LAA Closure with the Amulet Device Sameer A






- Slides: 24

LAA Closure with the Amulet Device Sameer A. Gafoor, MD 1 Swedish Heart and Vascular: Ming Zhang, Paul Huang, Darryl Wells, Adam Zivin, Eric Williams, John Petersen II, Madalena Petrescu, Nimish Muni, , Eric Lehr, Sam Youssef, Glenn Barnhart, Irina Penev, Amanda Ray, Cale Peterson, Julia Antonyuk, Thearry Deap, Zachary Newhart, 2 CVC Frankfurt: Jennifer Franke, Simon Lam, Stefan Bertog, Laura Vaskelyte, Ilona Hofmann, Markus Reinartz, Horst Sievert Swedish Heart and Vascular: Swedish Medical Center, Seattle, WA, USA CVC: Cardio. Vascular Center Frankfurt, Germany

Disclosures Consulting/Speaker Boston Scientific Abbott Vascular Medtronic Examples of relationships are: Advisory Board/Board Member, Consultant, Honoraria, Research Support, Speaker’s Bureau, Stockholder Please list full company name

• Device • Case • Data

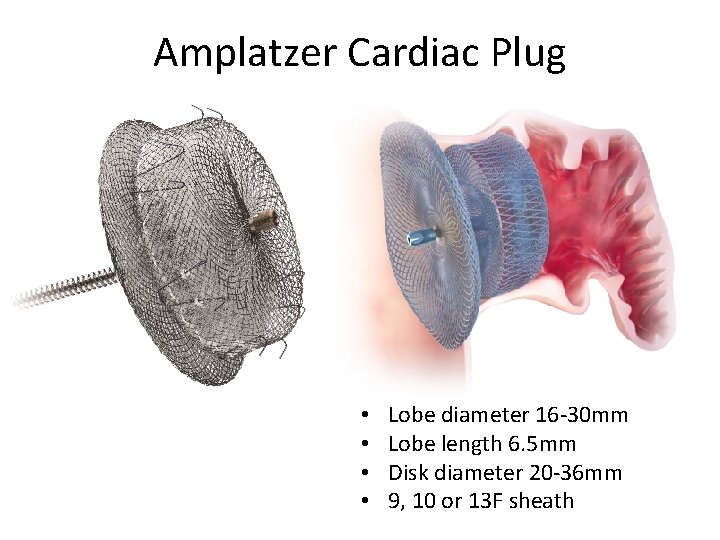
Amplatzer Cardiac Plug • • Lobe diameter 16 -30 mm Lobe length 6. 5 mm Disk diameter 20 -36 mm 9, 10 or 13 F sheath

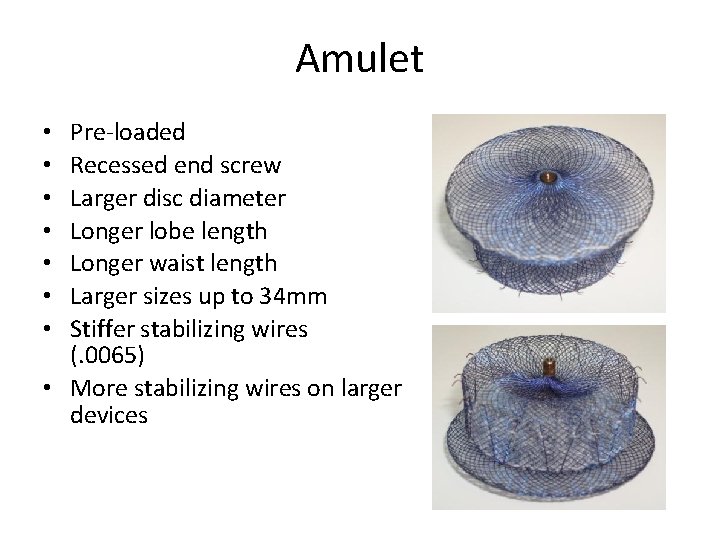
Amulet Pre-loaded Recessed end screw Larger disc diameter Longer lobe length Longer waist length Larger sizes up to 34 mm Stiffer stabilizing wires (. 0065) • More stabilizing wires on larger devices • •

Recessed End Screw • Recessed end screw creates a uniform surface in the left atrium 90 days — Preclinical canine model 6

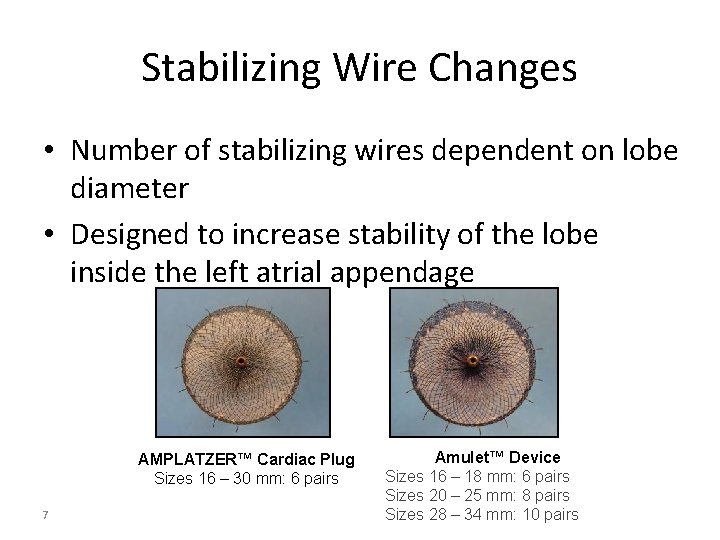
Stabilizing Wire Changes • Number of stabilizing wires dependent on lobe diameter • Designed to increase stability of the lobe inside the left atrial appendage AMPLATZER™ Cardiac Plug Sizes 16 – 30 mm: 6 pairs 7 Amulet™ Device Sizes 16 – 18 mm: 6 pairs Sizes 20 – 25 mm: 8 pairs Sizes 28 – 34 mm: 10 pairs

Device Specifications Feature Size / Lobe Diameter (mm) Disc Diameter AMPLATZER™ Amulet™ 16 18 20 22 25 28 31 34 AMPLATZER™ Cardiac Plug 16 18 20 22 24 Lobe + 6 mm Lobe + 7 mm Lobe Length 7. 5 mm 10 mm 6. 5 mm Waist Length 5. 5 mm 8 mm 4 mm Stabilizing Wires Sheath Diameter 8 6 pairs 8 pairs 12 F 14 F (with Adaptor) Lobe + 4 mm 10 pairs 14 F 26 28 Lobe + 6 mm 6 pairs 9 F 10 F 13 F 30

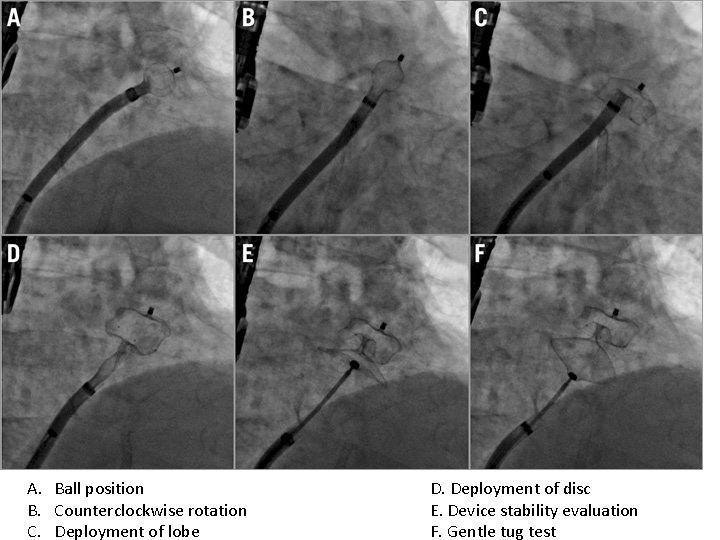
A. Ball position B. Counterclockwise rotation C. Deployment of lobe D. Deployment of disc E. Device stability evaluation F. Gentle tug test

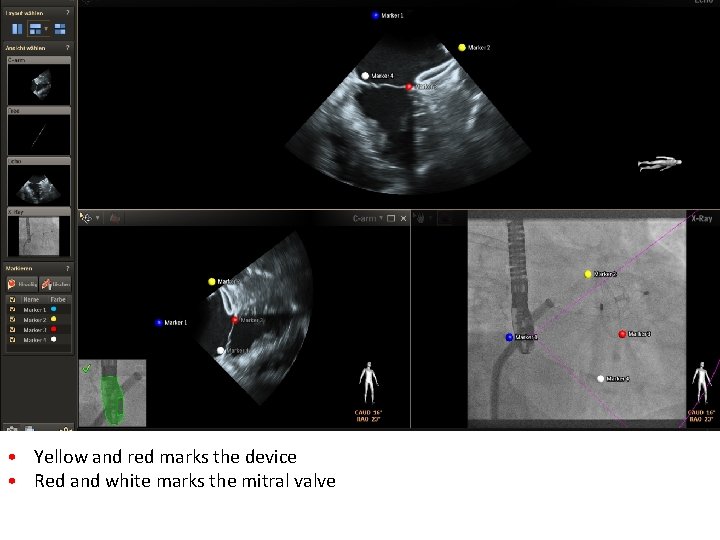
• Yellow and red marks the device • Red and white marks the mitral valve

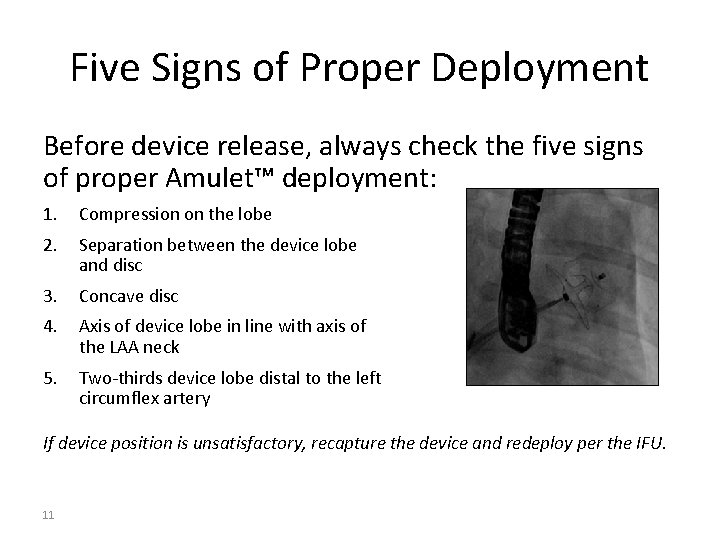
Five Signs of Proper Deployment Before device release, always check the five signs of proper Amulet™ deployment: 1. Compression on the lobe 2. Separation between the device lobe and disc 3. Concave disc 4. Axis of device lobe in line with axis of the LAA neck 5. Two-thirds device lobe distal to the left circumflex artery If device position is unsatisfactory, recapture the device and redeploy per the IFU. 11

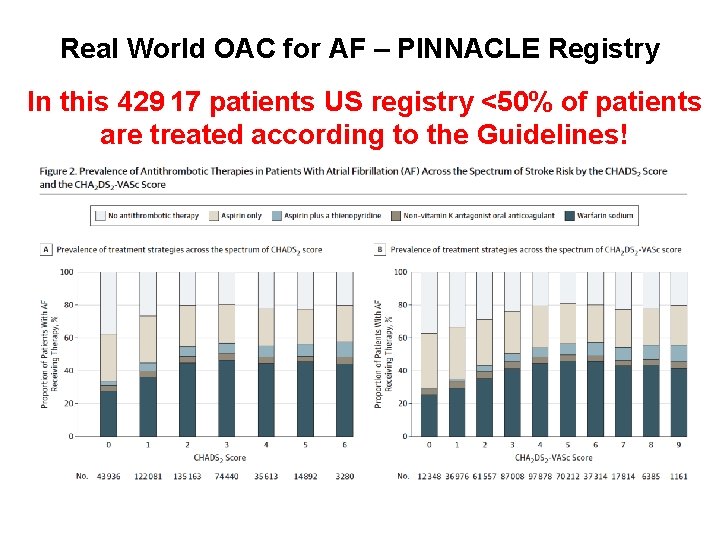
Real World OAC for AF – PINNACLE Registry In this 429 17 patients US registry <50% of patients are treated according to the Guidelines! From: OAC Therapy Prescription in Patients With AF Across the Spectrum of Stroke JAMA Cardiol. 2016; 1(1): 55 -62 Risk: Insights From the NCDR PINNACLE Registry

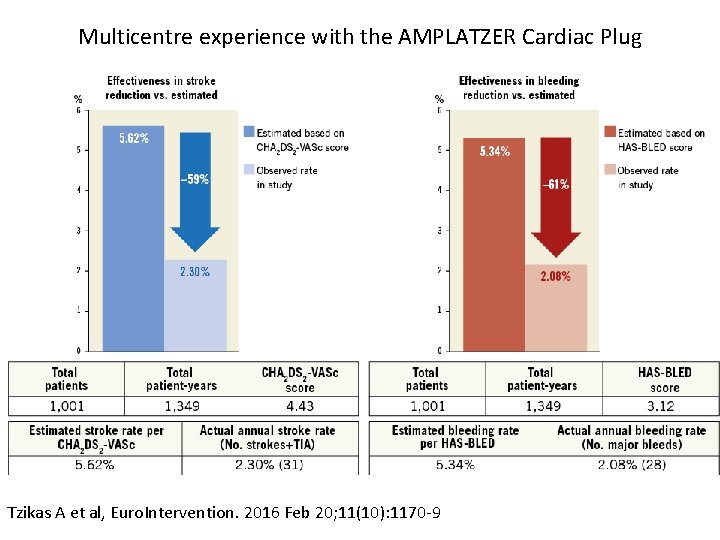
Multicentre experience with the AMPLATZER Cardiac Plug Tzikas A et al, Euro. Intervention. 2016 Feb 20; 11(10): 1170 -9

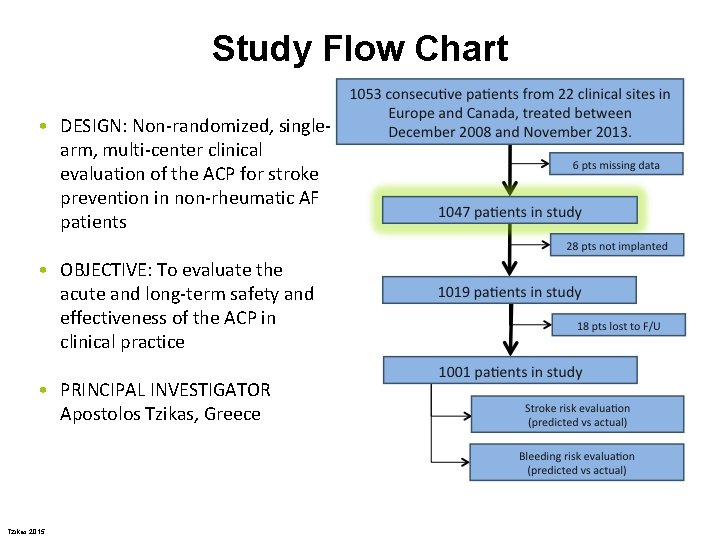
Study Flow Chart • DESIGN: Non-randomized, singlearm, multi-center clinical evaluation of the ACP for stroke prevention in non-rheumatic AF patients • OBJECTIVE: To evaluate the acute and long-term safety and effectiveness of the ACP in clinical practice • PRINCIPAL INVESTIGATOR Apostolos Tzikas, Greece Tzikas 2015

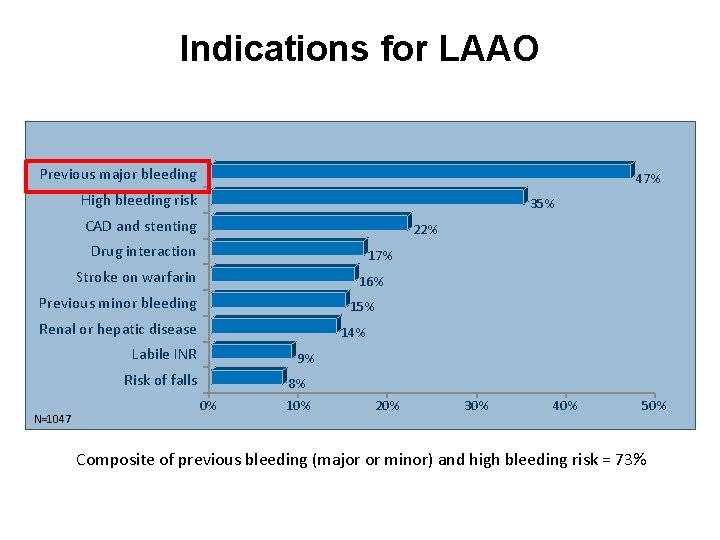
Indications for LAAO Previous major bleeding 47% High bleeding risk 35% CAD and stenting 22% Drug interaction 17% Stroke on warfarin 16% Previous minor bleeding 15% Renal or hepatic disease 14% Labile INR 9% Risk of falls N=1047 0% 8% 10% 20% 30% 40% 50% Composite of previous bleeding (major or minor) and high bleeding risk = 73%

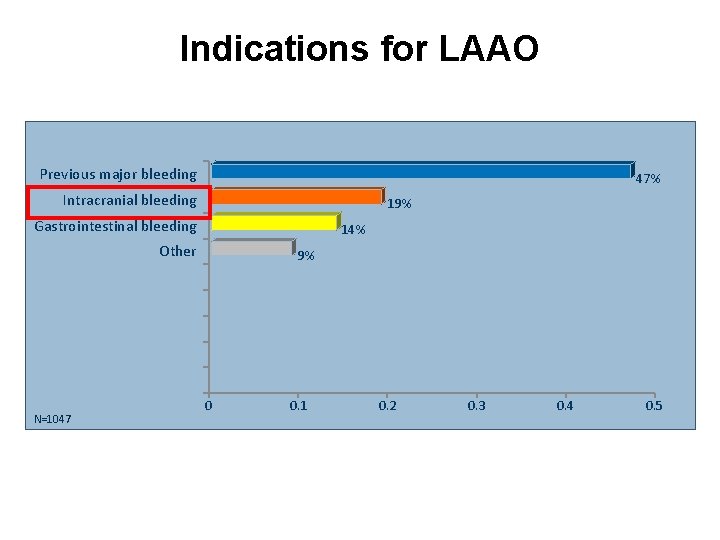
Indications for LAAO Previous major bleeding 47% Intracranial bleeding 19% Gastrointestinal bleeding 14% Other N=1047 9% 0 0. 1 0. 2 0. 3 0. 4 0. 5

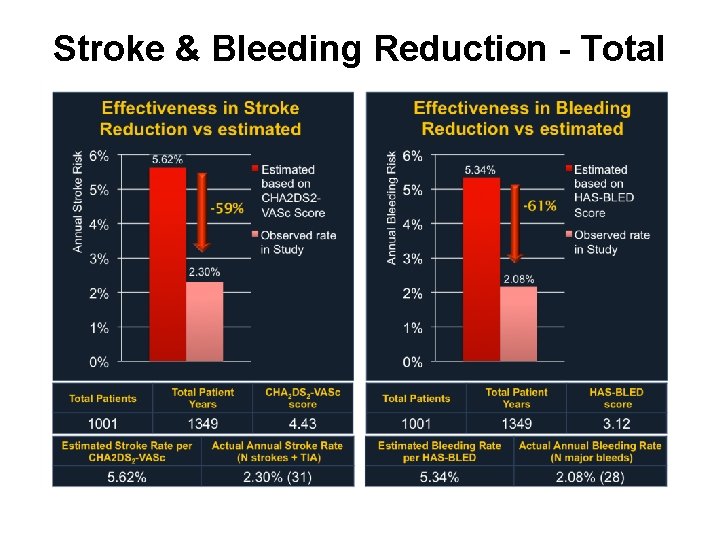
Stroke & Bleeding Reduction - Total

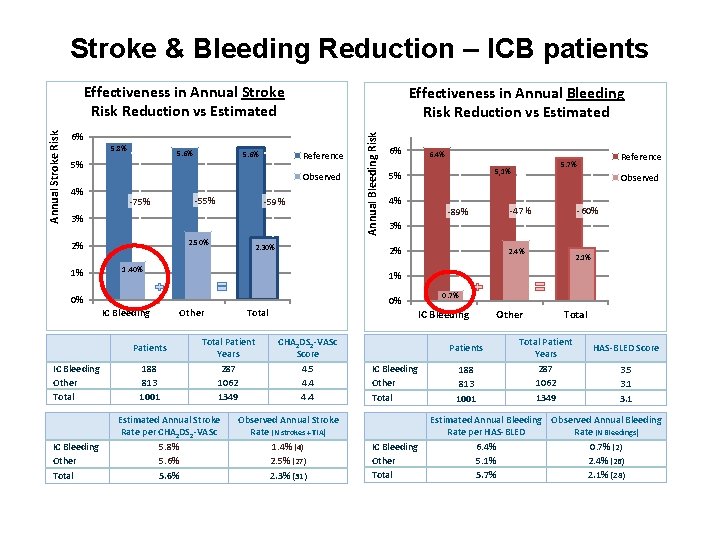
Stroke & Bleeding Reduction – ICB patients Effectiveness in Annual Bleeding Risk Reduction vs Estimated 6% 5. 8% 5. 6% 5% 5. 6% Reference Observed 4% -75% -59% 3% 2. 50% 2% 1% 0% IC Bleeding Other Total 188 813 1001 6% 6. 4% 4% -89% Reference 5. 7% 5, 1% 5% Observed -60% -47% 3% 2% 1. 40% Patients IC Bleeding Other Total 2. 30% Annual Bleeding Risk Annual Stroke Risk Effectiveness in Annual Stroke Risk Reduction vs Estimated 2. 4% 2. 1% 1% Other Total Patient Years 287 1062 1349 Estimated Annual Stroke Rate per CHA 2 DS 2 -VASc 5. 8% 5. 6% 0% 0. 7% IC Bleeding CHA 2 DS 2 -VASc Score 4. 5 4. 4 Observed Annual Stroke Rate (N strokes + TIA) 1. 4% (4) 2. 5% (27) 2. 3% (31) Patients IC Bleeding Other Total 188 813 1001 Other Total Patient Years 287 1062 1349 HAS-BLED Score 3. 5 3. 1 Estimated Annual Bleeding Observed Annual Bleeding Rate per HAS-BLED Rate (N Bleedings) 6. 4% 0. 7% (2) 5. 1% 2. 4% (26) 5. 7% 2. 1% (28)

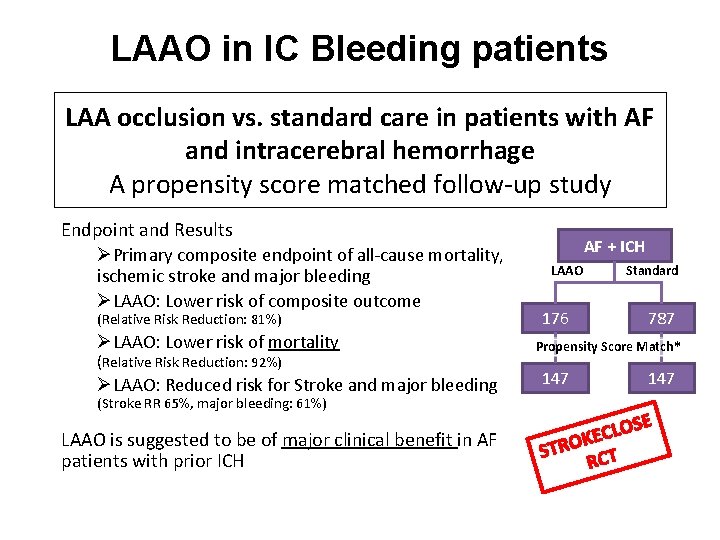
LAAO in IC Bleeding patients LAA occlusion vs. standard care in patients with AF and intracerebral hemorrhage A propensity score matched follow-up study Endpoint and Results ØPrimary composite endpoint of all-cause mortality, ischemic stroke and major bleeding ØLAAO: Lower risk of composite outcome (Relative Risk Reduction: 81%) ØLAAO: Lower risk of mortality (Relative Risk Reduction: 92%) ØLAAO: Reduced risk for Stroke and major bleeding (Stroke RR 65%, major bleeding: 61%) LAAO is suggested to be of major clinical benefit in AF patients with prior ICH J-E Nielsen-Kudsk – Euro. PCR 2016 AF + ICH LAAO Standard 176 787 Propensity Score Match* 147 OS L C E K STRO CT R E

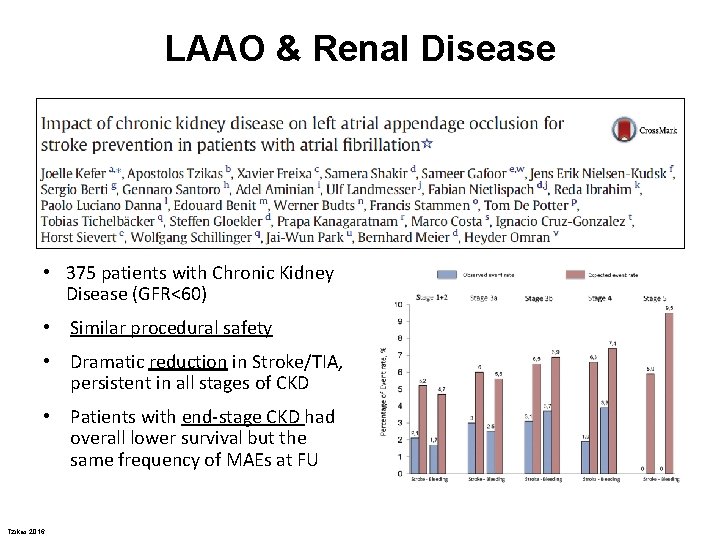
LAAO & Renal Disease • 375 patients with Chronic Kidney Disease (GFR<60) • Similar procedural safety • Dramatic reduction in Stroke/TIA, persistent in all stages of CKD • Patients with end-stage CKD had overall lower survival but the same frequency of MAEs at FU Tzikas 2016 Int J Cardiol 2016

LAAO in the Elderly • LAAO was associated with: Ø similar procedural success in patients aged <75 and ≥ 75 years Ø older patients had a higher incidence of cardiac tamponade • At follow-up: Ø stroke and major bleeding rates were similar among groups Tzikas 2016 Am J Card 2016

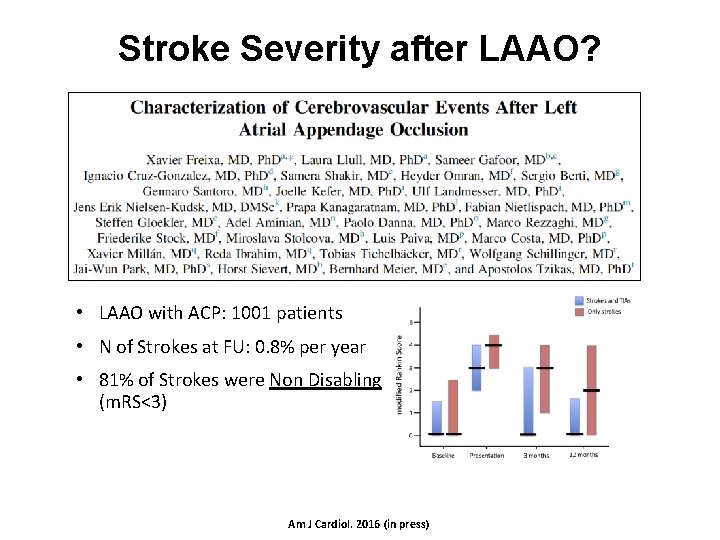
Stroke Severity after LAAO? • LAAO with ACP: 1001 patients • N of Strokes at FU: 0. 8% per year • 81% of Strokes were Non Disabling (m. RS<3) Am J Cardiol. 2016 (in press)

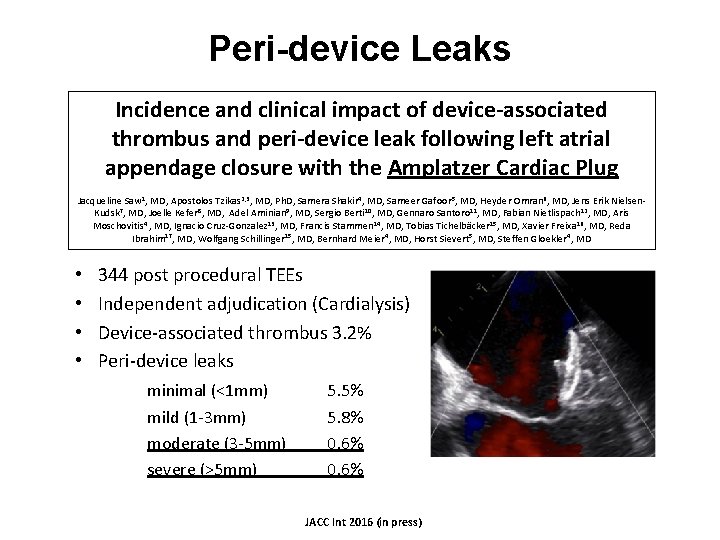
Peri-device Leaks Incidence and clinical impact of device-associated thrombus and peri-device leak following left atrial appendage closure with the Amplatzer Cardiac Plug Jacqueline Saw 1, MD, Apostolos Tzikas 2, 3, MD, Ph. D, Samera Shakir 4, MD, Sameer Gafoor 5, MD, Heyder Omran 6, MD, Jens Erik Nielsen. Kudsk 7, MD, Joelle Kefer 8, MD, Adel Aminian 9, MD, Sergio Berti 10, MD, Gennaro Santoro 11, MD, Fabian Nietlispach 12, MD, Aris Moschovitis 4 , MD, Ignacio Cruz-Gonzalez 13, MD, Francis Stammen 14, MD, Tobias Tichelbäcker 15, MD, Xavier Freixa 16, MD, Reda Ibrahim 17, MD, Wolfgang Schillinger 15, MD, Bernhard Meier 4, MD, Horst Sievert 5, MD, Steffen Gloekler 4, MD • • 344 post procedural TEEs Independent adjudication (Cardialysis) Device-associated thrombus 3. 2% Peri-device leaks minimal (<1 mm) mild (1 -3 mm) moderate (3 -5 mm) severe (>5 mm) 5. 5% 5. 8% 0. 6% JACC Int 2016 (in press)

Thank You