Light is an electromagnetic wave EM wave a











- Slides: 24


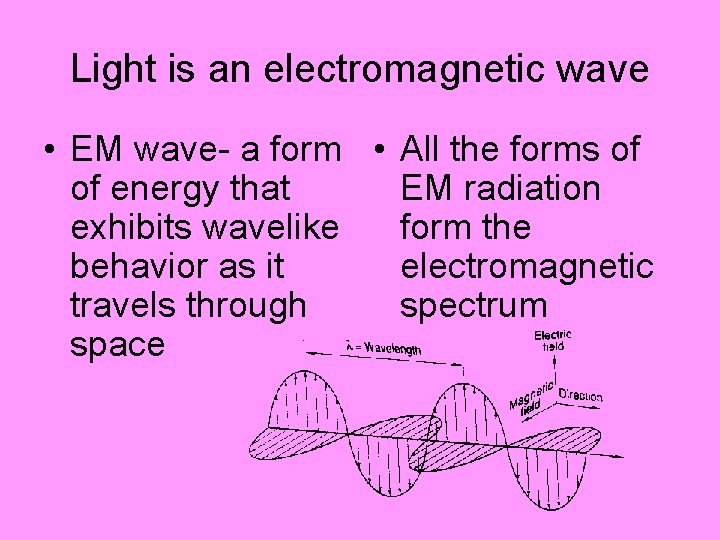
Light is an electromagnetic wave • EM wave- a form • All the forms of of energy that EM radiation exhibits wavelike form the behavior as it electromagnetic travels through spectrum space

Properties of EM waves • Speed- all forms of • Frequency- the # of EM radiation travel waves that pass a at 3 x 108 m/s in a stationary pt. in vacuum one second • Wavelength-the • Amplitude- the distance between 2 height of a wave consecutive waves measured from the origin to its crest

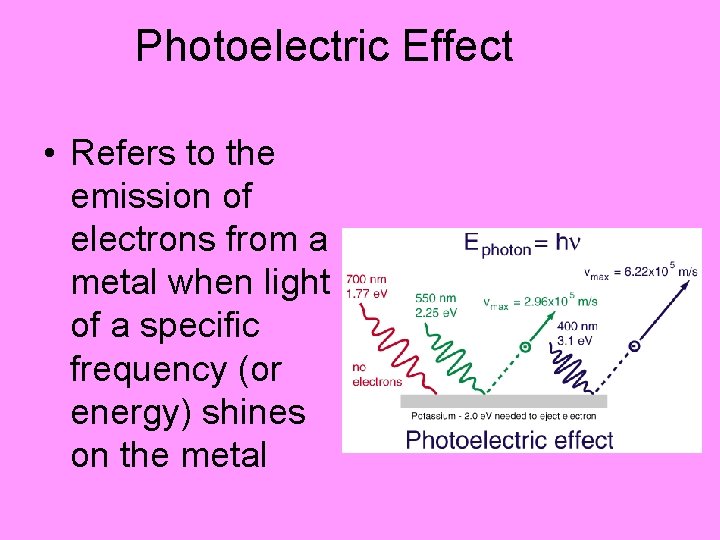
Photoelectric Effect • Refers to the emission of electrons from a metal when light of a specific frequency (or energy) shines on the metal

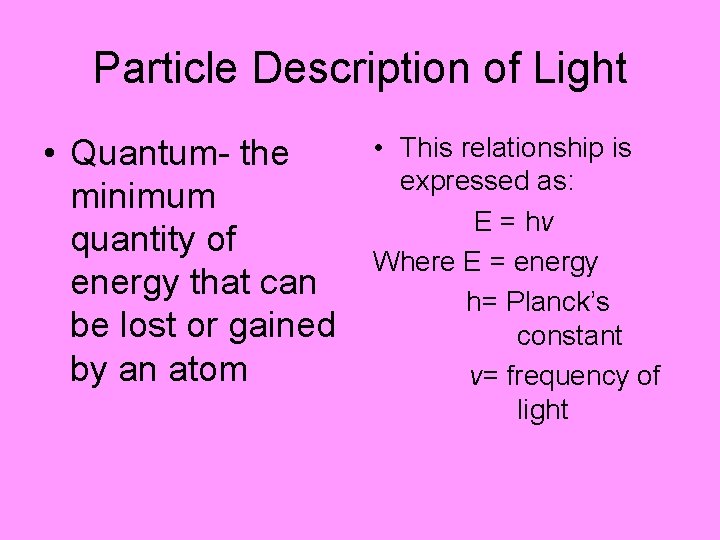
Particle Description of Light • Quantum- the minimum quantity of energy that can be lost or gained by an atom • This relationship is expressed as: E = hv Where E = energy h= Planck’s constant v= frequency of light

The Dual Nature of Light • Light exhibits • Photon- a many wavelike particle of EM properties but radiation can also be having zero thought of as a mass and stream of carrying a particles called quantum of photons. energy

Bohr’s Model • The electron can circle the nucleus only in allowed paths or orbits • In an orbit, an electron has a fixed energy • The lowest energy state is closest to the nucleus • An electron can move to a higher orbital if it gains the amount of energy equal to the difference in energy between the initial orbit and the higher energy orbit

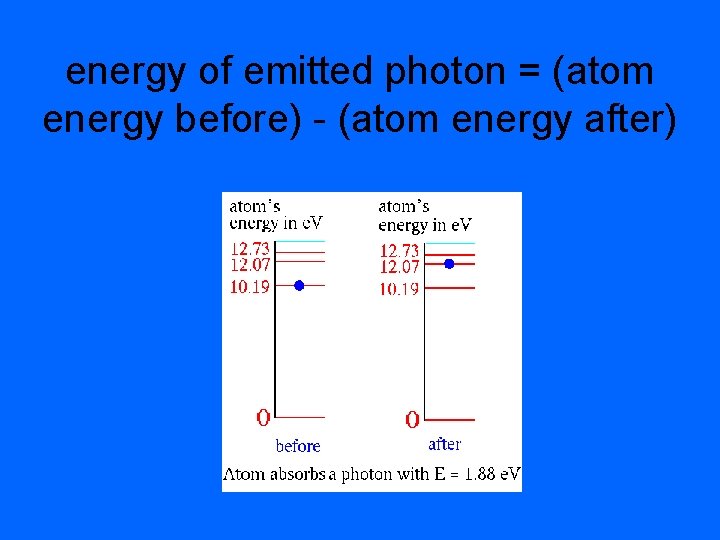
Line Emission Spectra • The lowest energy state of an electron is the ground state. • A state in which that atom has a higher energy potential is called an excited state. • As the excited electron falls back to its ground state, it releases EM radiation of an energy that corresponds to the amount of energy gained to reach the excited state.

energy of emitted photon = (atom energy before) - (atom energy after)

• When the EM radiation released is passed through a prism or diffraction grating- it is separated into a series of specific frequencies of visible light. • This is referred to as a line emission spectra


De. Broglie’s Idea • Suggested that electrons could also have a wave nature much like light. This was based on the fact that: v Electrons can be diffracted (bending) v Electrons exhibit interference (overlapping that results in a reduction of energy)

• It is impossible to determine simultaneously both the position and velocity of an electron.

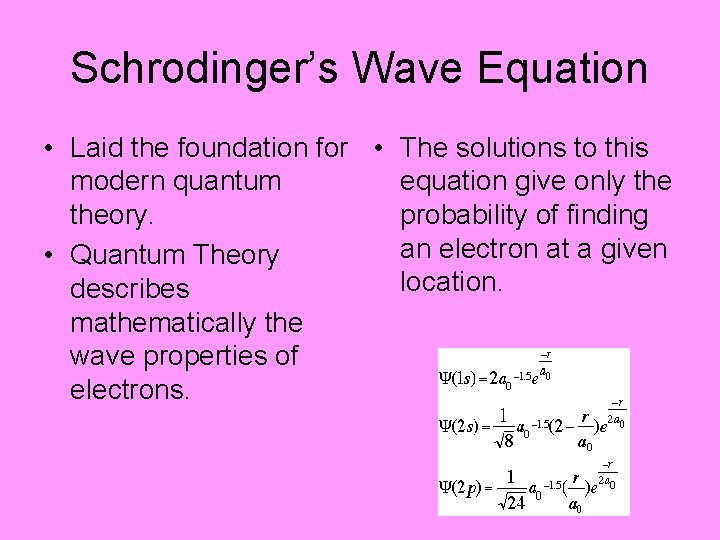
Schrodinger’s Wave Equation • Laid the foundation for • The solutions to this modern quantum equation give only theory. probability of finding an electron at a given • Quantum Theory location. describes mathematically the wave properties of electrons.

• Electrons do not travel in neat orbits • They exist in three-dimensional regions called orbits that indicate the probable location of an electron.

The location of electrons within the atom can be described using quantum numbers: v. Principle v. Orbital (Angular Momentum) v. Magnetic v. Spin

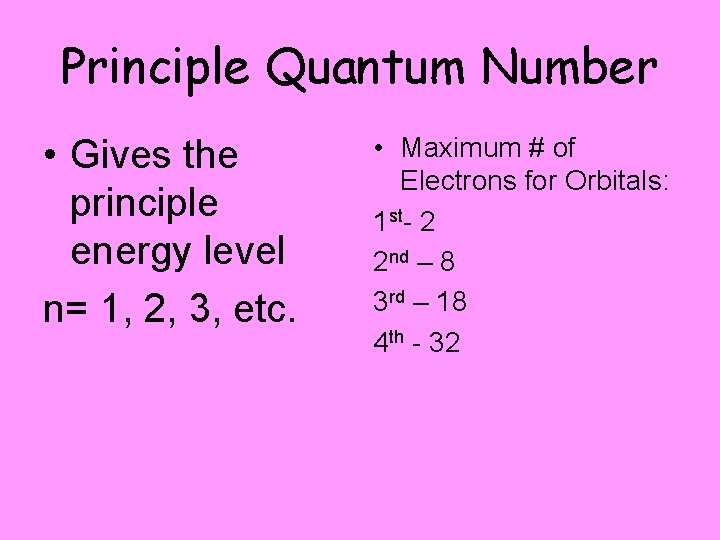
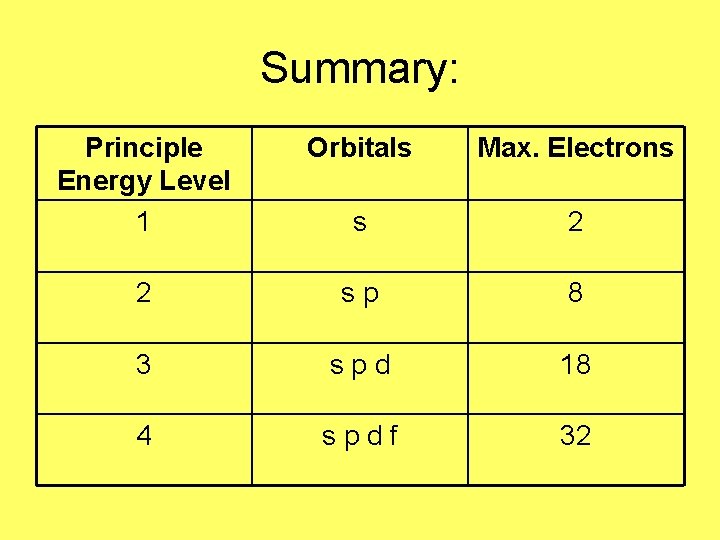
Principle Quantum Number • Gives the principle energy level n= 1, 2, 3, etc. • Maximum # of Electrons for Orbitals: 1 st- 2 2 nd – 8 3 rd – 18 4 th - 32

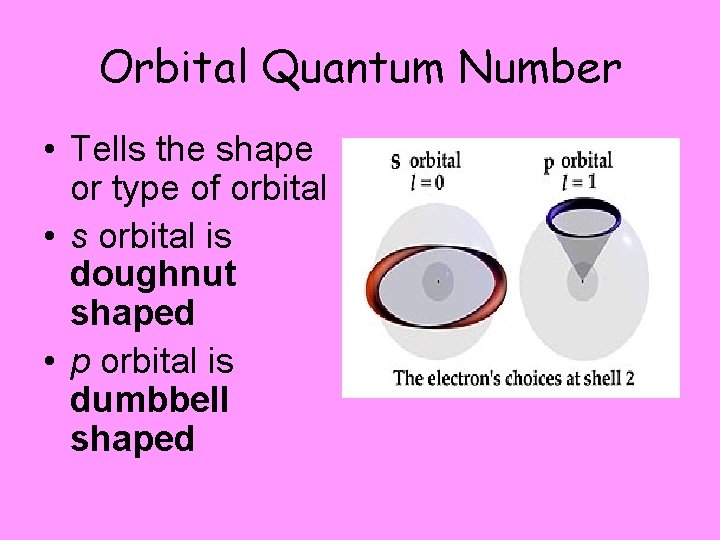
Orbital Quantum Number • Tells the shape or type of orbital • s orbital is doughnut shaped • p orbital is dumbbell shaped

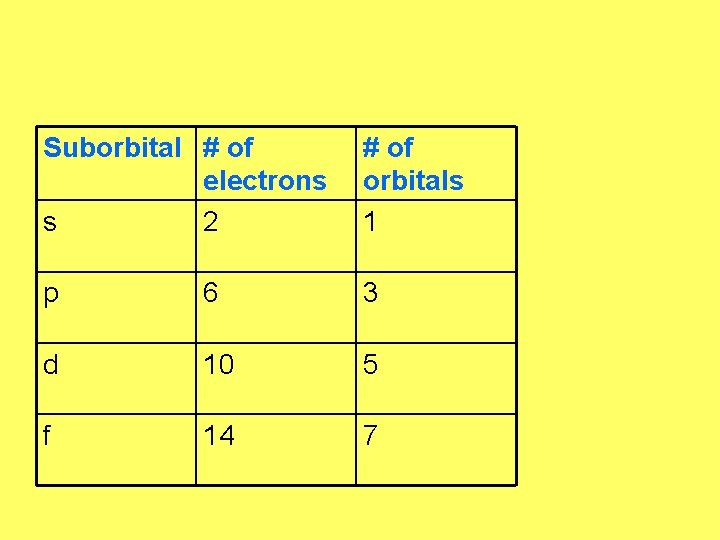
Suborbital # of electrons s 2 # of orbitals 1 p 6 3 d 10 5 f 14 7

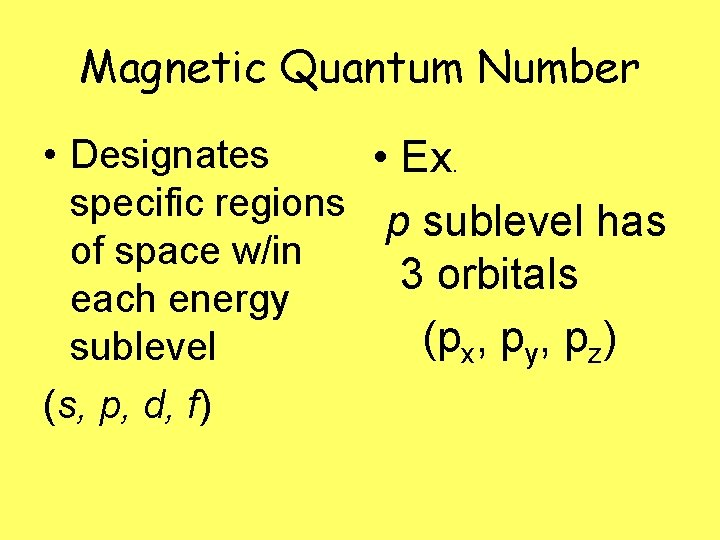
Magnetic Quantum Number • Designates • Ex. specific regions p sublevel has of space w/in 3 orbitals each energy (px, py, pz) sublevel (s, p, d, f)

Spin Quantum Number • Designates direction of electron spin • Electrons w/in an orbital spin in opposite directions

Governing Rules and Principles • Pauli Exclusion Principle- only 2 electrons in each orbital • Aufbau Principle- electrons must occupy lower energy orbitals first • Hunds Rule- a second electron can not be added to an orbital until each orbital in a sublevel contains an electron


Summary: Principle Energy Level 1 Orbitals Max. Electrons s 2 2 sp 8 3 spd 18 4 spdf 32