Light and Electrons Electromagnetic Radiation Light is electromagnetic



![Electron Orbitals Example: What is the non-core electron orbital notation for gold? [Xe] . Electron Orbitals Example: What is the non-core electron orbital notation for gold? [Xe] .](https://slidetodoc.com/presentation_image_h/7635790a7134b102faa44de514c90db9/image-35.jpg)

- Slides: 37

Light and Electrons

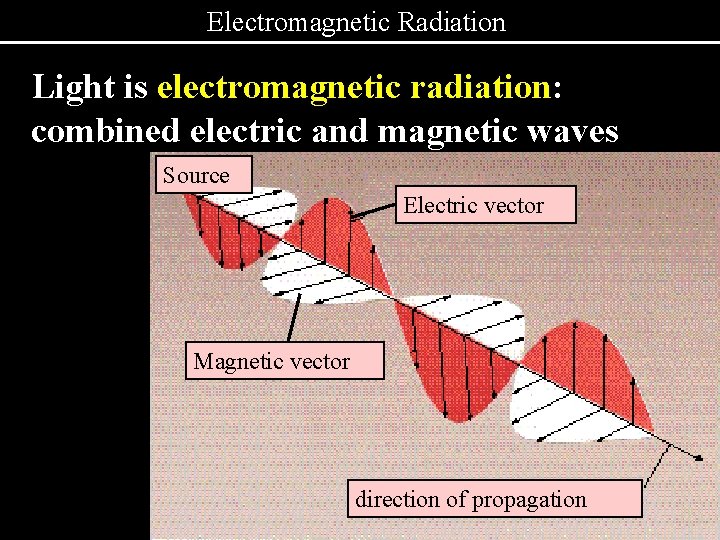
Electromagnetic Radiation Light is electromagnetic radiation: combined electric and magnetic waves Source Electric vector Magnetic vector direction of propagation

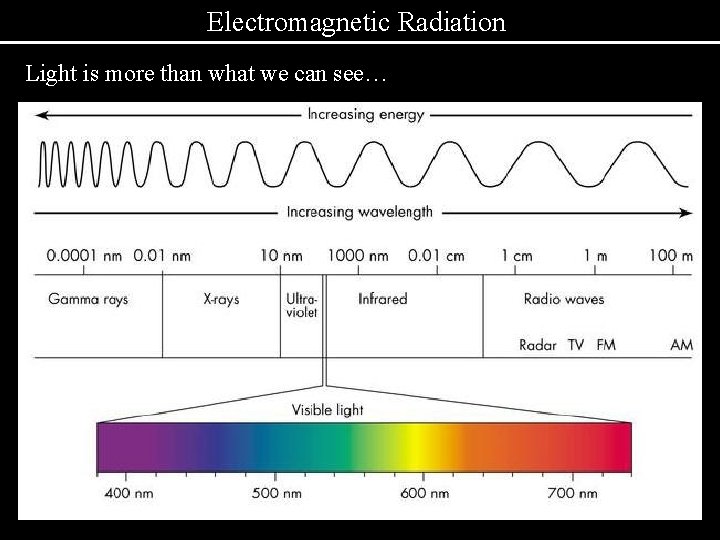
Electromagnetic Radiation Light is more than what we can see…

Electromagnetic Radiation Subatomic particles (electron, photon, proton, etc) exhibit both PARTICLE and WAVE properties. This is known as Wave. Particle Duality. Diffraction: wave-like Photoelectric Effect: particle-like

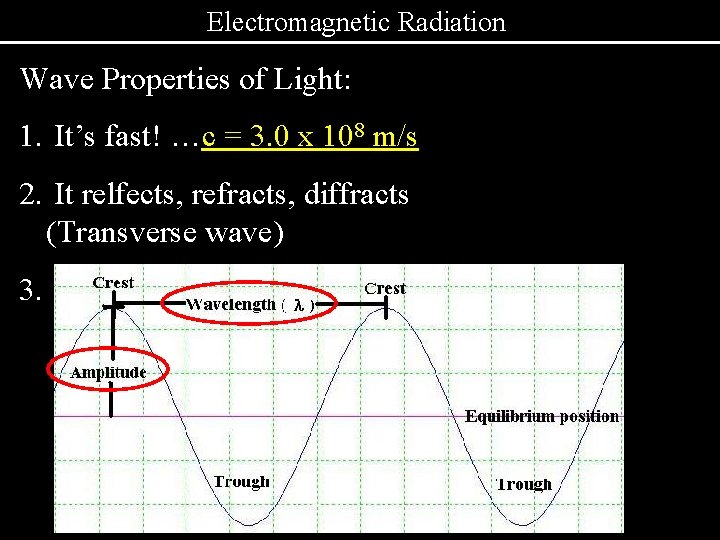
Electromagnetic Radiation Wave Properties of Light: 1. It’s fast! …c = 3. 0 x 108 m/s 2. It relfects, refracts, diffracts (Transverse wave) 3.

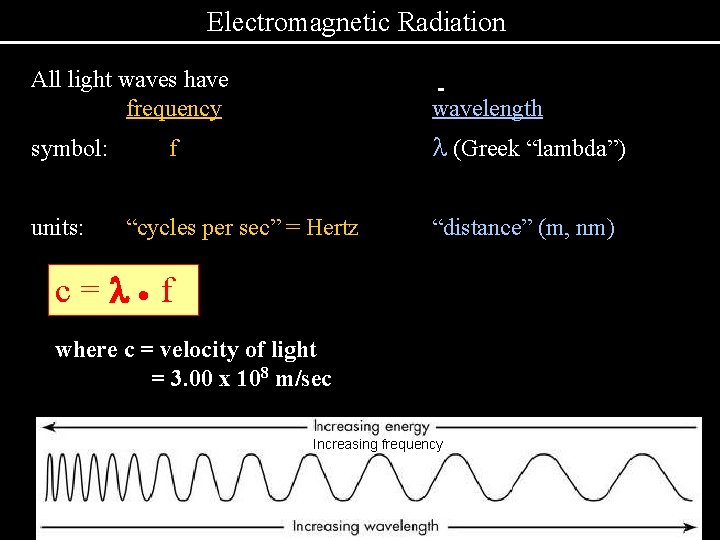
Electromagnetic Radiation All light waves have frequency wavelength symbol: l (Greek “lambda”) units: f “cycles per sec” = Hertz “distance” (m, nm) c=l· f where c = velocity of light = 3. 00 x 108 m/sec Increasing frequency

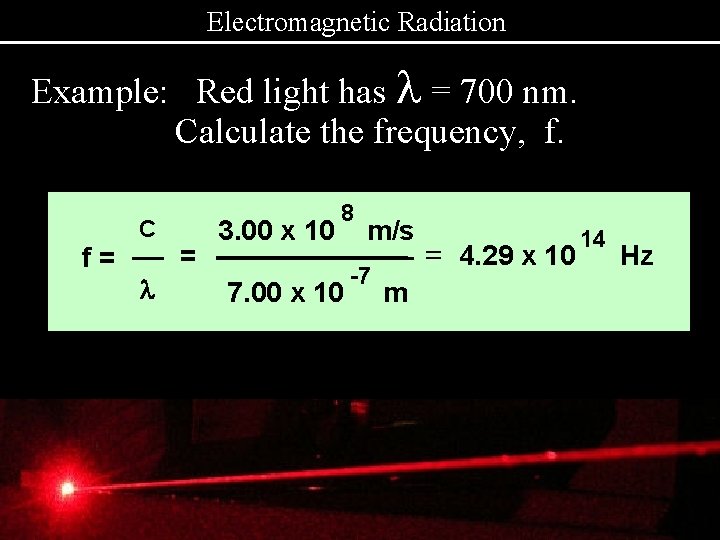
Electromagnetic Radiation Example: Red light has l = 700 nm. Calculate the frequency, f. f= C l = 3. 00 x 10 8 7. 00 x 10 m/s -7 m = 4. 29 x 10 14 Hz

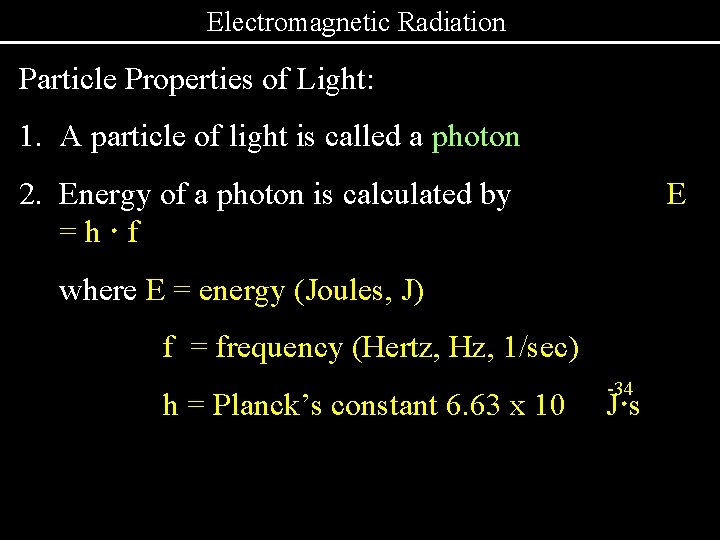
Electromagnetic Radiation Particle Properties of Light: 1. A particle of light is called a photon 2. Energy of a photon is calculated by =h·f E where E = energy (Joules, J) f = frequency (Hertz, Hz, 1/sec) h = Planck’s constant 6. 63 x 10 -34 J·s

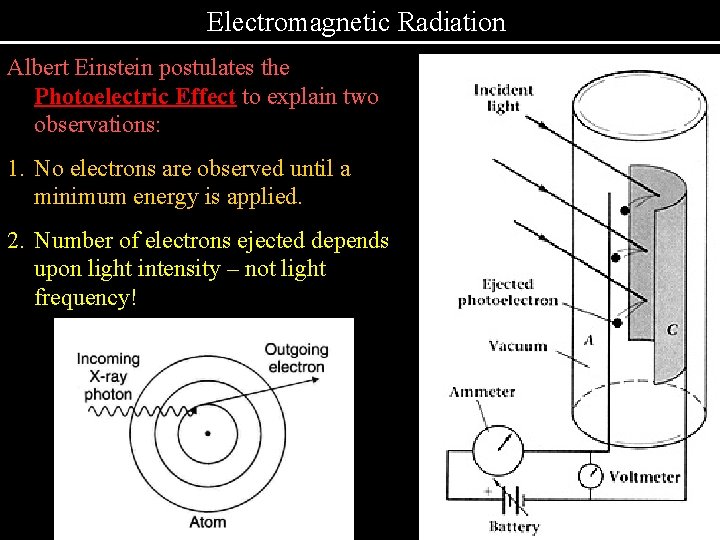
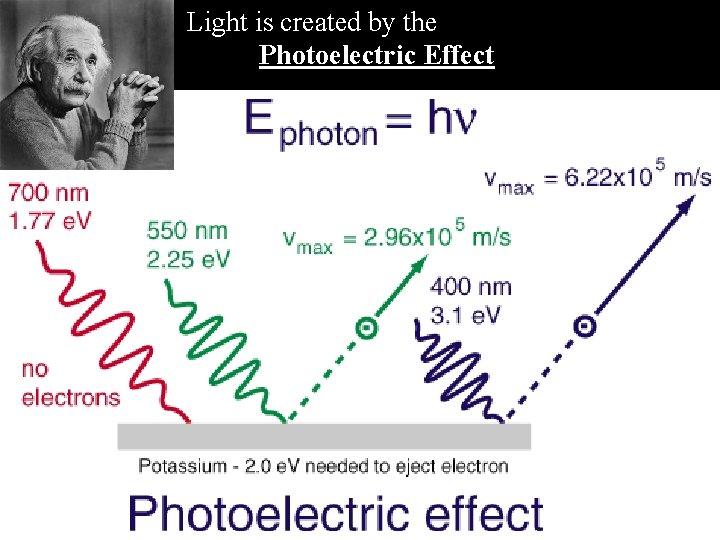
Electromagnetic Radiation Albert Einstein postulates the Photoelectric Effect to explain two observations: 1. No electrons are observed until a minimum energy is applied. 2. Number of electrons ejected depends upon light intensity – not light frequency!

Light is created by the Photoelectric Effect

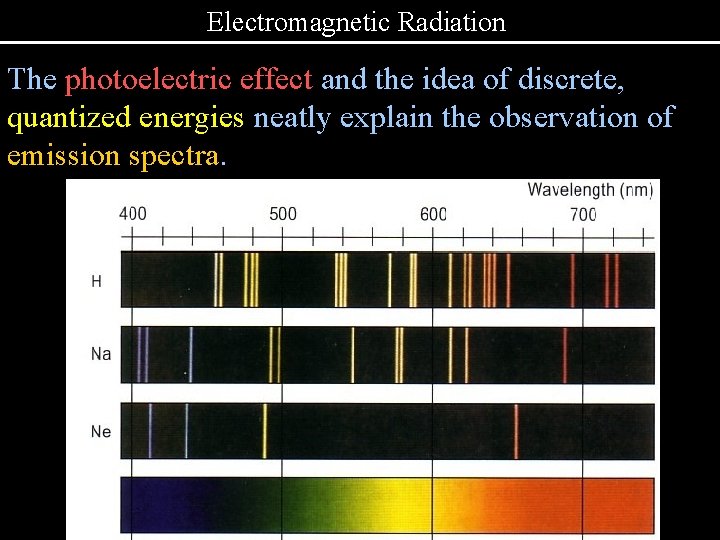
Electromagnetic Radiation The photoelectric effect and the idea of discrete, quantized energies neatly explain the observation of emission spectra.

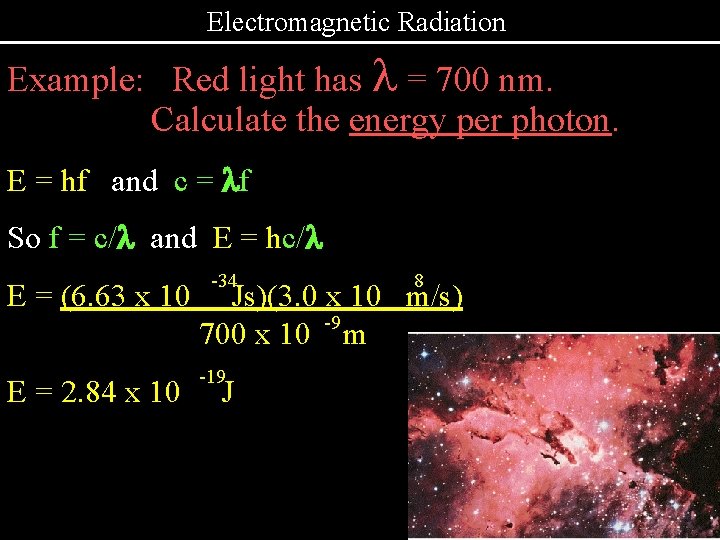
Electromagnetic Radiation Example: Red light has l = 700 nm. Calculate the energy per photon. E = hf and c = lf So f = c/l and E = hc/l -34 8 E = (6. 63 x 10 Js)(3. 0 x 10 m/s) -9 700 x 10 m E = 2. 84 x 10 -19 J

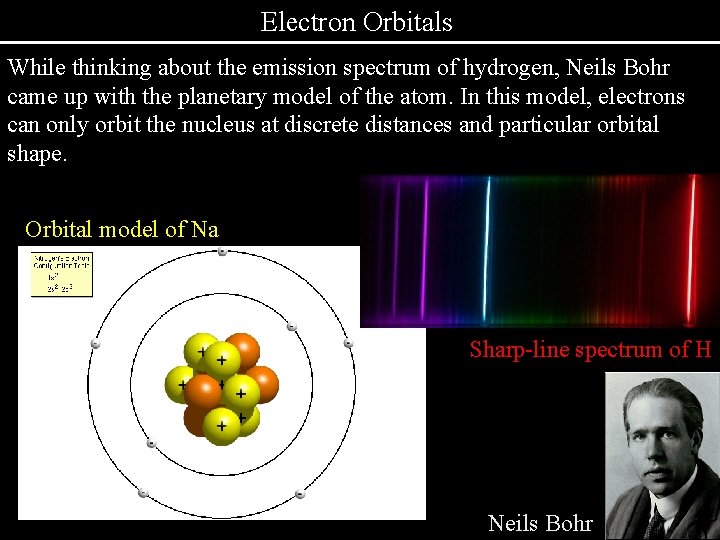
Electron Orbitals While thinking about the emission spectrum of hydrogen, Neils Bohr came up with the planetary model of the atom. In this model, electrons can only orbit the nucleus at discrete distances and particular orbital shape. Orbital model of Na Sharp-line spectrum of H Neils Bohr

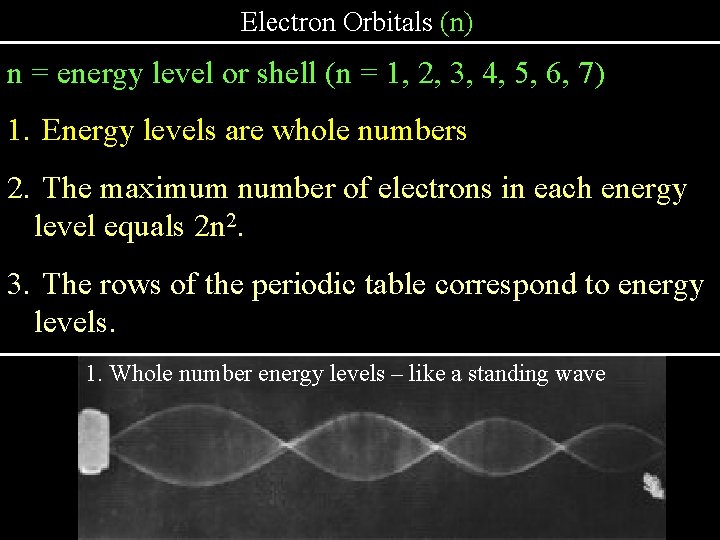
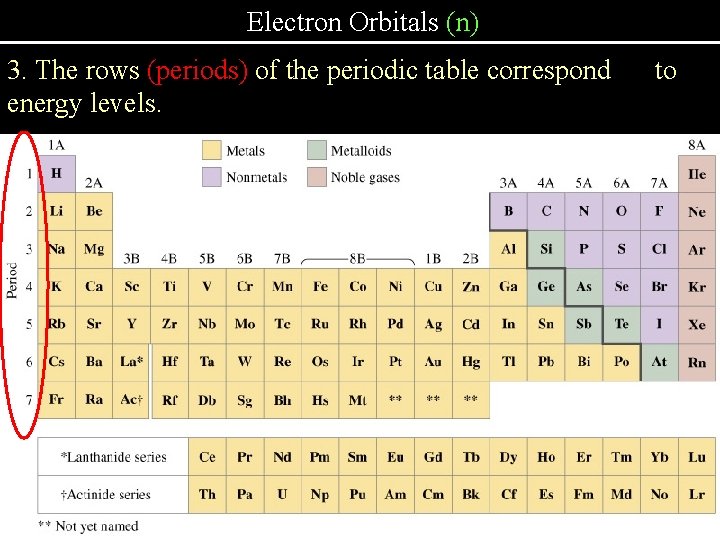
Electron Orbitals (n) n = energy level or shell (n = 1, 2, 3, 4, 5, 6, 7) 1. Energy levels are whole numbers 2. The maximum number of electrons in each energy level equals 2 n 2. 3. The rows of the periodic table correspond to energy levels. 1. Whole number energy levels – like a standing wave

Electron Orbitals (n) 3. The rows (periods) of the periodic table correspond energy levels. to

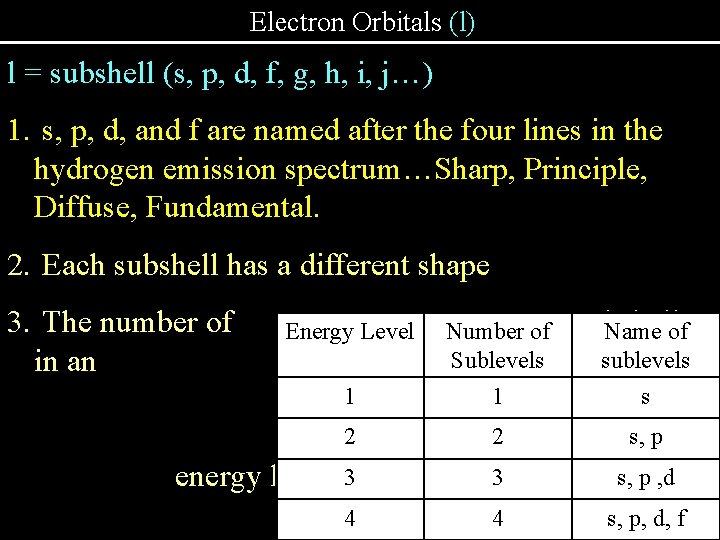
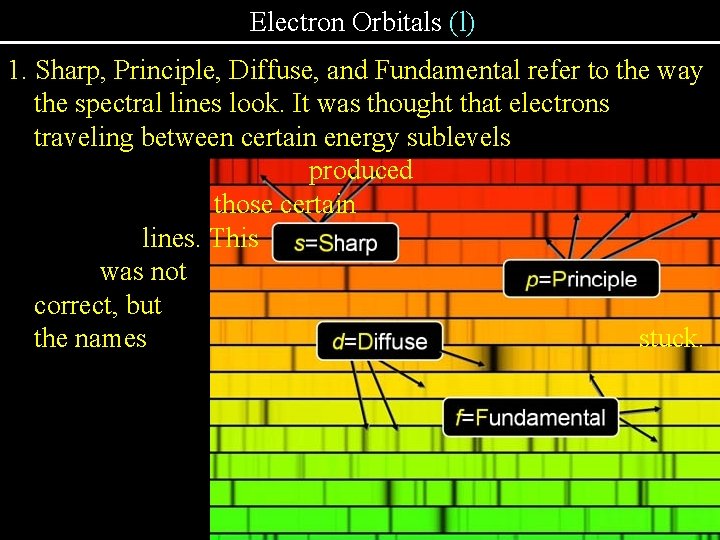
Electron Orbitals (l) l = subshell (s, p, d, f, g, h, i, j…) 1. s, p, d, and f are named after the four lines in the hydrogen emission spectrum…Sharp, Principle, Diffuse, Fundamental. 2. Each subshell has a different shape 3. The number of in an Energy Level Number of Sublevels energy equal 1 to the 2 number of the 2 3 energy level. 3 1 4 4 subshells Name of sublevels level is s s, p , d s, p, d, f

Electron Orbitals (l) 1. Sharp, Principle, Diffuse, and Fundamental refer to the way the spectral lines look. It was thought that electrons traveling between certain energy sublevels produced those certain lines. This was not correct, but the names stuck.

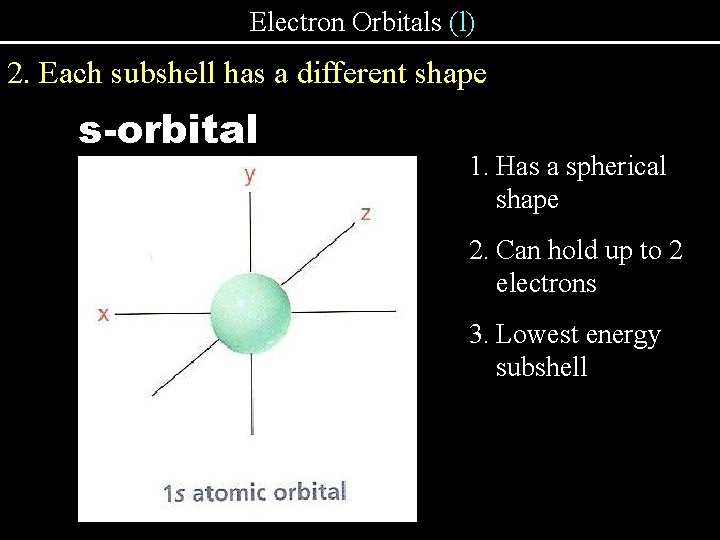
Electron Orbitals (l) 2. Each subshell has a different shape s-orbital 1. Has a spherical shape 2. Can hold up to 2 electrons 3. Lowest energy subshell

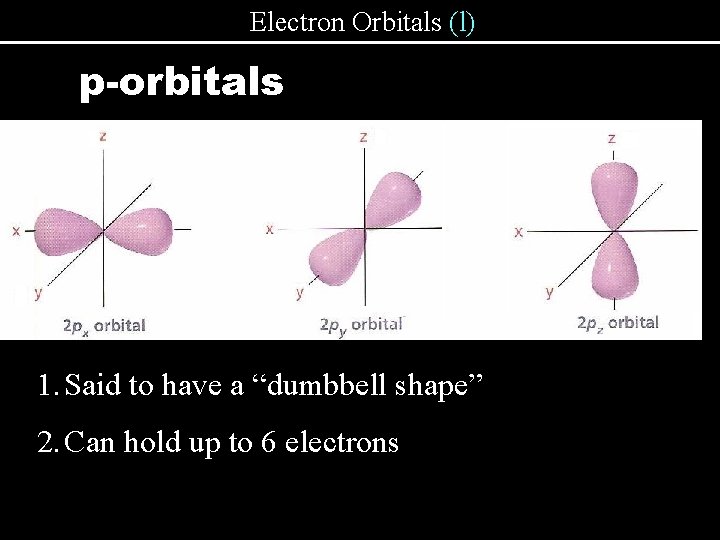
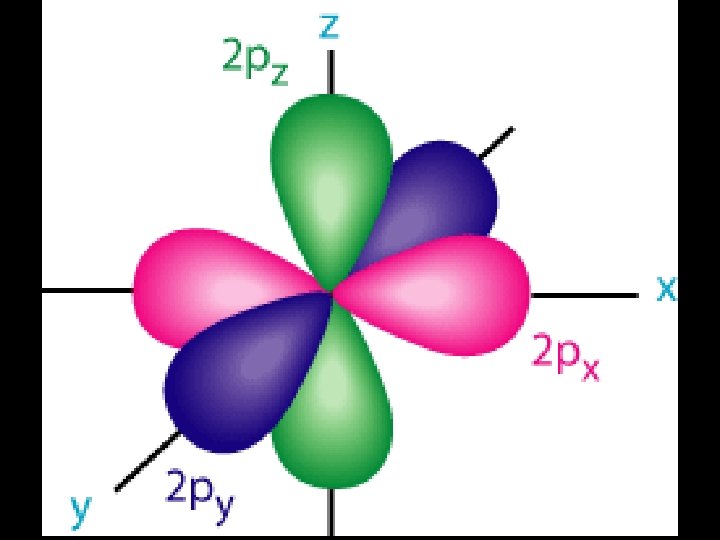
Electron Orbitals (l) p-orbitals 1. Said to have a “dumbbell shape” 2. Can hold up to 6 electrons


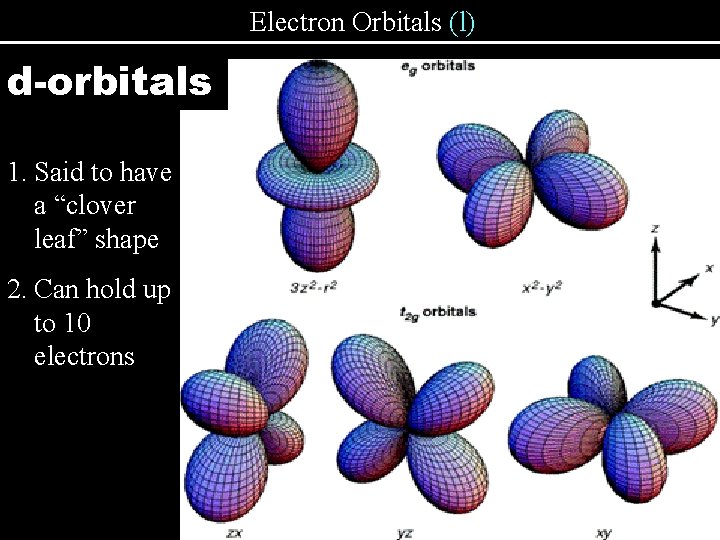
Electron Orbitals (l) d-orbitals 1. Said to have a “clover leaf” shape 2. Can hold up to 10 electrons

d-orbitals combined orbitals

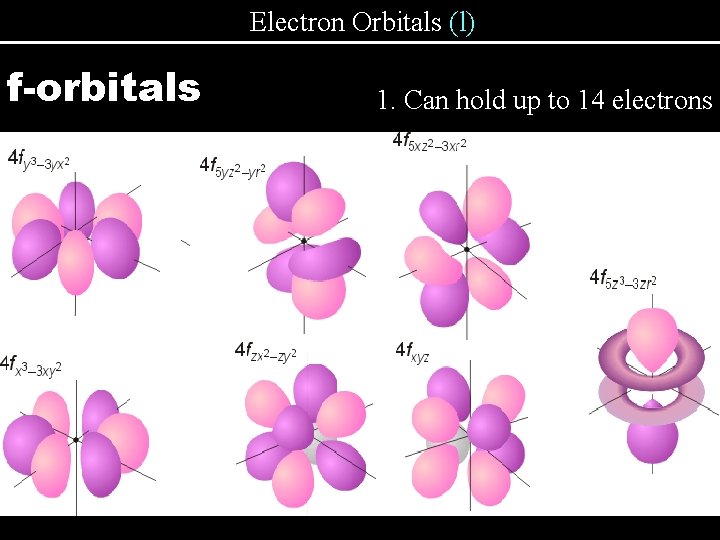
Electron Orbitals (l) f-orbitals 1. Can hold up to 14 electrons

f-orbitals combined orbitals

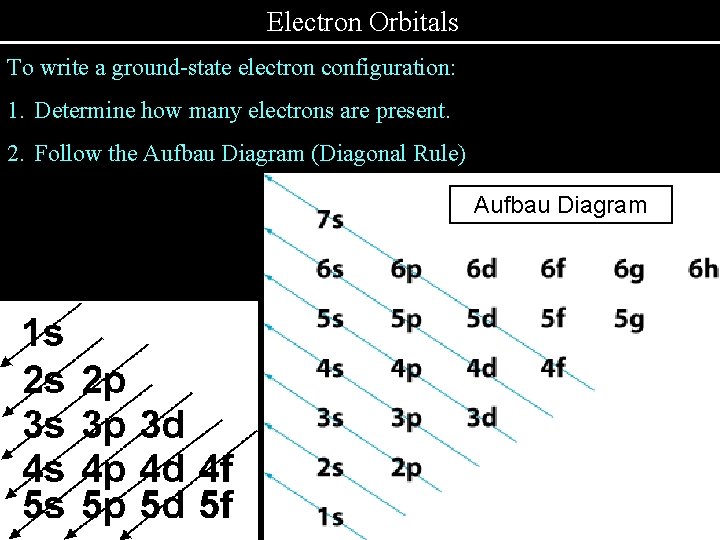
Electron Orbitals To write a ground-state electron configuration: 1. Determine how many electrons are present. 2. Follow the Aufbau Diagram (Diagonal Rule) Aufbau Diagram

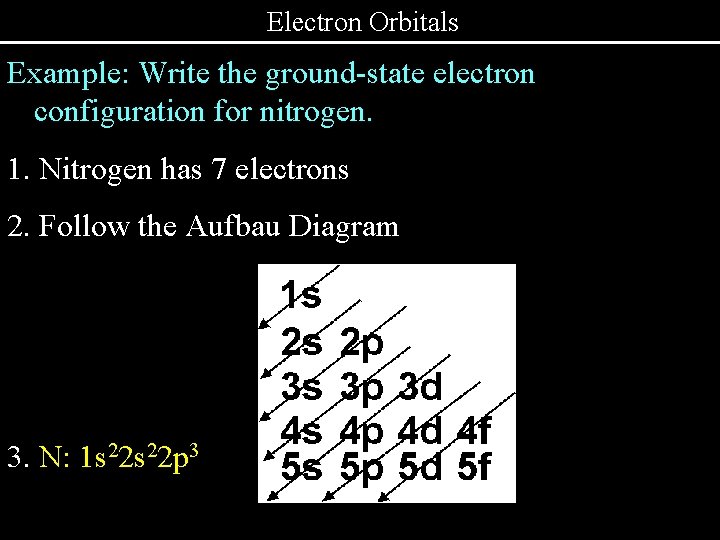
Electron Orbitals Example: Write the ground-state electron configuration for nitrogen. 1. Nitrogen has 7 electrons 2. Follow the Aufbau Diagram 3. N: 1 s 22 p 3

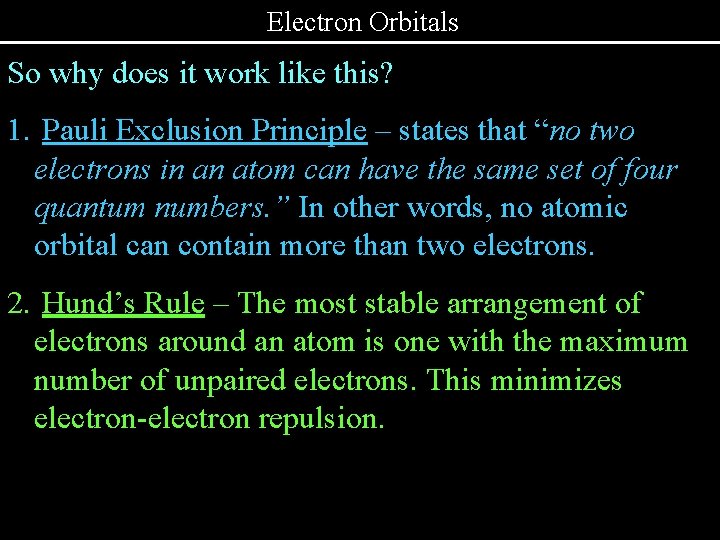
Electron Orbitals So why does it work like this? 1. Pauli Exclusion Principle – states that “no two electrons in an atom can have the same set of four quantum numbers. ” In other words, no atomic orbital can contain more than two electrons. 2. Hund’s Rule – The most stable arrangement of electrons around an atom is one with the maximum number of unpaired electrons. This minimizes electron-electron repulsion.

Electron Orbitals So why does it work like this? (cont. ) 3. Aufbau Principle – Electrons occupy the lowest energy state possible. 4. Heisenberg Uncertainty Principle – The orbitals are probabilities – not shapes in space like planetary orbits. The uncertainty principle states that you cannot know the location and velocity of an electron simultaneously.

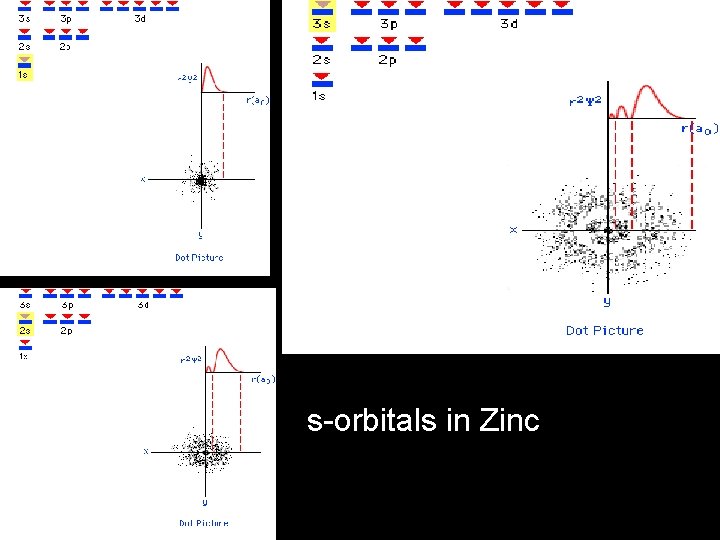
s-orbitals in Zinc

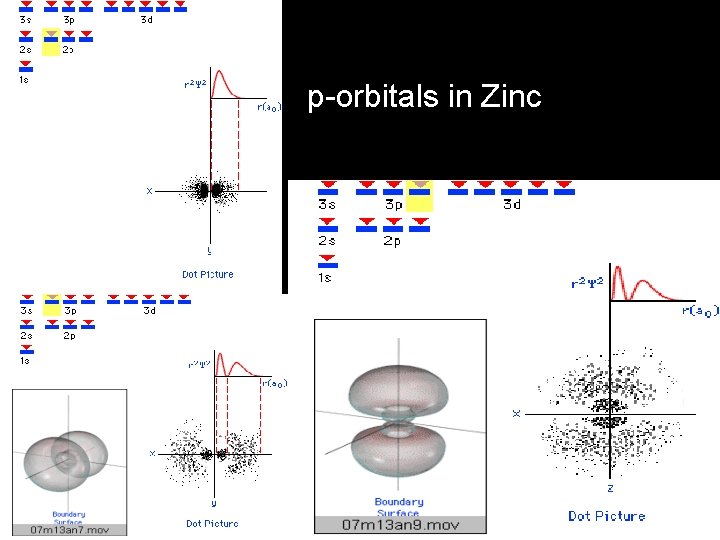
p-orbitals in Zinc

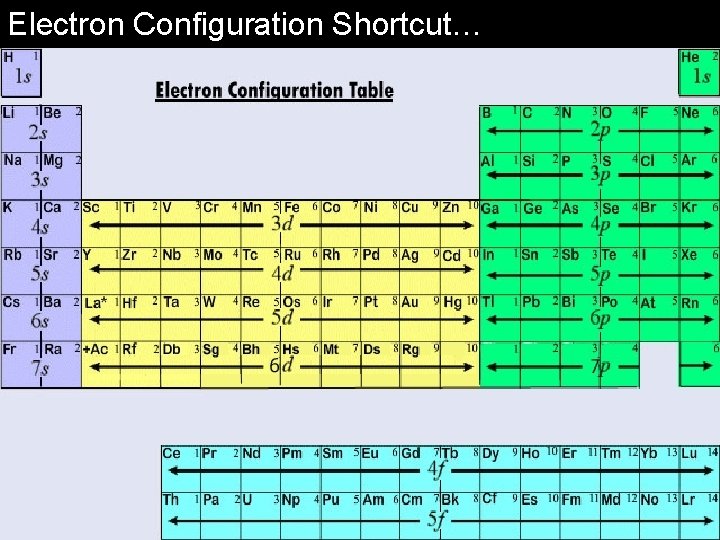
Electron Configuration Shortcut…

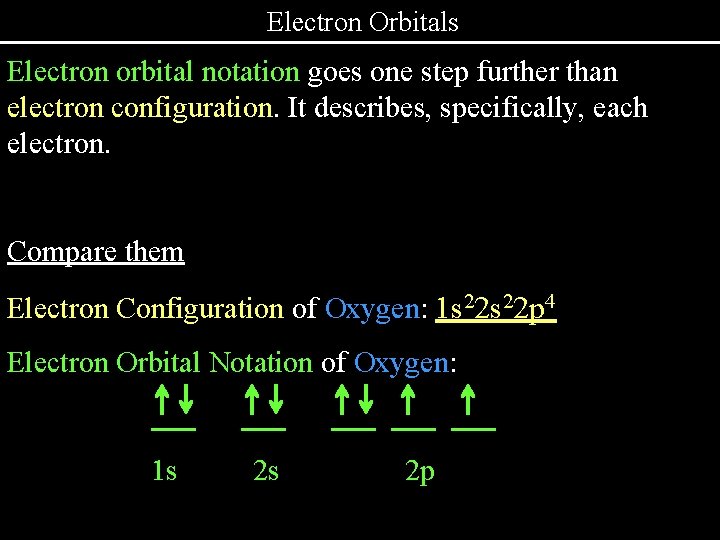
Electron Orbitals Electron orbital notation goes one step further than electron configuration. It describes, specifically, each electron. Compare them Electron Configuration of Oxygen: 1 s 22 p 4 Electron Orbital Notation of Oxygen: . 1 s . 2 s . . 2 p .

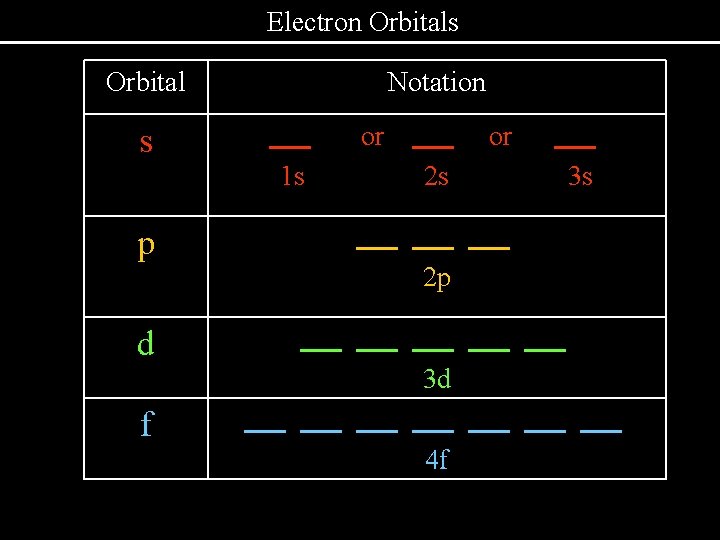
Electron Orbitals Orbital s Notation. or 1 s 2 s. p . 3 s . . . . 2 p. d f . or . 3 d . . . 4 f .

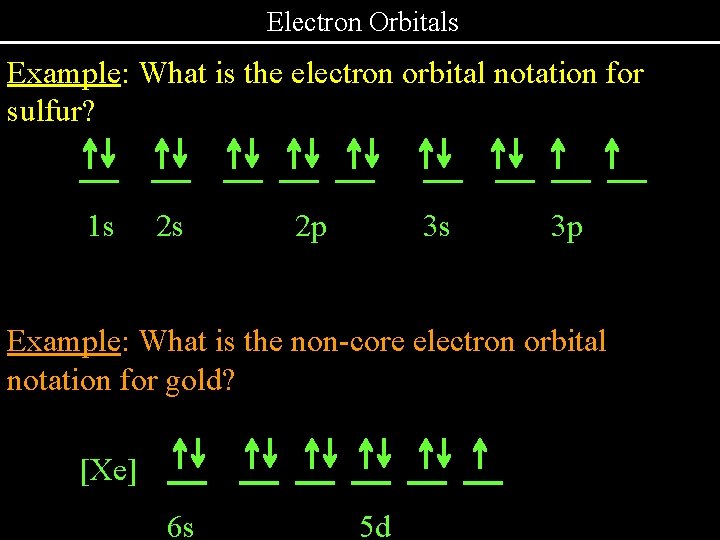
Electron Orbitals Example: What is the electron orbital notation for sulfur? . 1 s . . . 2 s . . 2 p . 3 s . 3 p Example: What is the non-core electron orbital notation for gold? [Xe] . 6 s . . . 5 d . . .
![Electron Orbitals Example What is the noncore electron orbital notation for gold Xe Electron Orbitals Example: What is the non-core electron orbital notation for gold? [Xe] .](https://slidetodoc.com/presentation_image_h/7635790a7134b102faa44de514c90db9/image-35.jpg)
Electron Orbitals Example: What is the non-core electron orbital notation for gold? [Xe] . . 6 s . . 5 d Electrons are more stable in full or half-full orbitals. …or more likely, [Xe] . 6 s . . . 5 d . .

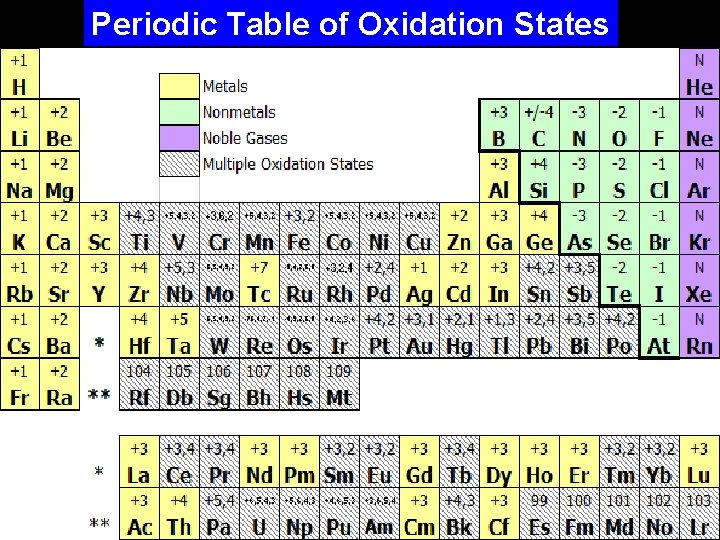
Electron Orbitals Octet Rule: Atoms will gain or lose electrons to achieve a full valence shell (usually this means 8 electrons). Oxidation State: The value of the charge on an ion (positive or negative), after the atom has achieved a full valence shell. - metals tend to lose electrons, forming positive (+) ions (cations). - non-metals tend to gain electrons, forming negative (-) ions (anions).

Electron Orbitals Periodic Table of Oxidation States