Lection 9 Dynamic methods to construct phase diagrams
- Slides: 26

Lection 9 Dynamic methods to construct phase diagrams Dynamic methods of phase equilibrium studies – DTA, HF-DSC a. Unary system; b. Binary and ternary systems c. TGA; d. Heat capacity measurements using DSC v

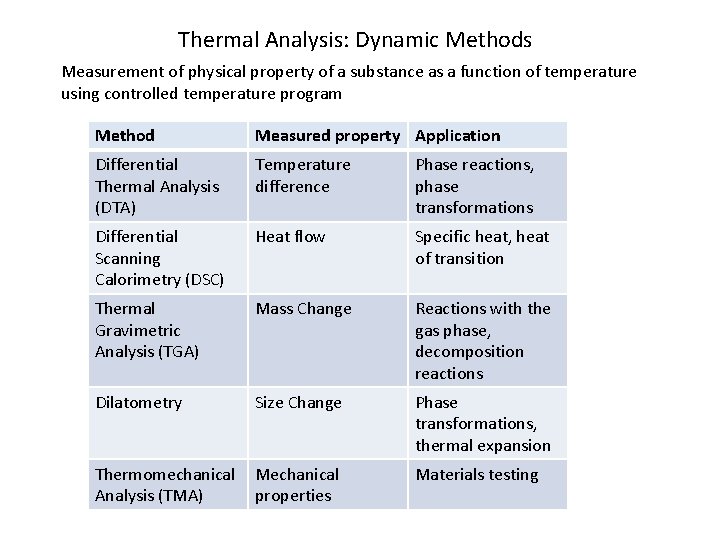
Thermal Analysis: Dynamic Methods Measurement of physical property of a substance as a function of temperature using controlled temperature program Method Measured property Application Differential Thermal Analysis (DTA) Temperature difference Phase reactions, phase transformations Differential Scanning Calorimetry (DSC) Heat flow Specific heat, heat of transition Thermal Gravimetric Analysis (TGA) Mass Change Reactions with the gas phase, decomposition reactions Dilatometry Size Change Phase transformations, thermal expansion Thermomechanical Analysis (TMA) Mechanical properties Materials testing

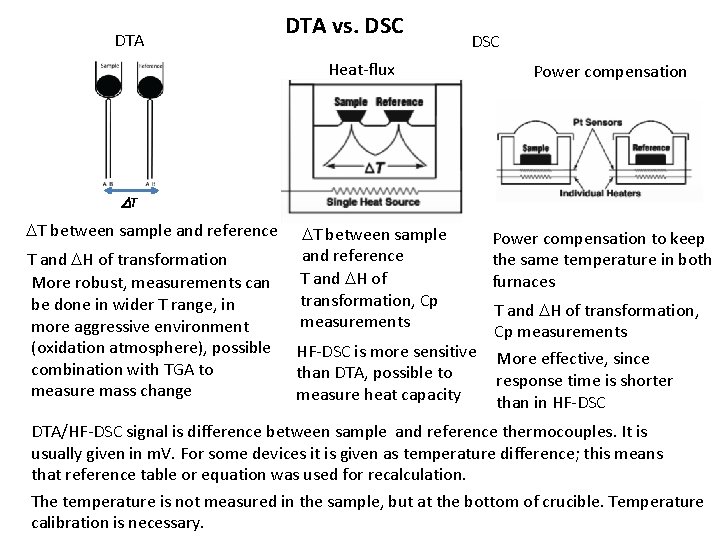
DTA vs. DSC Heat-flux Power compensation DT DT between sample and reference T and DH of transformation More robust, measurements can be done in wider T range, in more aggressive environment (oxidation atmosphere), possible combination with TGA to measure mass change DT between sample and reference T and DH of transformation, Cp measurements Power compensation to keep the same temperature in both furnaces HF-DSC is more sensitive than DTA, possible to measure heat capacity More effective, since response time is shorter than in HF-DSC T and DH of transformation, Cp measurements DTA/HF-DSC signal is difference between sample and reference thermocouples. It is usually given in m. V. For some devices it is given as temperature difference; this means that reference table or equation was used for recalculation. The temperature is not measured in the sample, but at the bottom of crucible. Temperature calibration is necessary.

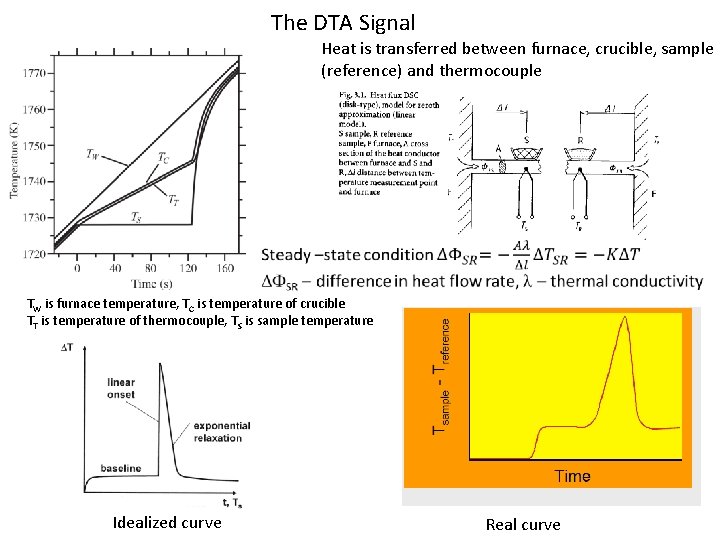
The DTA Signal Heat is transferred between furnace, crucible, sample (reference) and thermocouple TW is furnace temperature, TC is temperature of crucible TT is temperature of thermocouple, TS is sample temperature Idealized curve Real curve

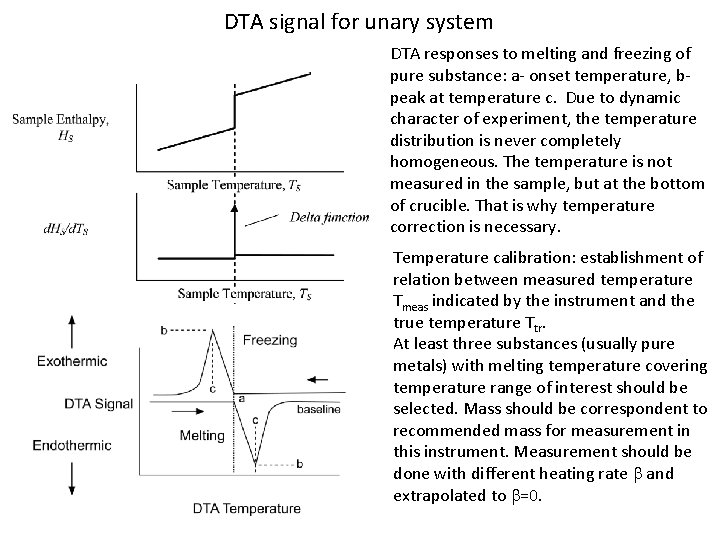
DTA signal for unary system DTA responses to melting and freezing of pure substance: a- onset temperature, bpeak at temperature c. Due to dynamic character of experiment, the temperature distribution is never completely homogeneous. The temperature is not measured in the sample, but at the bottom of crucible. That is why temperature correction is necessary. Temperature calibration: establishment of relation between measured temperature Tmeas indicated by the instrument and the true temperature Ttr. At least three substances (usually pure metals) with melting temperature covering temperature range of interest should be selected. Mass should be correspondent to recommended mass for measurement in this instrument. Measurement should be done with different heating rate b and extrapolated to b=0.

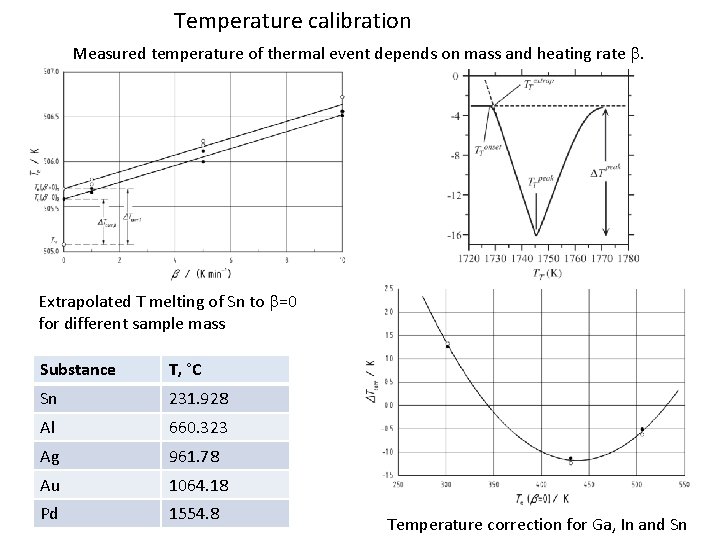
Temperature calibration Measured temperature of thermal event depends on mass and heating rate b. Extrapolated T melting of Sn to b=0 for different sample mass Substance T, °C Sn 231. 928 Al 660. 323 Ag 961. 78 Au 1064. 18 Pd 1554. 8 Temperature correction for Ga, In and Sn

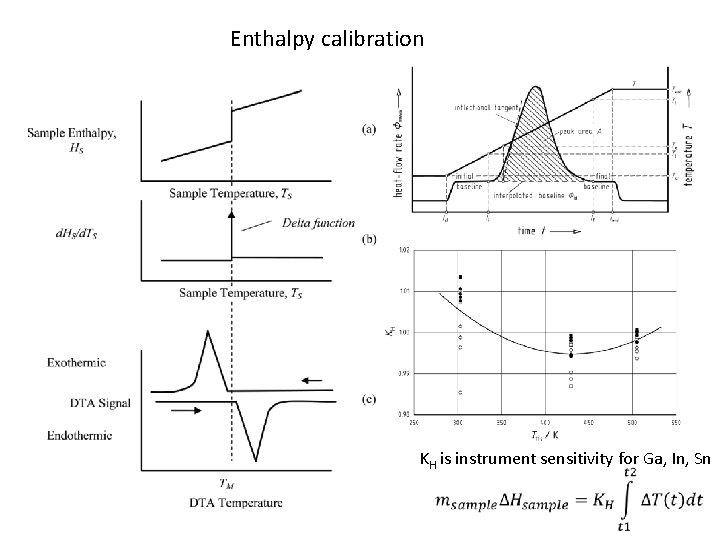
Enthalpy calibration KH is instrument sensitivity for Ga, In, Sn

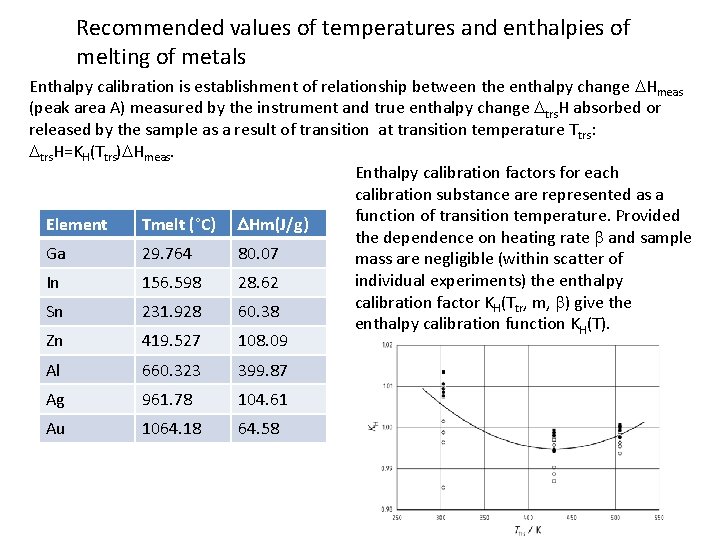
Recommended values of temperatures and enthalpies of melting of metals Enthalpy calibration is establishment of relationship between the enthalpy change DHmeas (peak area A) measured by the instrument and true enthalpy change Dtrs. H absorbed or released by the sample as a result of transition at transition temperature T trs: Dtrs. H=KH(Ttrs)DHmeas. Enthalpy calibration factors for each calibration substance are represented as a function of transition temperature. Provided Element Tmelt (°C) DHm(J/g) the dependence on heating rate b and sample Ga 29. 764 80. 07 mass are negligible (within scatter of individual experiments) the enthalpy In 156. 598 28. 62 calibration factor KH(Ttr, m, b) give the Sn 231. 928 60. 38 enthalpy calibration function KH(T). Zn 419. 527 108. 09 Al 660. 323 399. 87 Ag 961. 78 104. 61 Au 1064. 18 64. 58

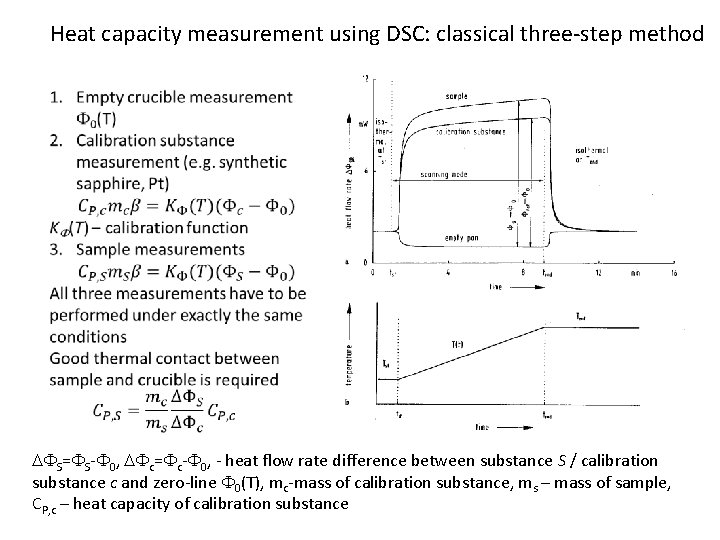
Heat capacity measurement using DSC: classical three-step method DFS=FS-F 0, DFc=Fc-F 0, - heat flow rate difference between substance S / calibration substance c and zero-line F 0(T), mc-mass of calibration substance, ms – mass of sample, CP, c – heat capacity of calibration substance

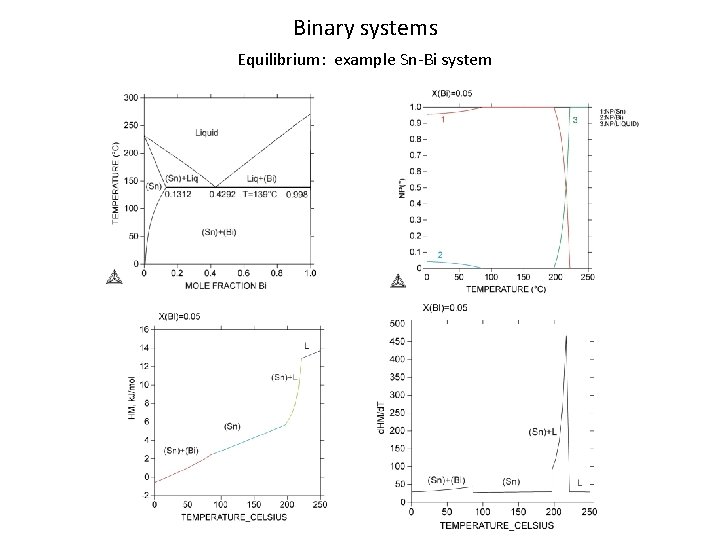
Binary systems Equilibrium: example Sn-Bi system

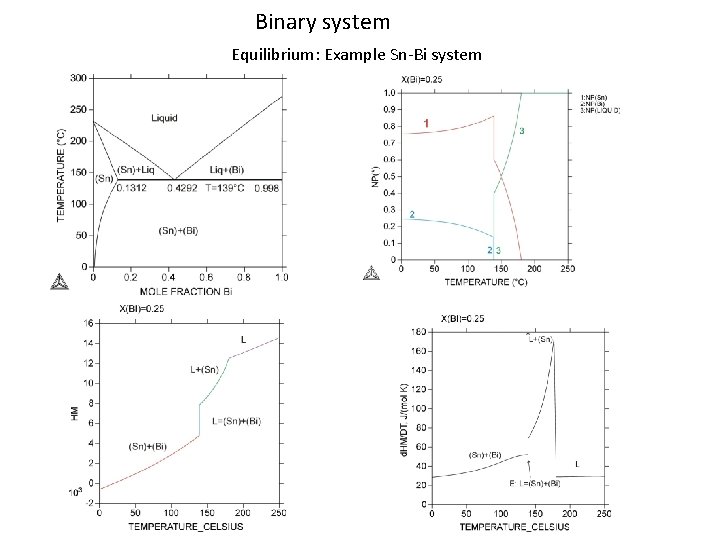
Binary system Equilibrium: Example Sn-Bi system

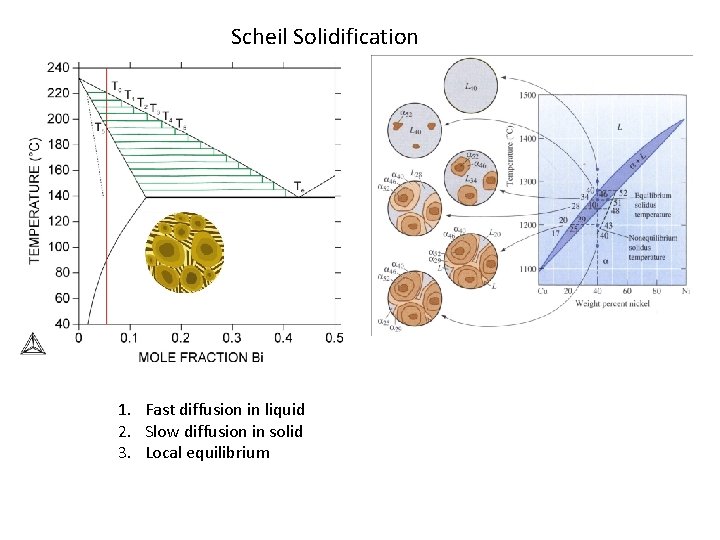
Scheil Solidification 1. Fast diffusion in liquid 2. Slow diffusion in solid 3. Local equilibrium

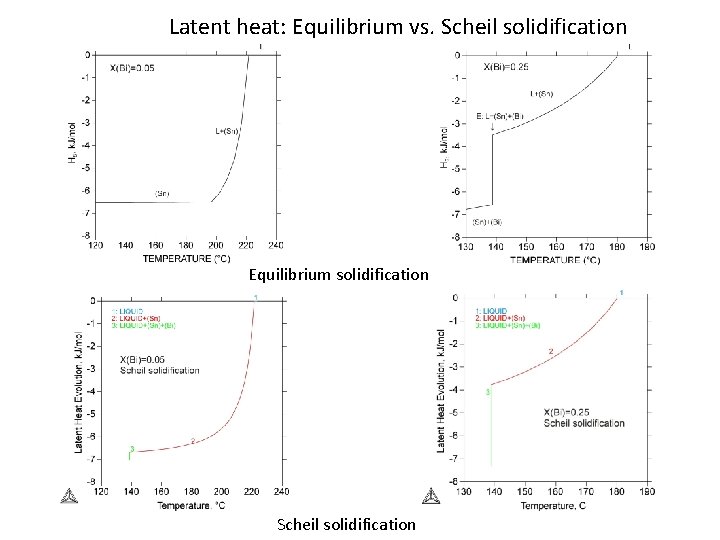
Latent heat: Equilibrium vs. Scheil solidification Equilibrium solidification Scheil solidification

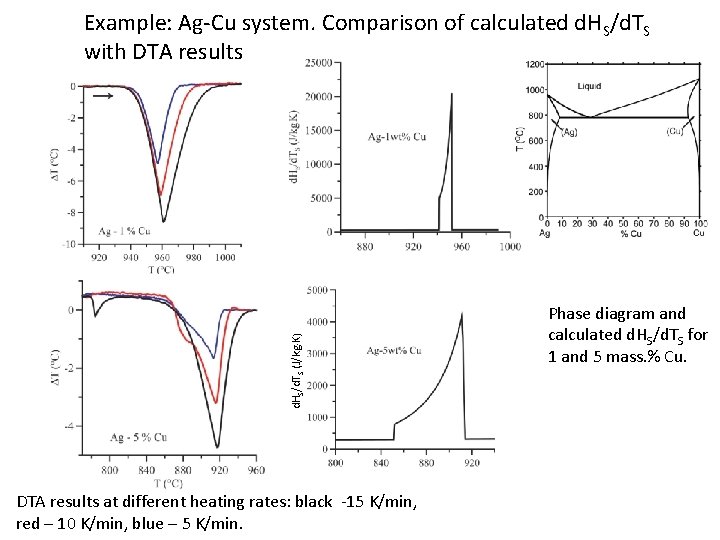
d. HS/d. TS (J/kg K) Example: Ag-Cu system. Comparison of calculated d. HS/d. TS with DTA results at different heating rates: black -15 K/min, red – 10 K/min, blue – 5 K/min. Phase diagram and calculated d. HS/d. TS for 1 and 5 mass. % Cu.

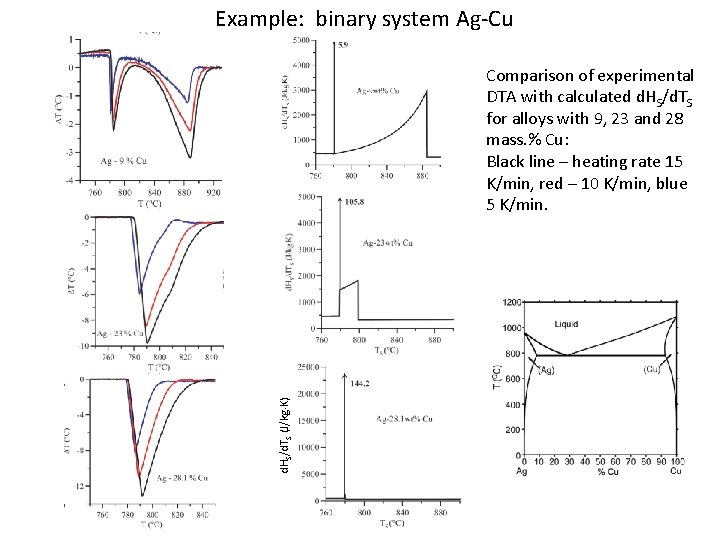
Example: binary system Ag-Cu d. HS/d. TS (J/kg K) Comparison of experimental DTA with calculated d. HS/d. TS for alloys with 9, 23 and 28 mass. % Cu: Black line – heating rate 15 K/min, red – 10 K/min, blue 5 K/min.

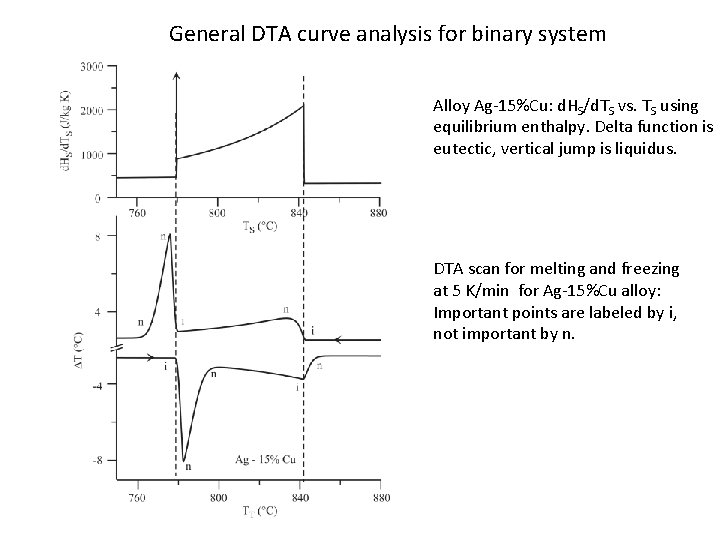
General DTA curve analysis for binary system Alloy Ag-15%Cu: d. HS/d. TS vs. TS using equilibrium enthalpy. Delta function is eutectic, vertical jump is liquidus. DTA scan for melting and freezing at 5 K/min for Ag-15%Cu alloy: Important points are labeled by i, not important by n.

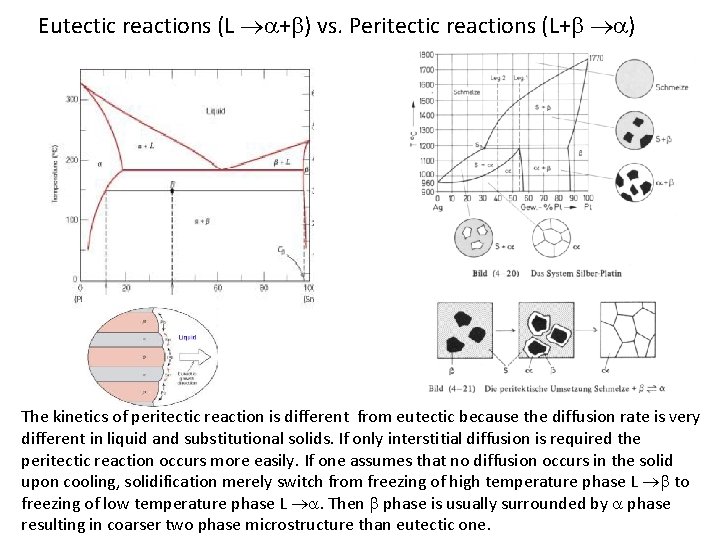
Eutectic reactions (L a+b) vs. Peritectic reactions (L+b a) The kinetics of peritectic reaction is different from eutectic because the diffusion rate is very different in liquid and substitutional solids. If only interstitial diffusion is required the peritectic reaction occurs more easily. If one assumes that no diffusion occurs in the solid upon cooling, solidification merely switch from freezing of high temperature phase L b to freezing of low temperature phase L a. Then b phase is usually surrounded by a phase resulting in coarser two phase microstructure than eutectic one.

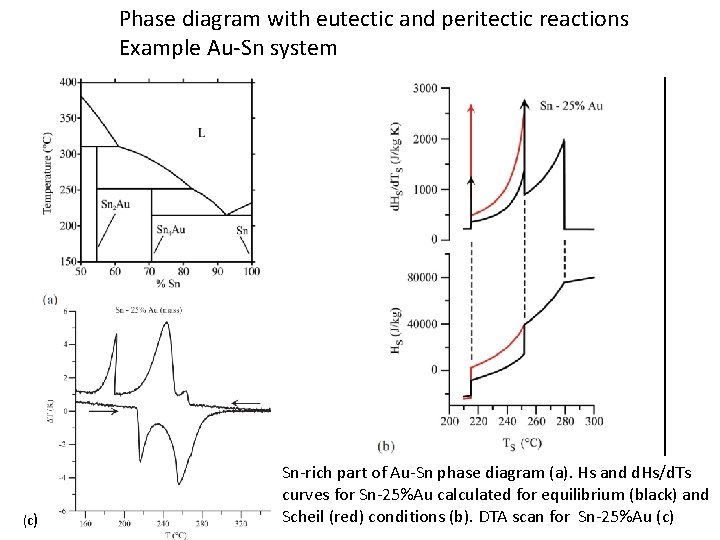
Phase diagram with eutectic and peritectic reactions Example Au-Sn system (c) Sn-rich part of Au-Sn phase diagram (a). Hs and d. Hs/d. Ts curves for Sn-25%Au calculated for equilibrium (black) and Scheil (red) conditions (b). DTA scan for Sn-25%Au (c)

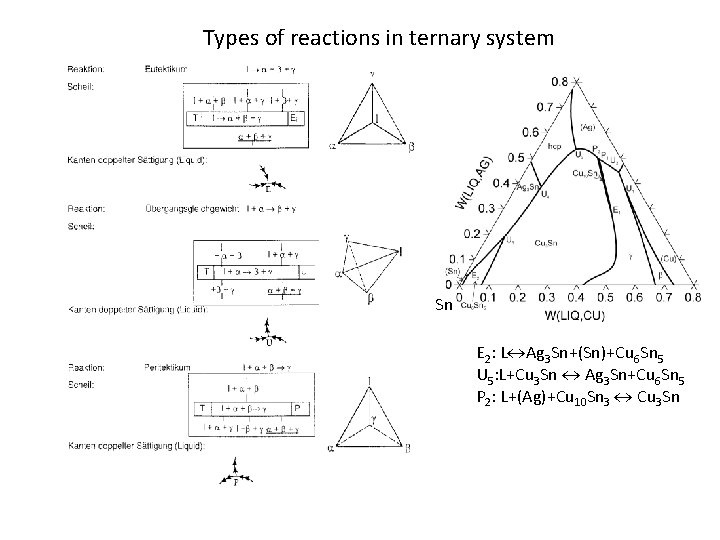
Types of reactions in ternary system Sn E 2: L Ag 3 Sn+(Sn)+Cu 6 Sn 5 U 5: L+Cu 3 Sn Ag 3 Sn+Cu 6 Sn 5 P 2: L+(Ag)+Cu 10 Sn 3 Cu 3 Sn

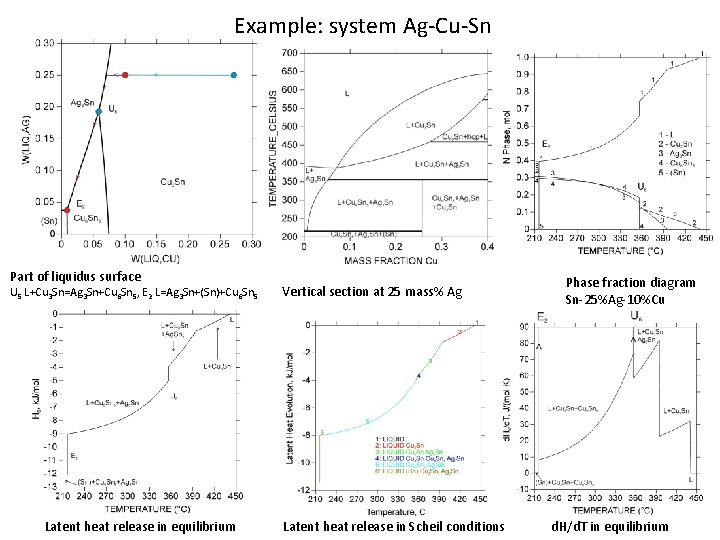
Example: system Ag-Cu-Sn Part of liquidus surface U 5 L+Cu 3 Sn=Ag 3 Sn+Cu 6 Sn 5, E 2 L=Ag 3 Sn+(Sn)+Cu 6 Sn 5 Latent heat release in equilibrium Vertical section at 25 mass% Ag Latent heat release in Scheil conditions Phase fraction diagram Sn-25%Ag-10%Cu d. H/d. T in equilibrium

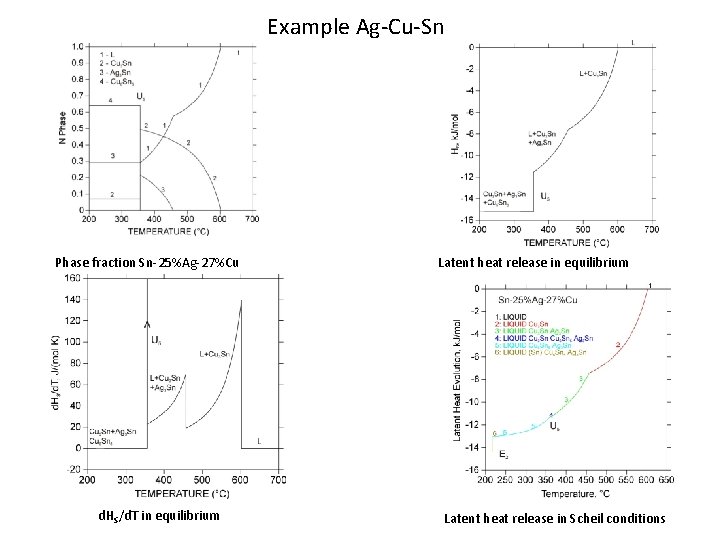
Example Ag-Cu-Sn Phase fraction Sn-25%Ag-27%Cu d. HS/d. T in equilibrium Latent heat release in Scheil conditions

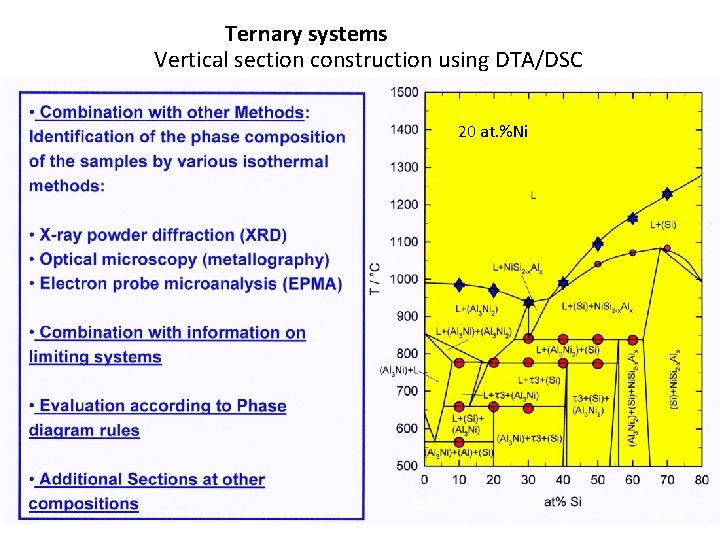
Ternary systems Vertical section construction using DTA/DSC 20 at. %Ni

TGA: Thermo-Gravimetric Analysis Determination of the mass as function of temperature. It is applicable for all reaction involving gas phase Decomposition reactions Vaporization and sublimation Desorption Oxidation and reduction Corrosion Small sample Gas flow essential Different temperature modes Linear heating, isothermal, stepwise isothermal

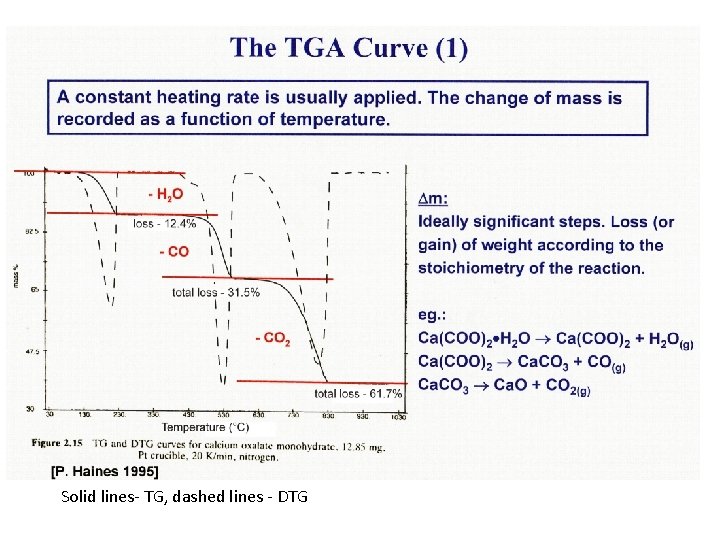
Solid lines- TG, dashed lines - DTG

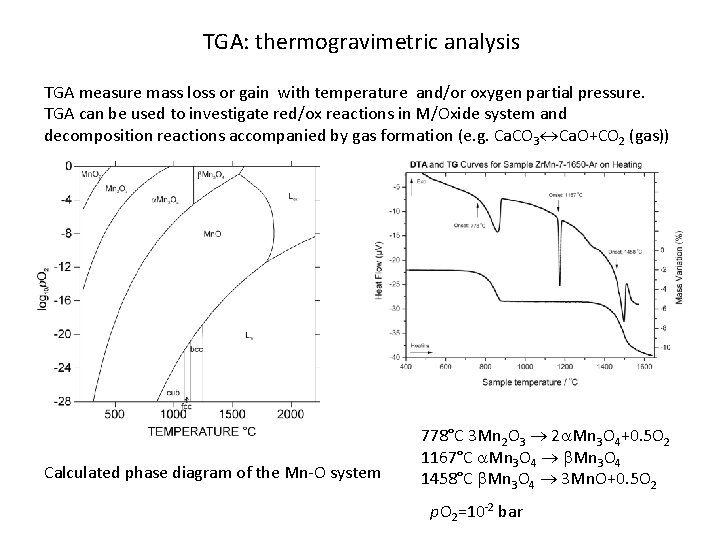
TGA: thermogravimetric analysis TGA measure mass loss or gain with temperature and/or oxygen partial pressure. TGA can be used to investigate red/ox reactions in M/Oxide system and decomposition reactions accompanied by gas formation (e. g. Ca. CO 3 Ca. O+CO 2 (gas)) Calculated phase diagram of the Mn-O system 778°C 3 Mn 2 O 3 2 a. Mn 3 O 4+0. 5 O 2 1167°C a. Mn 3 O 4 b. Mn 3 O 4 1458°C b. Mn 3 O 4 3 Mn. O+0. 5 O 2 p. O 2=10 -2 bar

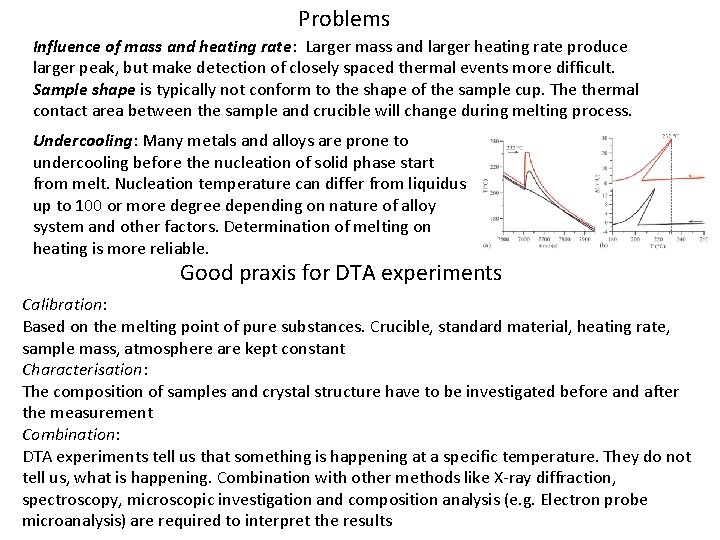
Problems Influence of mass and heating rate: Larger mass and larger heating rate produce larger peak, but make detection of closely spaced thermal events more difficult. Sample shape is typically not conform to the shape of the sample cup. The thermal contact area between the sample and crucible will change during melting process. Undercooling: Many metals and alloys are prone to undercooling before the nucleation of solid phase start from melt. Nucleation temperature can differ from liquidus up to 100 or more degree depending on nature of alloy system and other factors. Determination of melting on heating is more reliable. Good praxis for DTA experiments Calibration: Based on the melting point of pure substances. Crucible, standard material, heating rate, sample mass, atmosphere are kept constant Characterisation: The composition of samples and crystal structure have to be investigated before and after the measurement Combination: DTA experiments tell us that something is happening at a specific temperature. They do not tell us, what is happening. Combination with other methods like X-ray diffraction, spectroscopy, microscopic investigation and composition analysis (e. g. Electron probe microanalysis) are required to interpret the results