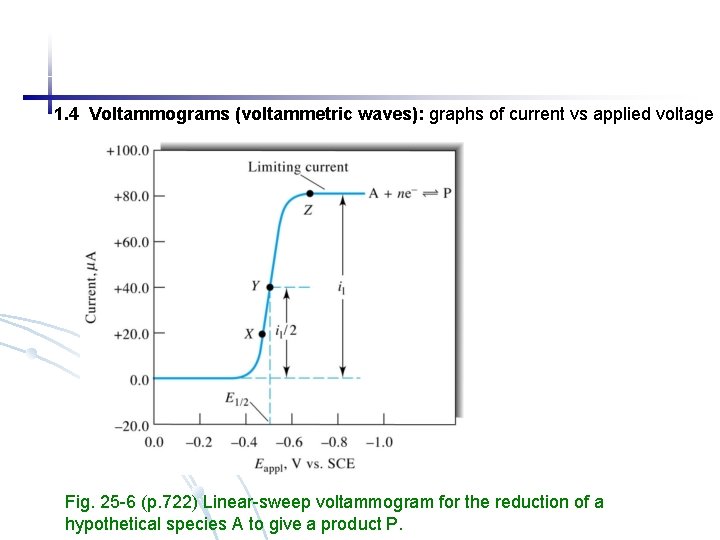
1. 4 Voltammograms (voltammetric waves): graphs of current vs applied voltage Fig. 25 -6 (p. 722) Linear-sweep voltammogram for the reduction of a hypothetical species A to give a product P.

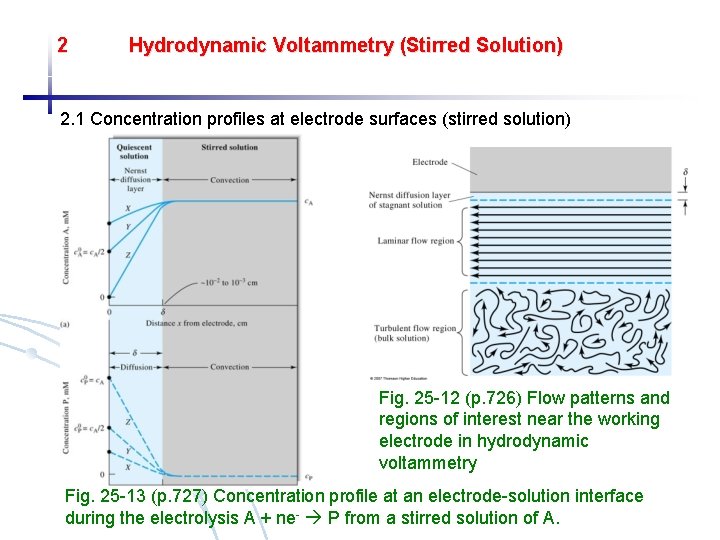
2 Hydrodynamic Voltammetry (Stirred Solution) 2. 1 Concentration profiles at electrode surfaces (stirred solution) Fig. 25 -12 (p. 726) Flow patterns and regions of interest near the working electrode in hydrodynamic voltammetry Fig. 25 -13 (p. 727) Concentration profile at an electrode-solution interface during the electrolysis A + ne- P from a stirred solution of A.

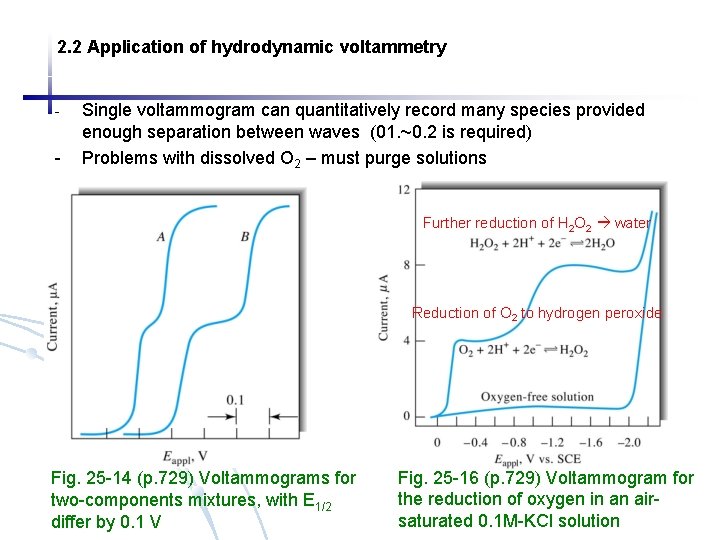
2. 2 Application of hydrodynamic voltammetry - - Single voltammogram can quantitatively record many species provided enough separation between waves (01. ~0. 2 is required) Problems with dissolved O 2 – must purge solutions Further reduction of H 2 O 2 water Reduction of O 2 to hydrogen peroxide Fig. 25 -14 (p. 729) Voltammograms for two-components mixtures, with E 1/2 differ by 0. 1 V Fig. 25 -16 (p. 729) Voltammogram for the reduction of oxygen in an airsaturated 0. 1 M-KCl solution

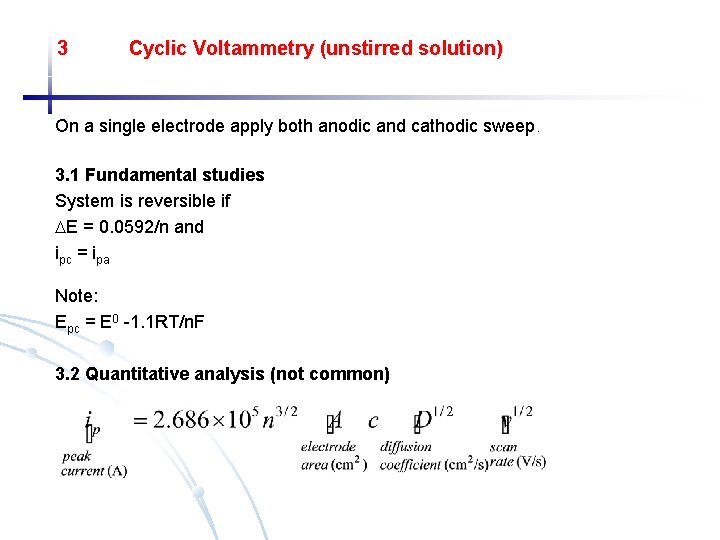
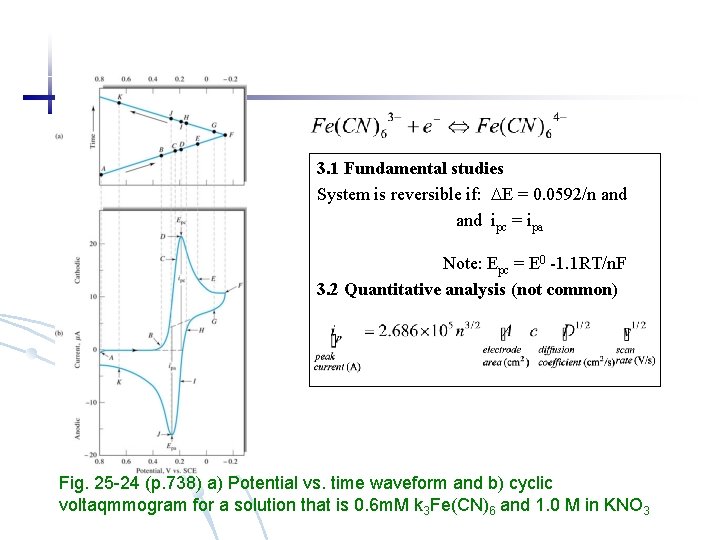
3 Cyclic Voltammetry (unstirred solution) On a single electrode apply both anodic and cathodic sweep. 3. 1 Fundamental studies System is reversible if E = 0. 0592/n and ipc = ipa Note: Epc = E 0 -1. 1 RT/n. F 3. 2 Quantitative analysis (not common)

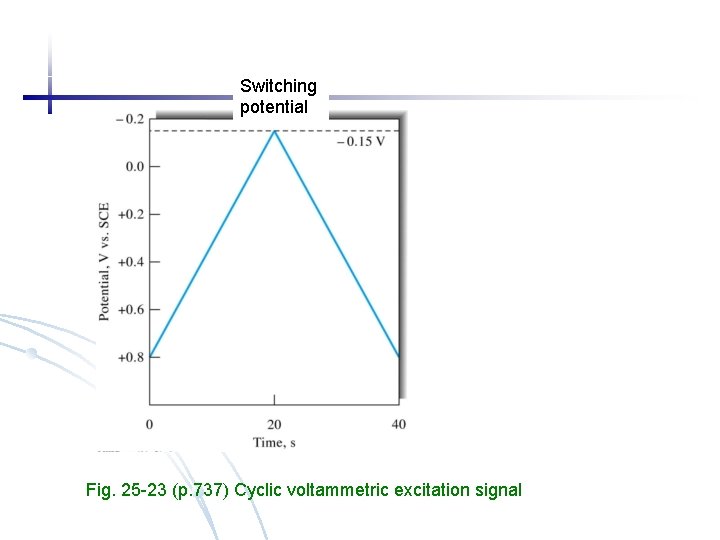
Switching potential Fig. 25 -23 (p. 737) Cyclic voltammetric excitation signal

3. 1 Fundamental studies System is reversible if: E = 0. 0592/n and ipc = ipa Note: Epc = E 0 -1. 1 RT/n. F 3. 2 Quantitative analysis (not common) Fig. 25 -24 (p. 738) a) Potential vs. time waveform and b) cyclic voltaqmmogram for a solution that is 0. 6 m. M k 3 Fe(CN)6 and 1. 0 M in KNO 3