LAA Closure Lessons from the Pivotal Studies Three






- Slides: 18

LAA Closure: Lessons from the Pivotal Studies & Three Advisory Panels Keith Dawkins MD FRCP FACC FSCAI Global Chief Medical Officer Executive Vice President Boston Scientific Corporation

Conflict of Interest Boston Scientific Corporation Employee Stockholder

Global Burden of Atrial Fibrillation Individuals with AF = 33. 5 million Incidence ↑ Prevalence ↑ Disease Burden ↑ AF-Related Mortality ↑ Chugh SS: Circ 2014; 129: 837 -847

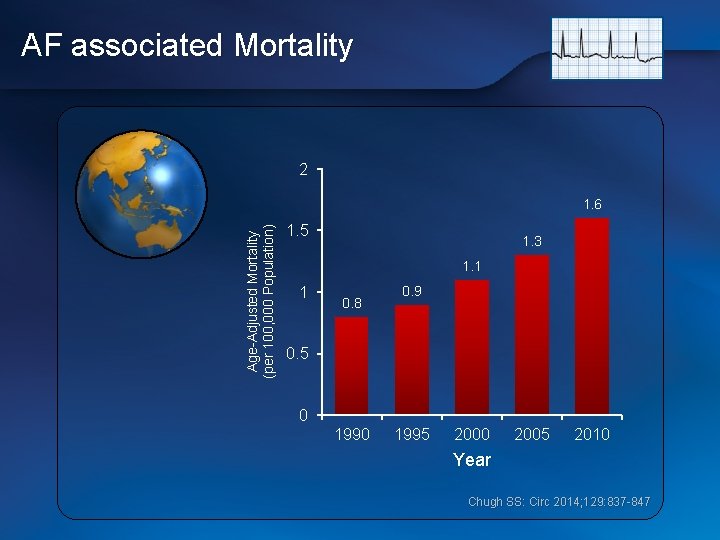
AF associated Mortality 2 Age-Adjusted Mortality (per 100, 000 Population) 1. 6 1. 5 1. 3 1. 1 1 0. 8 0. 9 0. 5 0 1995 2000 2005 2010 Year Chugh SS: Circ 2014; 129: 837 -847

Atrial Fibrillation & Anticoagulation Atrial Fibrillation is the most common sustained cardiac arrhythmia Fivefold increase in stroke when AF is present INR levels outside therapeutic range (2. 0 -3. 0) occurs in ~50% of patients According to HAS-BLED, 61% of pts currently on Warfarin for AF are at moderate risk of bleeding and an additional 19% are at high risk Risk of Thromboembolism in AF vs. Risk of Bleeding with Anticoagulants Camm AJ: Euro Heart J 2010; 31: 2369 -2429 Pisters R: Chest 2010; 138: 1093 -1100

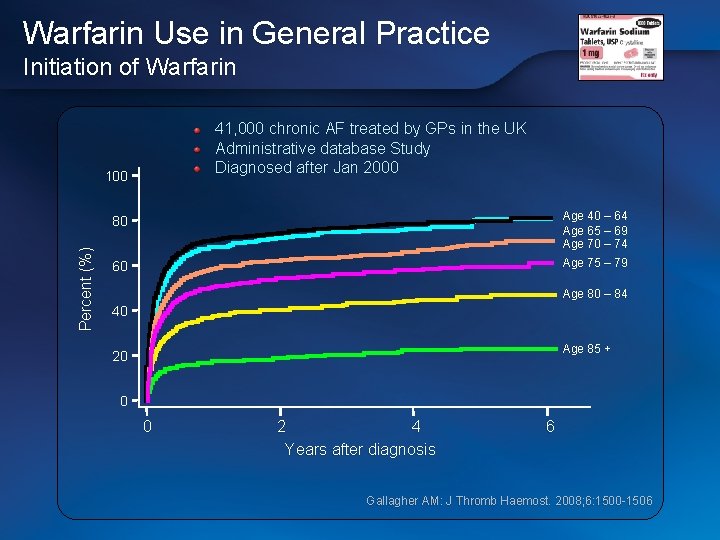
Warfarin Use in General Practice Initiation of Warfarin 41, 000 chronic AF treated by GPs in the UK Administrative database Study Diagnosed after Jan 2000 Percent (%) 100 80 Age 40 – 64 Age 65 – 69 Age 70 – 74 60 Age 75 – 79 Age 80 – 84 40 Age 85 + 20 0 0 2 4 Years after diagnosis 6 Gallagher AM: J Thromb Haemost. 2008; 6: 1500 -1506 Gallagher

Why do Physicians Underuse Warfarin in AF? Most Cited Reasons: Bleeding Risk Advanced Age Other factors influencing prescriptions: Previous Falls Perceived Fall Risk Comorbidities (cognitive impairment, alcohol) Inability to comply with monitoring/treatment Pugh D: Age and Ageing 2011; 40: 675 -683

Atrial Fibrillation & Anticoagulation High Prevalence High Cost Low Medication Adherence Poor Monitoring Behavior Poor Patient Understanding Kneeland PP: Patient Prefer Adherence 2010; 4: 51 -60

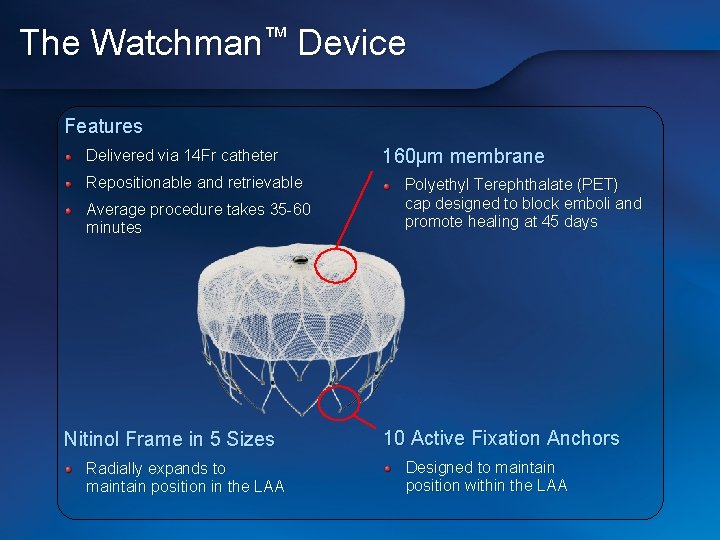
The Watchman™ Device Features Delivered via 14 Fr catheter Repositionable and retrievable Average procedure takes 35 -60 minutes Nitinol Frame in 5 Sizes Radially expands to maintain position in the LAA 160μm membrane Polyethyl Terephthalate (PET) cap designed to block emboli and promote healing at 45 days 10 Active Fixation Anchors Designed to maintain position within the LAA

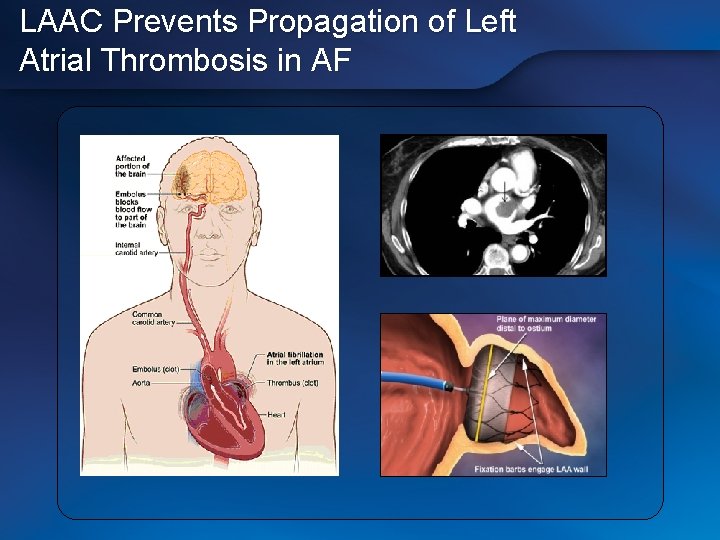
LAAC Prevents Propagation of Left Atrial Thrombosis in AF

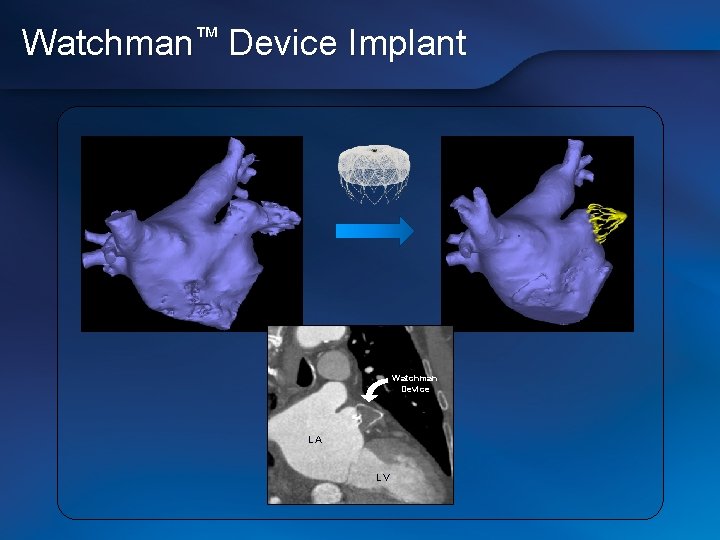
Watchman™ Device Implant Watchman Device LA LV

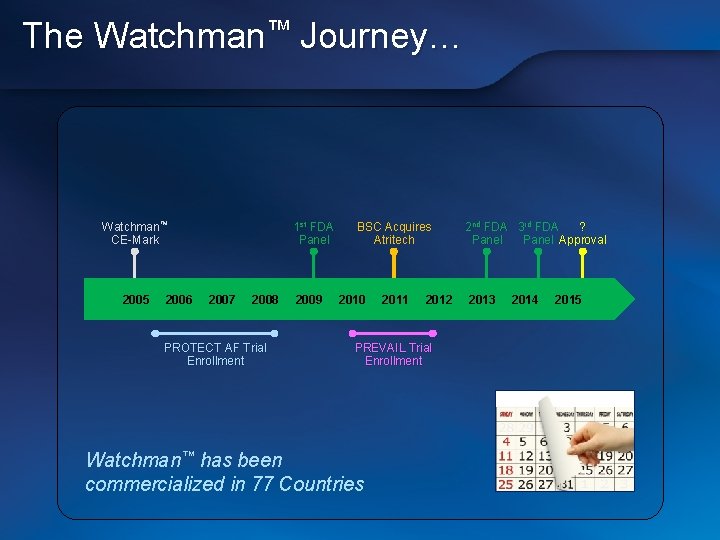
The Watchman™ Journey… Watchman™ CE-Mark 2005 2006 1 st FDA Panel 2007 2008 PROTECT AF Trial Enrollment 2009 BSC Acquires Atritech 2010 2011 2012 PREVAIL Trial Enrollment Watchman™ has been commercialized in 77 Countries 2 nd FDA 3 rd FDA ? Panel Approval 2013 2014 2015

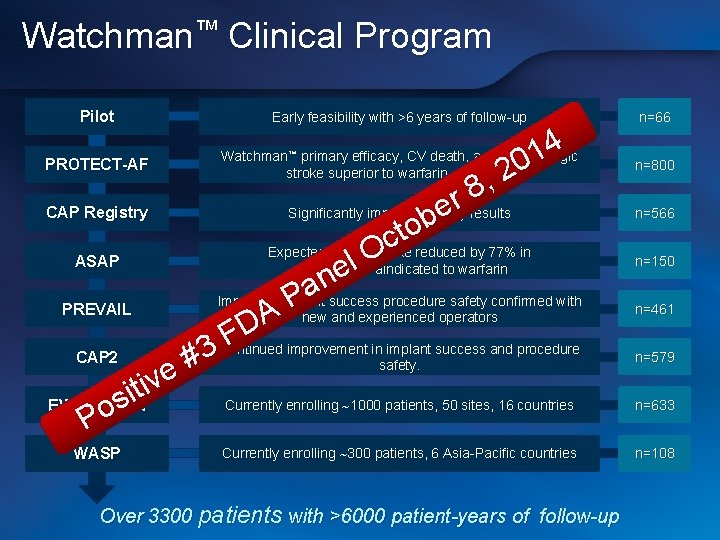
Watchman™ Clinical Program Pilot Early feasibility with >6 years of follow-up 4 1 PROTECT-AF 0 2 , 8 r results Significantly improved safety CAP Registry e b o t c Expected rate of stroke reduced by 77% in O ASAP l patients contraindicated to warfarin e n a Improved implant success procedure safety confirmed with P PREVAIL new and experienced operators A D F Continued improvement in implant success and procedure 3 CAP 2 safety. # e iv t i Currently enrolling 1000 patients, 50 sites, 16 countries EWOLUTION s o P Watchman™ primary efficacy, CV death, and hemorrhagic stroke superior to warfarin at 5 years WASP Currently enrolling 300 patients, 6 Asia-Pacific countries Over 3300 patients with >6000 patient-years of follow-up n=66 n=800 n=566 n=150 n=461 n=579 n=633 n=108

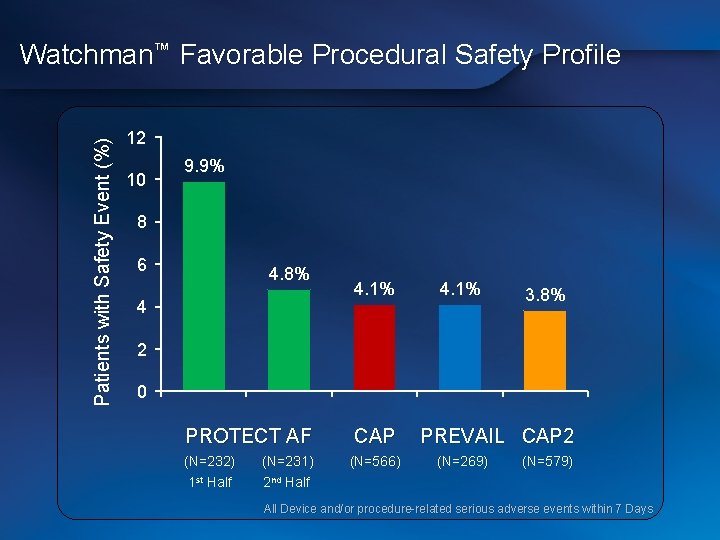
Patients with Safety Event (%) Watchman™ Favorable Procedural Safety Profile 12 10 9. 9% 8 6 4. 8% 4 4. 1% 3. 8% 2 0 PROTECT AF CAP (N=232) (N=231) 1 st Half 2 nd Half (N=566) PREVAIL CAP 2 (N=269) (N=579) All Device and/or procedure-related serious adverse events within 7 Days All Device and/or procedure-related serious adverse events within

Watchman™ Peer-Reviewed Publications

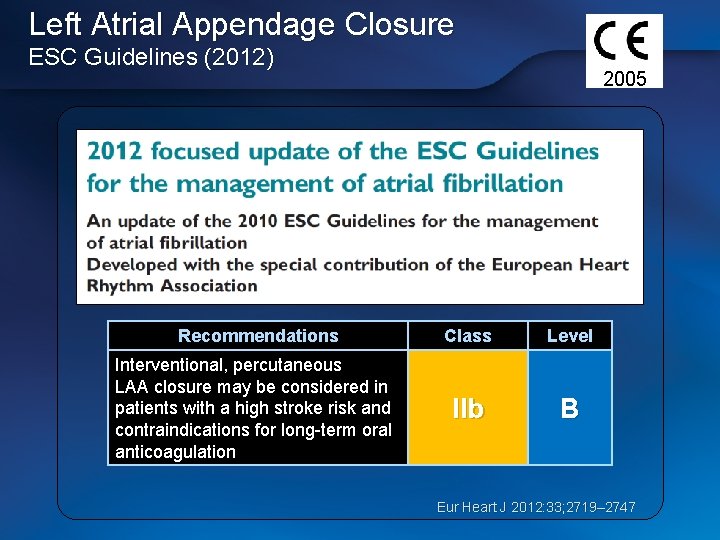
Left Atrial Appendage Closure ESC Guidelines (2012) 2005 Recommendations Class Level Interventional, percutaneous LAA closure may be considered in patients with a high stroke risk and contraindications for long-term oral anticoagulation IIb B Eur Heart J 2012: 33; 2719– 2747

Watchman™ Therapy… Is not designed to be a broad replacement for oral anticoagulation Is intended for patients: At an increased risk of stroke and systemic embolism Recommended and deemed suitable candidates for warfarin Having an appropriate rationale to seek a nonpharmacologic alternative to warfarin

Conclusions Eight clinical trials including 3, 000 patients and >6, 000 patient-years of data support Watchman™ use for the Primary Endpoint (All-Cause Stroke, Systemic Embolism, Cardiovascular death), with superiority for Cardiovascular Mortality, Hemorrhagic Stroke, and Disabling Stroke (post hoc analysis) We seek Watchman™ labeling related to the clinical trial population – i. e. patients with non-valvular atrial fibrillation at high risk based on CHADS 2 or CHA 2 DS 2 -VASc scores, who are suitable for Warfarin, and who have an appropriate rationale to seek an invasive and experimental non-pharma alternative to Warfarin Patients doing well on oral anticoagulants should continue on anticoagulants….