KJM 5120 and KJM 9120 Defects and Reactions







![Oxides with both oxygen deficiency and excess • Next we will solve for [Oi//]. Oxides with both oxygen deficiency and excess • Next we will solve for [Oi//].](https://slidetodoc.com/presentation_image_h2/cfbc6ea8ff18b01a4e989ac4b263add2/image-48.jpg)

- Slides: 53

KJM 5120 and KJM 9120 Defects and Reactions Ch 3. Thermodynamics Truls Norby Department of Chemistry University of Oslo Centre for Materials Science and Nanotechnology (SMN) FERMIO Oslo Research Park (Forskningsparken) truls. norby@kjemi. uio. no http: //folk. uio. no/trulsn

Defect thermodynamics • The main aim of this chapter is to learn to evaluate how defect concentrations vary with temperature and component activities (e. g. p. O 2). • The tool for this is the mass action law, via its equilibrium coefficients. • The chapter has an introduction to relevant thermodynamics. This may be trivial to some, and too brief and inaccessible for others. You may choose your level of approach based on your background aspirations. • BUT: Regardless of your understanding of thermodynamics, learning or accepting mass action law equilibrium constant expressions and how to write them up from a reaction equation is mandatory! • AND: Being able to combine such an equilibrium constant expression with a simplified electroneutrality condition is also mandatory. • These are the two central elements required as a minimum to make use of defect chemistry.

Equilibrium in defect chemical reactions The compendium recalls the first and second laws of thermodynamics and how they have lead to the understanding that a system is at equilibrium (no net reaction in any direction) when the Gibbs energy G = H – TS is at a minimum, i. e. when d. G = d. H – Td. S = 0. The compendium furthermore uses it on a simple example of vacancies in an elemental solid. It furthermore introduces a derivation of the mass action law and equilibrium constants based on thermodynamic arguments. Here, we will concentrate on an alternative derivation, namely from kinetics. This will help understand that equilibrium does not mean that nothing happens, just that reactions in both directions happen at equal rates.

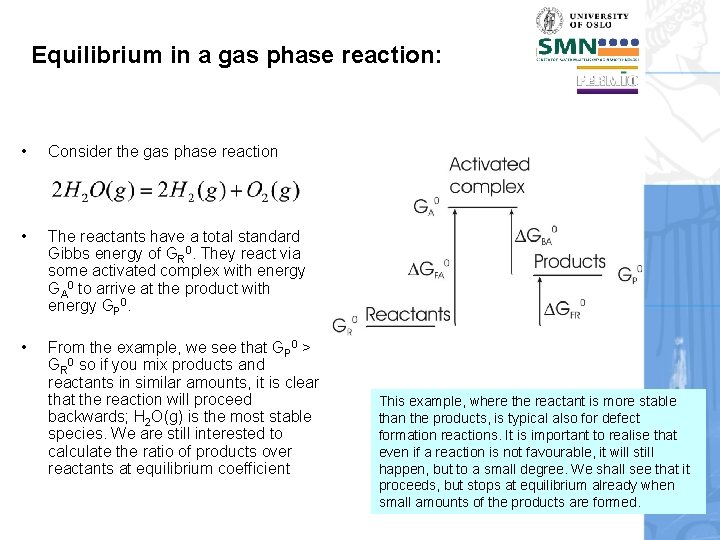
Equilibrium in a gas phase reaction: • Consider the gas phase reaction • The reactants have a total standard Gibbs energy of GR 0. They react via some activated complex with energy GA 0 to arrive at the product with energy GP 0. • From the example, we see that GP 0 > GR 0 so if you mix products and reactants in similar amounts, it is clear that the reaction will proceed backwards; H 2 O(g) is the most stable species. We are still interested to calculate the ratio of products over reactants at equilibrium coefficient This example, where the reactant is more stable than the products, is typical also for defect formation reactions. It is important to realise that even if a reaction is not favourable, it will still happen, but to a small degree. We shall see that it proceeds, but stops at equilibrium already when small amounts of the products are formed.

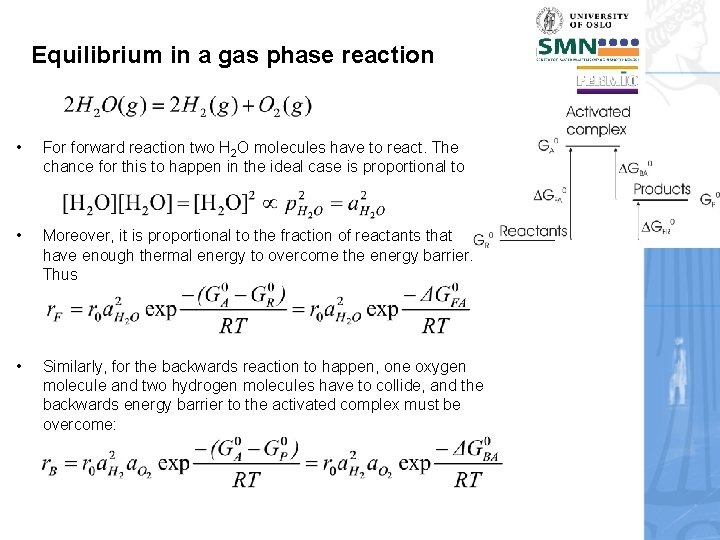
Equilibrium in a gas phase reaction • For forward reaction two H 2 O molecules have to react. The chance for this to happen in the ideal case is proportional to • Moreover, it is proportional to the fraction of reactants that have enough thermal energy to overcome the energy barrier. Thus • Similarly, for the backwards reaction to happen, one oxygen molecule and two hydrogen molecules have to collide, and the backwards energy barrier to the activated complex must be overcome:

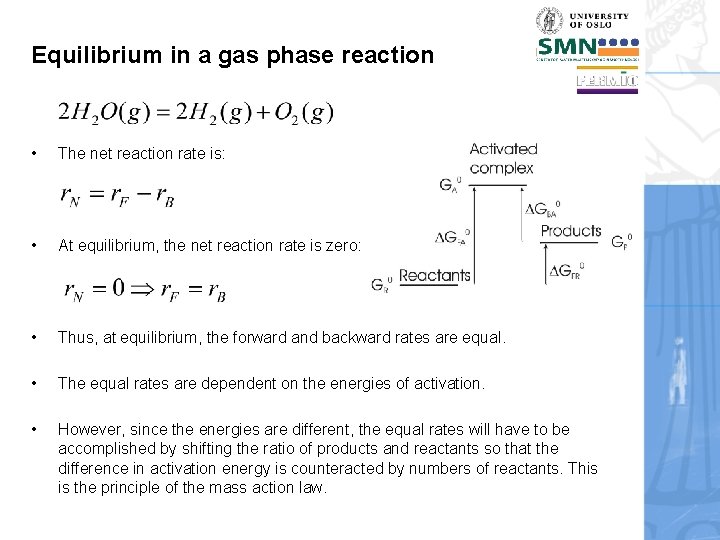
Equilibrium in a gas phase reaction • The net reaction rate is: • At equilibrium, the net reaction rate is zero: • Thus, at equilibrium, the forward and backward rates are equal. • The equal rates are dependent on the energies of activation. • However, since the energies are different, the equal rates will have to be accomplished by shifting the ratio of products and reactants so that the difference in activation energy is counteracted by numbers of reactants. This is the principle of the mass action law.

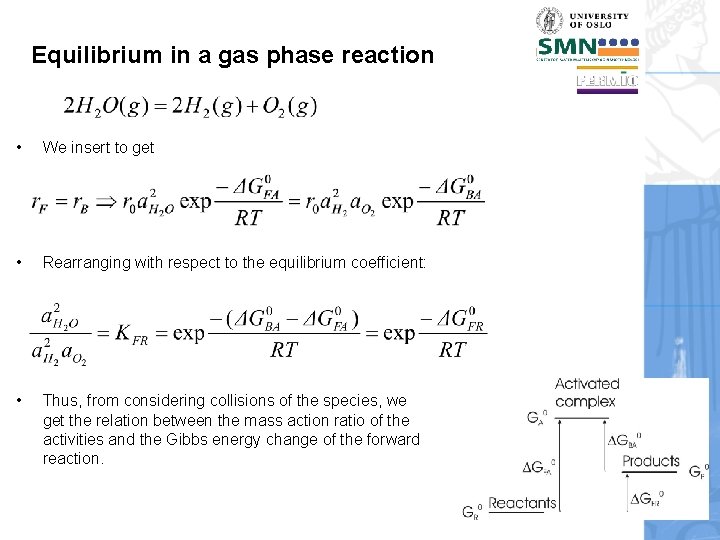
Equilibrium in a gas phase reaction • We insert to get • Rearranging with respect to the equilibrium coefficient: • Thus, from considering collisions of the species, we get the relation between the mass action ratio of the activities and the Gibbs energy change of the forward reaction.

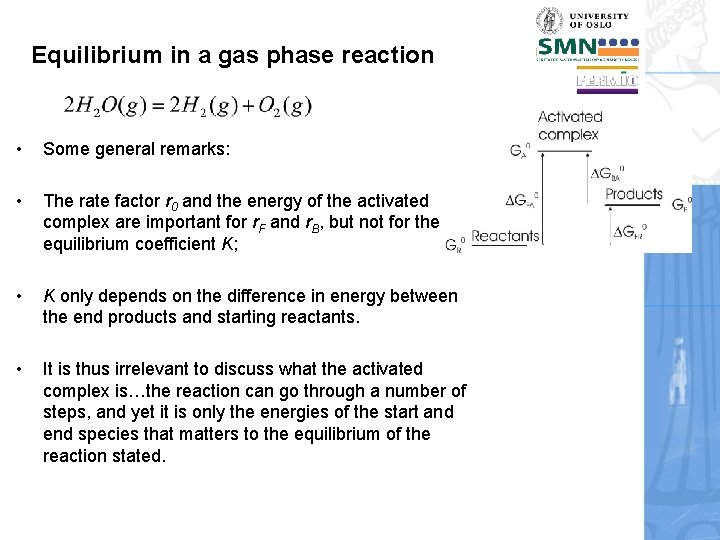
Equilibrium in a gas phase reaction • Some general remarks: • The rate factor r 0 and the energy of the activated complex are important for r. F and r. B, but not for the equilibrium coefficient K; • K only depends on the difference in energy between the end products and starting reactants. • It is thus irrelevant to discuss what the activated complex is…the reaction can go through a number of steps, and yet it is only the energies of the start and end species that matters to the equilibrium of the reaction stated.

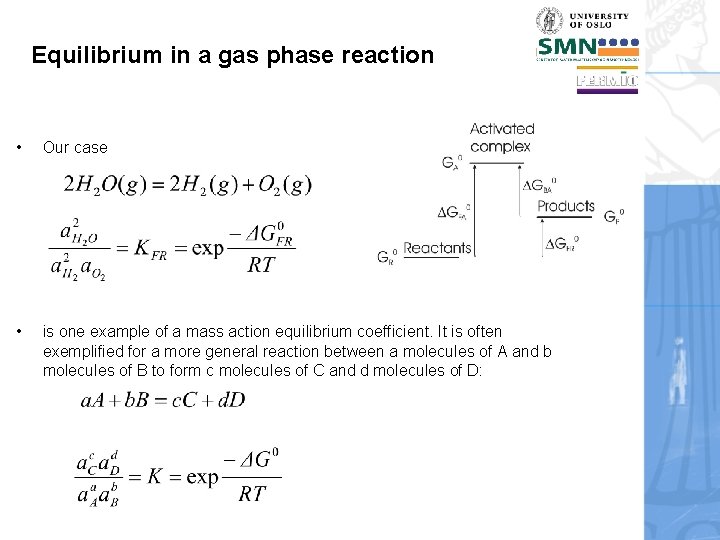
Equilibrium in a gas phase reaction • Our case • is one example of a mass action equilibrium coefficient. It is often exemplified for a more general reaction between a molecules of A and b molecules of B to form c molecules of C and d molecules of D:

Equilibrium based on the mass action law What we have done till now has been an attempt to show that chemical equilibrium is a result of forward and backward reactions happening at the same rate, due to a shift in the ratio of activities of the reactants and products. This shift – or ratio – is called the equilibrium coefficient. From the collision aspect of it it is called the law of mass action. It applies to all chemical reactions, also defect chemical reactions, where the derivation based on mass action may be more or less obvious. The compendium derives the equilibrium coefficients based purely on thermodynamics, in which the absolute and net rates become hidden, but where the statistical thermodynamics aspect of defects becomes better visualised.

Equilibrium constants and equilibrium coefficients • The K is often called a mass action or equilibrium constant because it has the important mission to tell us that a particular ratio of products and reactants is maintained constant at equilibrium. • However, the value changes with temperature. This is because the Gibbs energy is a balance between the relatively constant enthalpy change and the temperature dependent decrease in energy given by the entropy change: • Therefore, K’s are not constant (except at each temperature) and are therefore often instead referred to as coefficients.

Examples of defect equilibria in stoichiometric oxides and how dependencies on T are derived We will here, and in the compendium, go through many examples. However, be aware that the principles are taught in the first example – on Schottky defects in MO – so take that first example seriously. If you don’t understand that one, you won’t understand the rest.

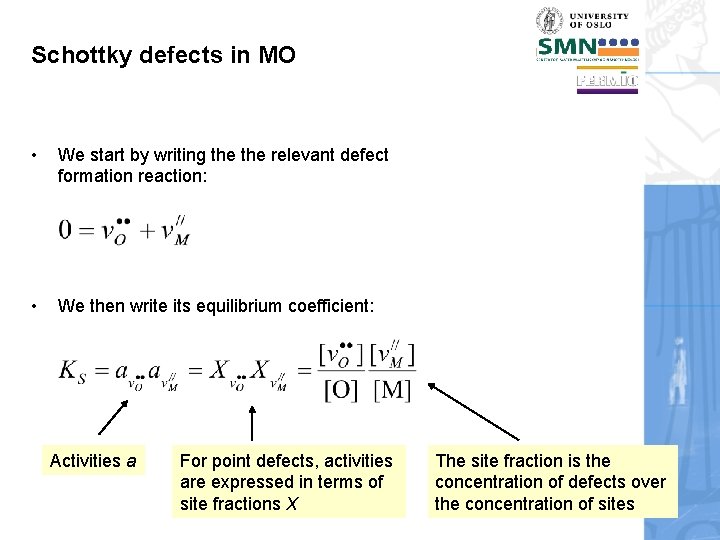
Schottky defects in MO • We start by writing the relevant defect formation reaction: • We then write its equilibrium coefficient: Activities a For point defects, activities are expressed in terms of site fractions X The site fraction is the concentration of defects over the concentration of sites

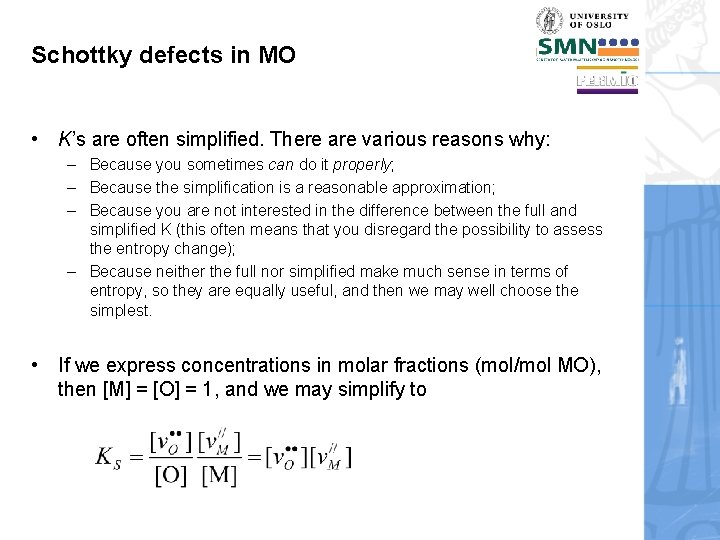
Schottky defects in MO • K’s are often simplified. There are various reasons why: – Because you sometimes can do it properly; – Because the simplification is a reasonable approximation; – Because you are not interested in the difference between the full and simplified K (this often means that you disregard the possibility to assess the entropy change); – Because neither the full nor simplified make much sense in terms of entropy, so they are equally useful, and then we may well choose the simplest. • If we express concentrations in molar fractions (mol/mol MO), then [M] = [O] = 1, and we may simplify to

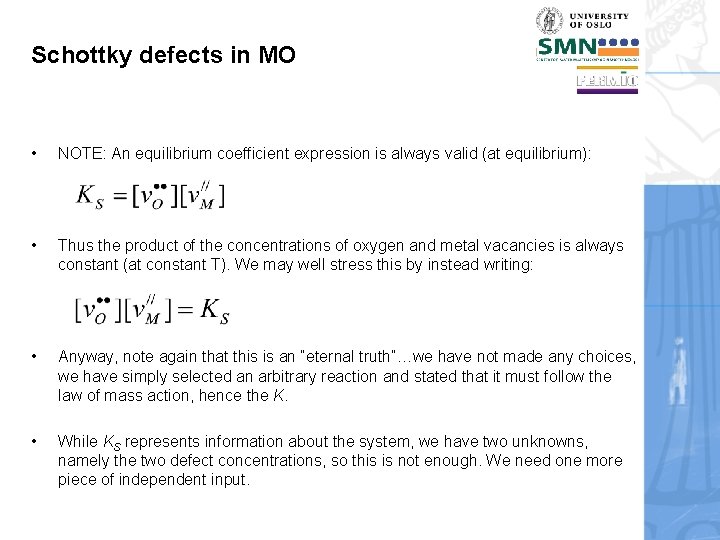
Schottky defects in MO • NOTE: An equilibrium coefficient expression is always valid (at equilibrium): • Thus the product of the concentrations of oxygen and metal vacancies is always constant (at constant T). We may well stress this by instead writing: • Anyway, note again that this is an “eternal truth”…we have not made any choices, we have simply selected an arbitrary reaction and stated that it must follow the law of mass action, hence the K. • While KS represents information about the system, we have two unknowns, namely the two defect concentrations, so this is not enough. We need one more piece of independent input.

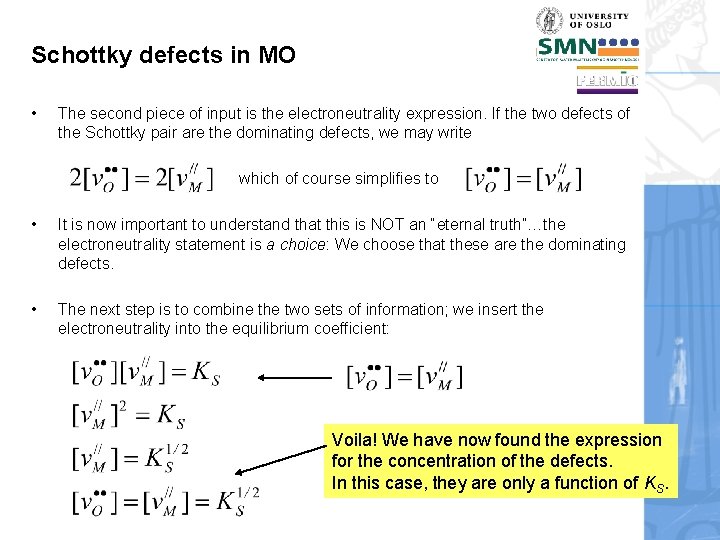
Schottky defects in MO • The second piece of input is the electroneutrality expression. If the two defects of the Schottky pair are the dominating defects, we may write which of course simplifies to • It is now important to understand that this is NOT an “eternal truth”…the electroneutrality statement is a choice: We choose that these are the dominating defects. • The next step is to combine the two sets of information; we insert the electroneutrality into the equilibrium coefficient: Voila! We have now found the expression for the concentration of the defects. In this case, they are only a function of KS.

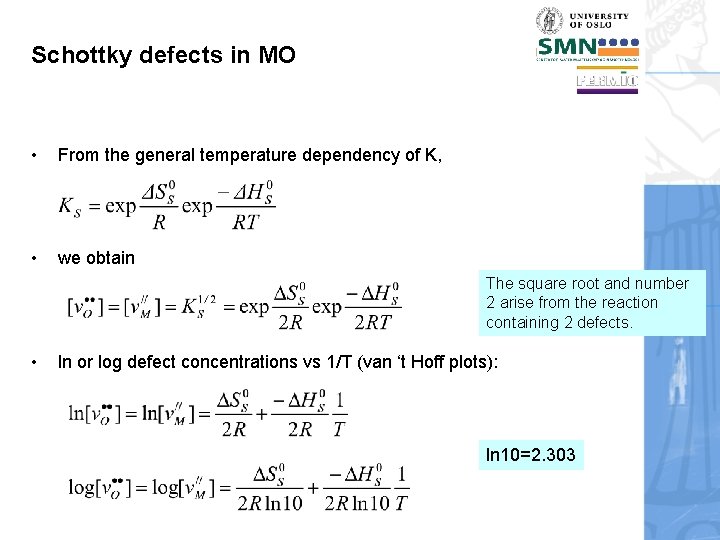
Schottky defects in MO • From the general temperature dependency of K, • we obtain The square root and number 2 arise from the reaction containing 2 defects. • ln or log defect concentrations vs 1/T (van ‘t Hoff plots): ln 10=2. 303

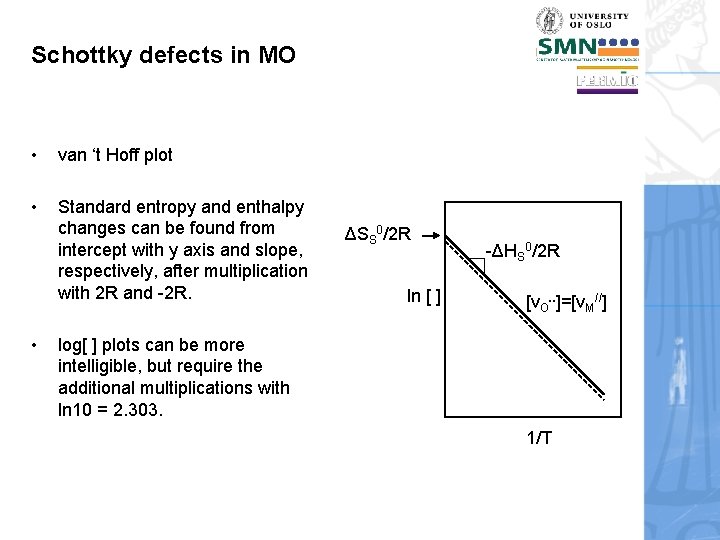
Schottky defects in MO • van ‘t Hoff plot • Standard entropy and enthalpy changes can be found from intercept with y axis and slope, respectively, after multiplication with 2 R and -2 R. • ΔSS 0/2 R ln [ ] -ΔHS 0/2 R [v. O. . ]=[v. M//] log[ ] plots can be more intelligible, but require the additional multiplications with ln 10 = 2. 303. 1/T

Before we move on… • Note that: – The solution we found assumes that the two Schottky defects are dominating. – The standard entropy and enthalpy changes of the Schottky reaction refer to the reaction when the reactants and products are in the standard state. For our defects, that means that the site fraction is unity! We recall that this is a hypothetical state, but nevertheless the state we have agreed on as standard. Therefore, the entropy as derived and used here is only valid if the defect concentrations are entered (plotted) in units of mole fraction (which in MO is the same as site fraction). We may also recall that the entropy change represents the change in vibrational entropy. – The model also assumes ideality, i. e. that the activities of defects are proportional to their concentrations. It is a dilute solution case. • Frenkel defect pair – Read the compendium text…. . similar to the Schottky case.

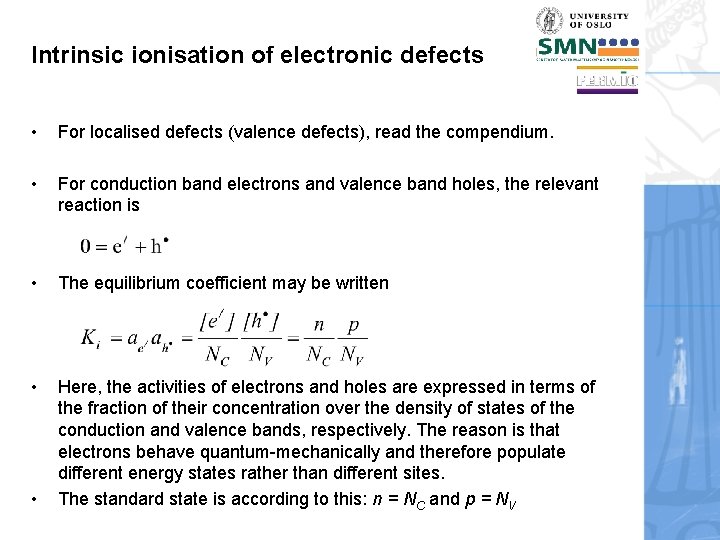
Intrinsic ionisation of electronic defects • For localised defects (valence defects), read the compendium. • For conduction band electrons and valence band holes, the relevant reaction is • The equilibrium coefficient may be written • Here, the activities of electrons and holes are expressed in terms of the fraction of their concentration over the density of states of the conduction and valence bands, respectively. The reason is that electrons behave quantum-mechanically and therefore populate different energy states rather than different sites. The standard state is according to this: n = NC and p = NV •

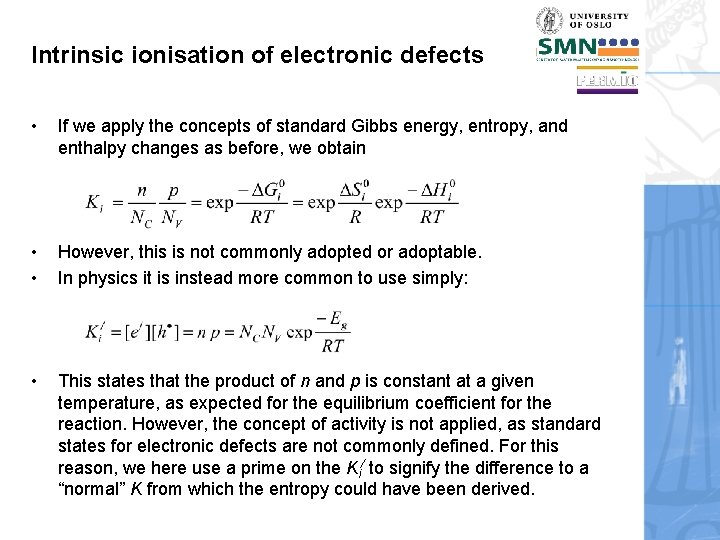
Intrinsic ionisation of electronic defects • If we apply the concepts of standard Gibbs energy, entropy, and enthalpy changes as before, we obtain • • However, this is not commonly adopted or adoptable. In physics it is instead more common to use simply: • This states that the product of n and p is constant at a given temperature, as expected for the equilibrium coefficient for the reaction. However, the concept of activity is not applied, as standard states for electronic defects are not commonly defined. For this reason, we here use a prime on the Ki/ to signify the difference to a “normal” K from which the entropy could have been derived.

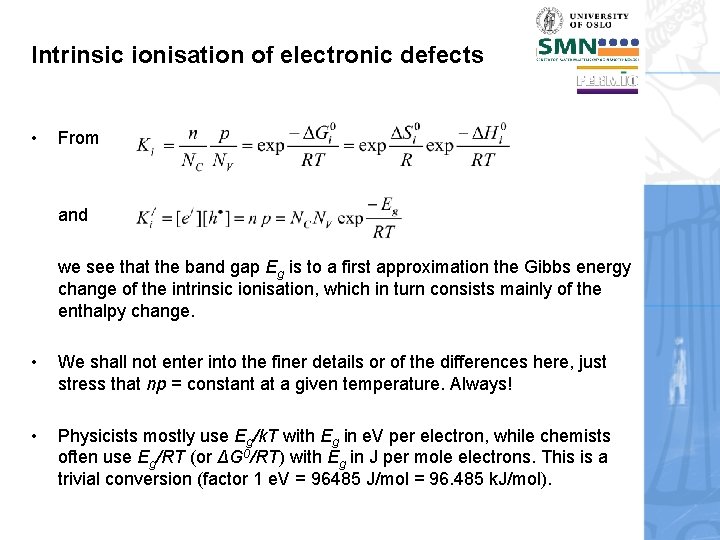
Intrinsic ionisation of electronic defects • From and we see that the band gap Eg is to a first approximation the Gibbs energy change of the intrinsic ionisation, which in turn consists mainly of the enthalpy change. • We shall not enter into the finer details or of the differences here, just stress that np = constant at a given temperature. Always! • Physicists mostly use Eg/k. T with Eg in e. V per electron, while chemists often use Eg/RT (or ΔG 0/RT) with Eg in J per mole electrons. This is a trivial conversion (factor 1 e. V = 96485 J/mol = 96. 485 k. J/mol).

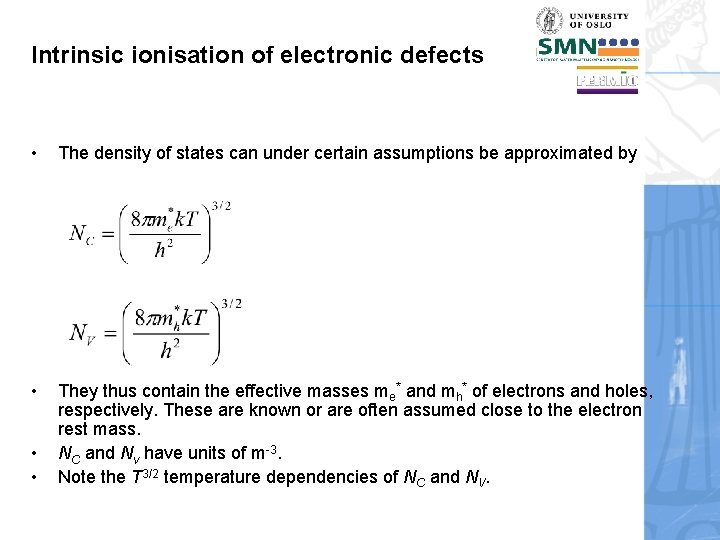
Intrinsic ionisation of electronic defects • The density of states can under certain assumptions be approximated by • They thus contain the effective masses me* and mh* of electrons and holes, respectively. These are known or are often assumed close to the electron rest mass. NC and Nv have units of m-3. Note the T 3/2 temperature dependencies of NC and NV. • •

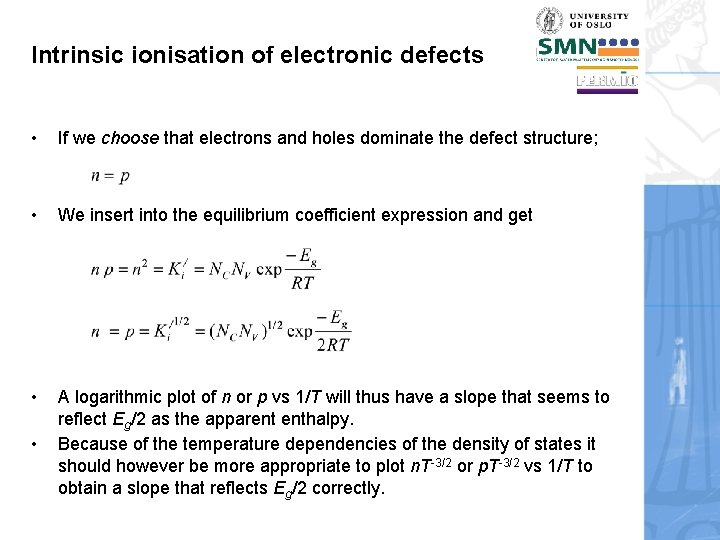
Intrinsic ionisation of electronic defects • If we choose that electrons and holes dominate the defect structure; • We insert into the equilibrium coefficient expression and get • A logarithmic plot of n or p vs 1/T will thus have a slope that seems to reflect Eg/2 as the apparent enthalpy. Because of the temperature dependencies of the density of states it should however be more appropriate to plot n. T-3/2 or p. T-3/2 vs 1/T to obtain a slope that reflects Eg/2 correctly. •

Examples of defect equilibria in non-stoichiometric oxides and how dependencies on T and p. O 2 are derived The next examples go by the same methodology as before…only now oxygen becomes a reactant or product. The defect concentrations become dependent on p. O 2 in addition to temperature.

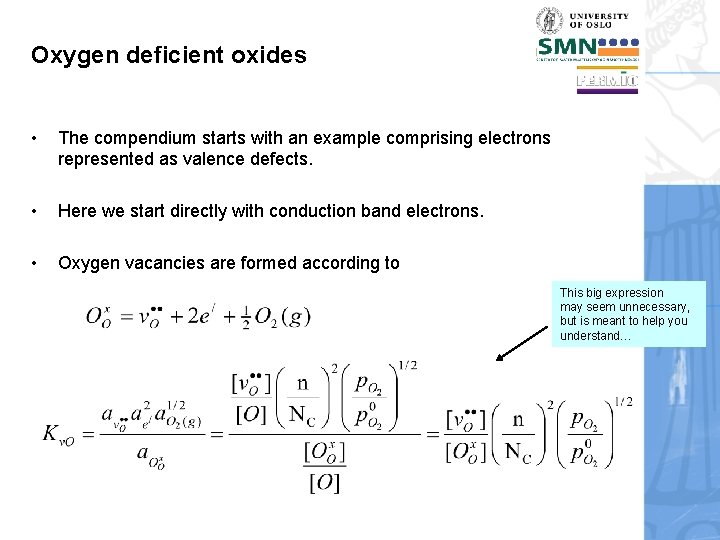
Oxygen deficient oxides • The compendium starts with an example comprising electrons represented as valence defects. • Here we start directly with conduction band electrons. • Oxygen vacancies are formed according to This big expression may seem unnecessary, but is meant to help you understand…

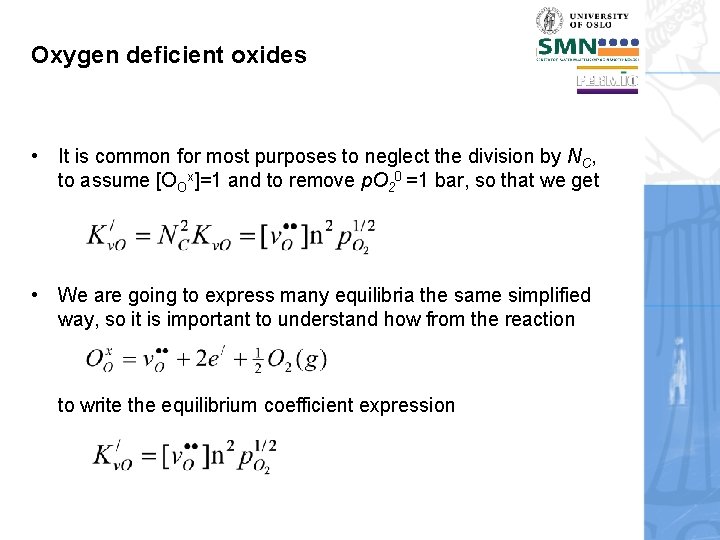
Oxygen deficient oxides • It is common for most purposes to neglect the division by NC, to assume [OOx]=1 and to remove p. O 20 =1 bar, so that we get • We are going to express many equilibria the same simplified way, so it is important to understand how from the reaction to write the equilibrium coefficient expression

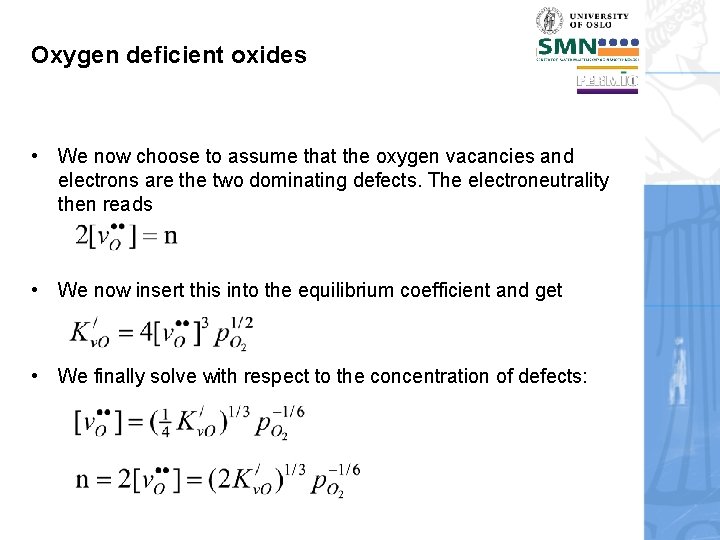
Oxygen deficient oxides • We now choose to assume that the oxygen vacancies and electrons are the two dominating defects. The electroneutrality then reads • We now insert this into the equilibrium coefficient and get • We finally solve with respect to the concentration of defects:

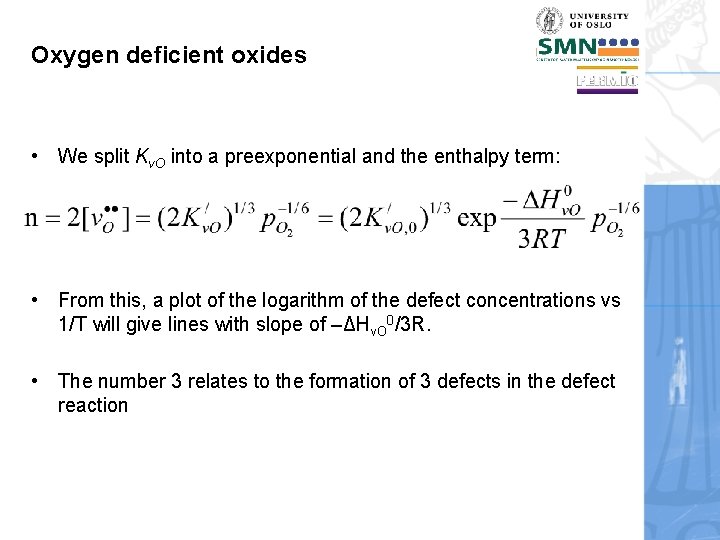
Oxygen deficient oxides • We split Kv. O into a preexponential and the enthalpy term: • From this, a plot of the logarithm of the defect concentrations vs 1/T will give lines with slope of –ΔHv. O 0/3 R. • The number 3 relates to the formation of 3 defects in the defect reaction

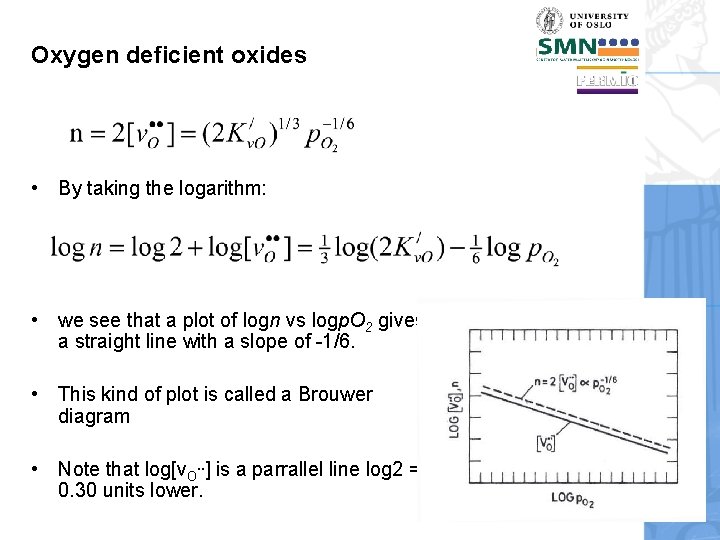
Oxygen deficient oxides • By taking the logarithm: • we see that a plot of logn vs logp. O 2 gives a straight line with a slope of -1/6. • This kind of plot is called a Brouwer diagram • Note that log[v. O. . ] is a parrallel line log 2 = 0. 30 units lower.

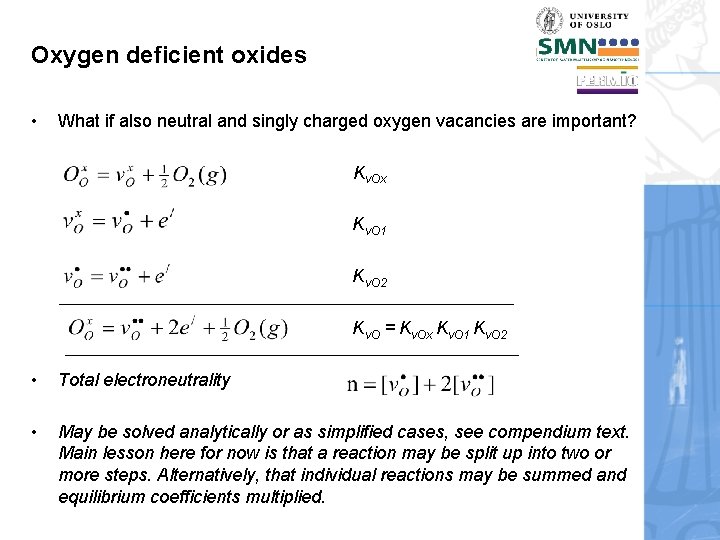
Oxygen deficient oxides • What if also neutral and singly charged oxygen vacancies are important? Kv. Ox K v. O 1 K v. O 2 K v. O = Kv. Ox Kv. O 1 Kv. O 2 • Total electroneutrality • May be solved analytically or as simplified cases, see compendium text. Main lesson here for now is that a reaction may be split up into two or more steps. Alternatively, that individual reactions may be summed and equilibrium coefficients multiplied.

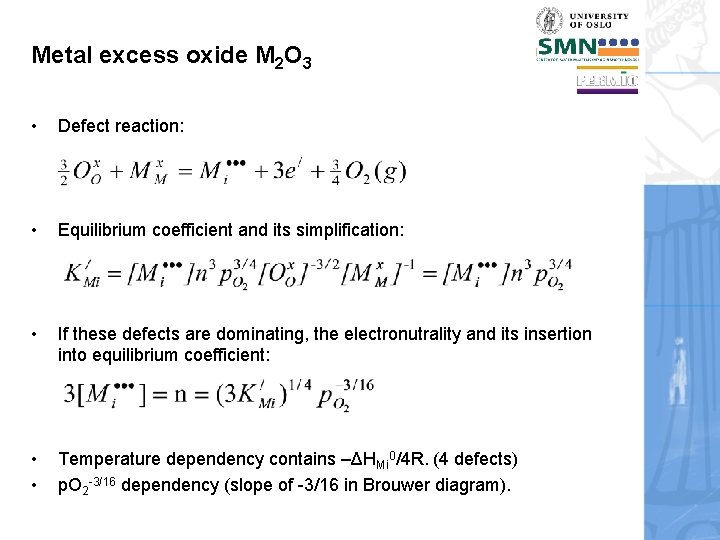
Metal excess oxide M 2 O 3 • Defect reaction: • Equilibrium coefficient and its simplification: • If these defects are dominating, the electronutrality and its insertion into equilibrium coefficient: • • Temperature dependency contains –ΔHMi 0/4 R. (4 defects) p. O 2 -3/16 dependency (slope of -3/16 in Brouwer diagram).

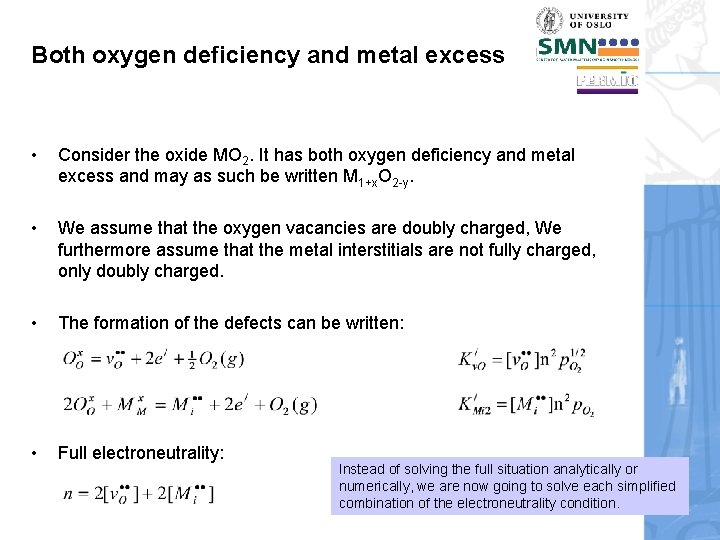
Both oxygen deficiency and metal excess Now we will take an example with two point defects, namely oxygen vacancies and metal interstitials, both compensating electrons. We will learn a way of determining the full Brouwer diagram for all defects. This is an important methodology, and this is where you learn it!

Both oxygen deficiency and metal excess • Consider the oxide MO 2. It has both oxygen deficiency and metal excess and may as such be written M 1+x. O 2 -y. • We assume that the oxygen vacancies are doubly charged, We furthermore assume that the metal interstitials are not fully charged, only doubly charged. • The formation of the defects can be written: • Full electroneutrality: Instead of solving the full situation analytically or numerically, we are now going to solve each simplified combination of the electroneutrality condition.

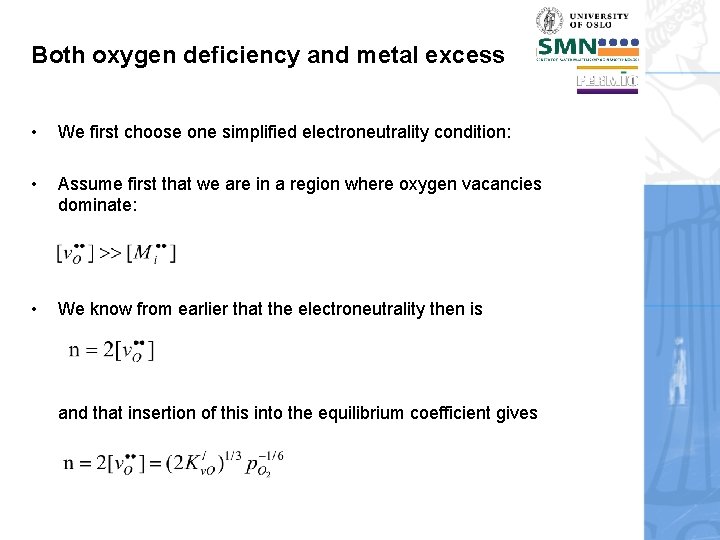
Both oxygen deficiency and metal excess • We first choose one simplified electroneutrality condition: • Assume first that we are in a region where oxygen vacancies dominate: • We know from earlier that the electroneutrality then is and that insertion of this into the equilibrium coefficient gives

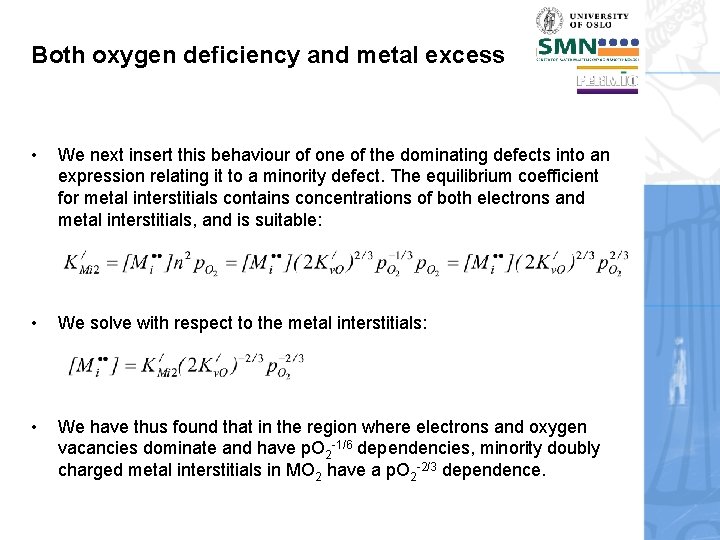
Both oxygen deficiency and metal excess • We next insert this behaviour of one of the dominating defects into an expression relating it to a minority defect. The equilibrium coefficient for metal interstitials contains concentrations of both electrons and metal interstitials, and is suitable: • We solve with respect to the metal interstitials: • We have thus found that in the region where electrons and oxygen vacancies dominate and have p. O 2 -1/6 dependencies, minority doubly charged metal interstitials in MO 2 have a p. O 2 -2/3 dependence.

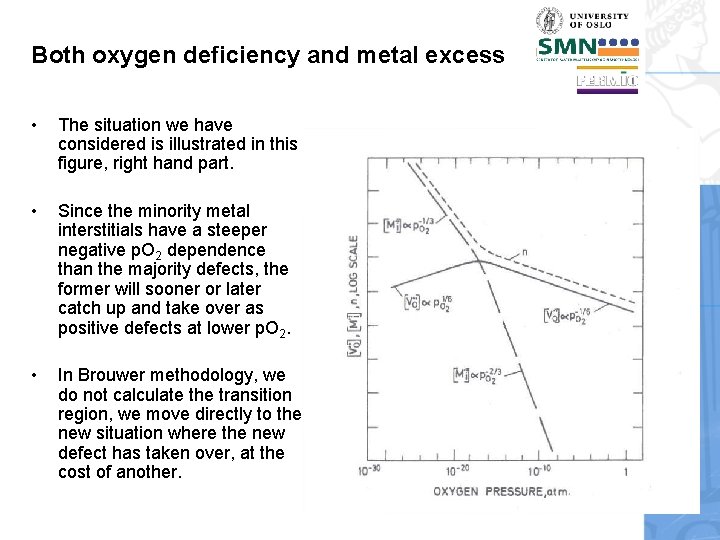
Both oxygen deficiency and metal excess • The situation we have considered is illustrated in this figure, right hand part. • Since the minority metal interstitials have a steeper negative p. O 2 dependence than the majority defects, the former will sooner or later catch up and take over as positive defects at lower p. O 2. • In Brouwer methodology, we do not calculate the transition region, we move directly to the new situation where the new defect has taken over, at the cost of another.

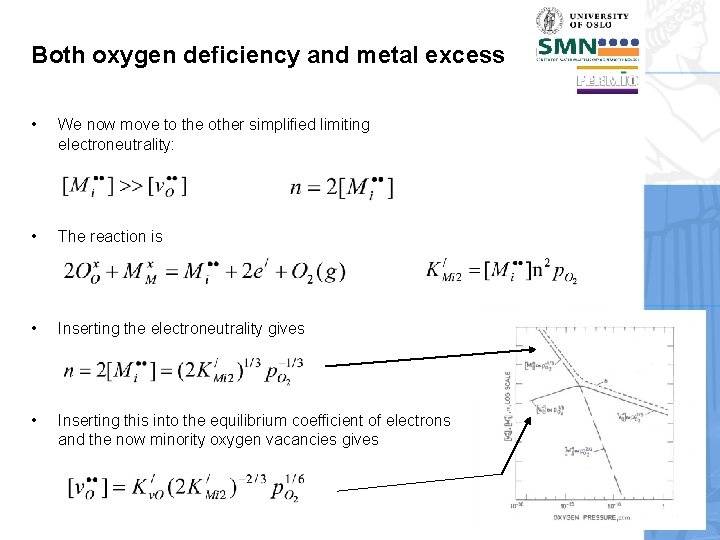
Both oxygen deficiency and metal excess • We now move to the other simplified limiting electroneutrality: • The reaction is • Inserting the electroneutrality gives • Inserting this into the equilibrium coefficient of electrons and the now minority oxygen vacancies gives

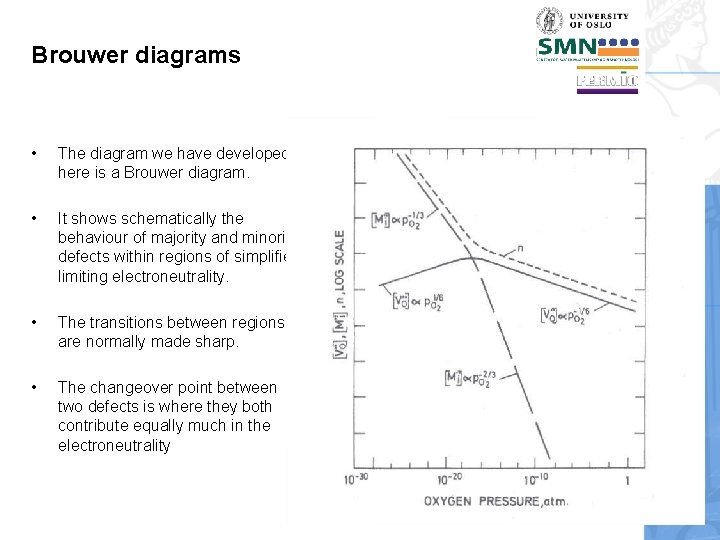
Brouwer diagrams • The diagram we have developed here is a Brouwer diagram. • It shows schematically the behaviour of majority and minority defects within regions of simplified limiting electroneutrality. • The transitions between regions are normally made sharp. • The changeover point between two defects is where they both contribute equally much in the electroneutrality

Rules for the methodology we have used 1. Write down the full electroneutrality of all defects you intend to consider. 2. Write a number of chemical reactions and equilibrium coefficients that relate the defects to each other. If there are n defects there must be n-1 independent reactions (meaning that no reaction must be a sum of other reactions). The nth reaction is the electroneutrality itself. 3. Choose one simplest possible set (normally a pair) of defects and formulate the simplified limiting electroneutrality assuming these are dominating. 4. Insert this into an equilibrium coefficient that contains these defects, and solve with respect to the dominating defect concentrations. 5. Insert the result from 4 into another equilibrium to determine the behaviour of a minority defect. If there are more minority defects, continue to insert and determine the behaviour of them all. 6. Extrapolate along e. g. p. O 2 to find which minority defect that takes over. Based on this, go back to Step 3, formulate a new simplified electroneutrality condition. Repeat this cycle in both directions of e. g. p. O 2 to explore all possible combinations of defects.

Metal deficiency and oxygen excess For these cases, see the compendium text.

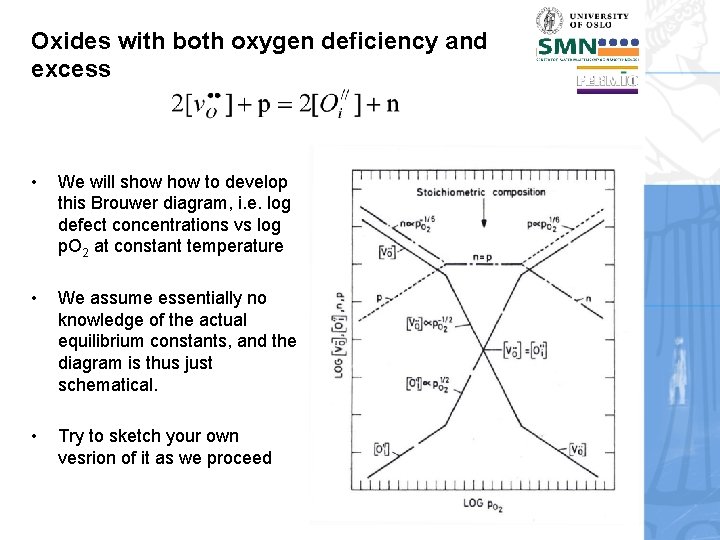
Oxides with both oxygen deficiency and excess The oxide we will use as example has oxygen vacancies and electrons at low p. O 2, and oxygen interstitials and holes at high p. O 2. At intermediate p. O 2 a stoichiometric defect disorder must be dominating. We will derive Brouwer diagrams quantitatively to understand the defect structure.

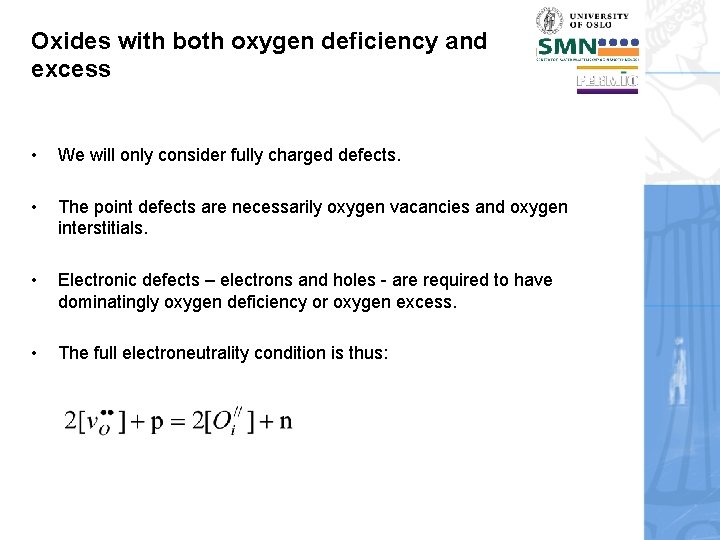
Oxides with both oxygen deficiency and excess • We will only consider fully charged defects. • The point defects are necessarily oxygen vacancies and oxygen interstitials. • Electronic defects – electrons and holes - are required to have dominatingly oxygen deficiency or oxygen excess. • The full electroneutrality condition is thus:

Oxides with both oxygen deficiency and excess • We will show to develop this Brouwer diagram, i. e. log defect concentrations vs log p. O 2 at constant temperature • We assume essentially no knowledge of the actual equilibrium constants, and the diagram is thus just schematical. • Try to sketch your own vesrion of it as we proceed

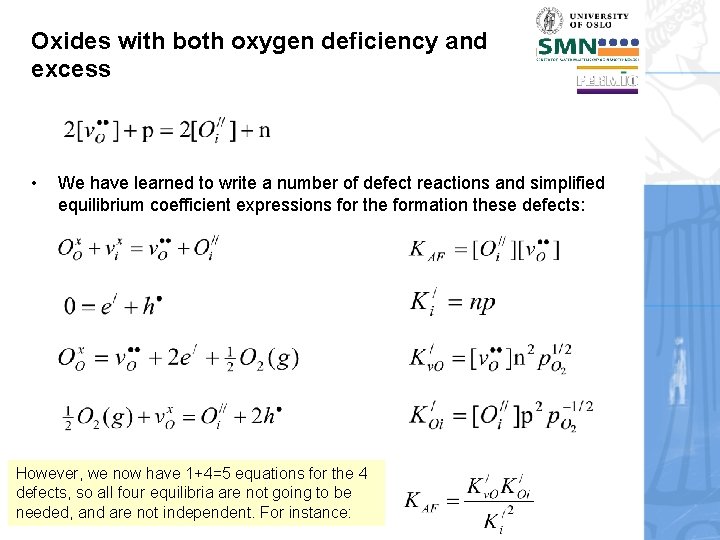
Oxides with both oxygen deficiency and excess • We have learned to write a number of defect reactions and simplified equilibrium coefficient expressions for the formation these defects: However, we now have 1+4=5 equations for the 4 defects, so all four equilibria are not going to be needed, and are not independent. For instance:

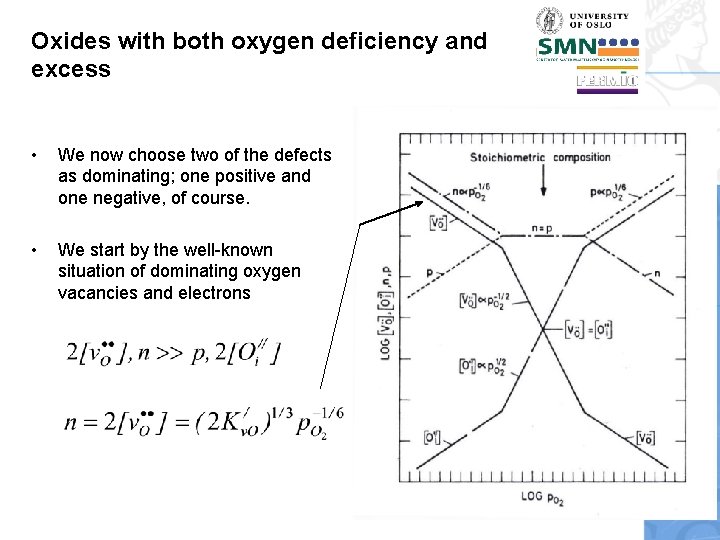
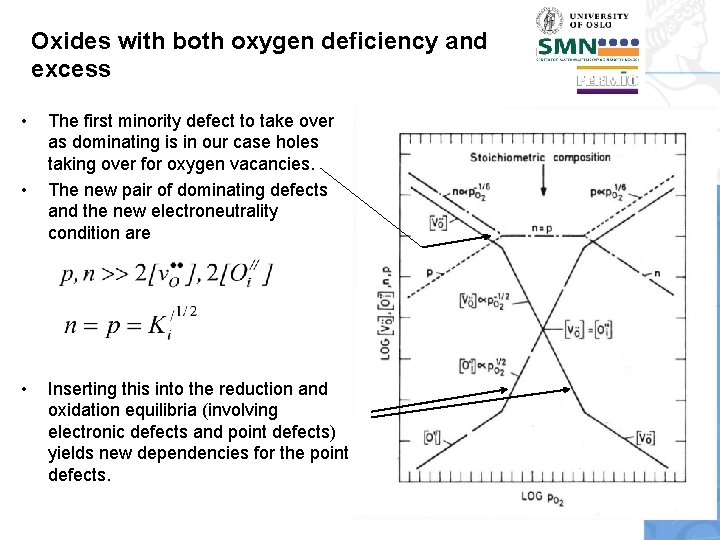
Oxides with both oxygen deficiency and excess • We now choose two of the defects as dominating; one positive and one negative, of course. • We start by the well-known situation of dominating oxygen vacancies and electrons

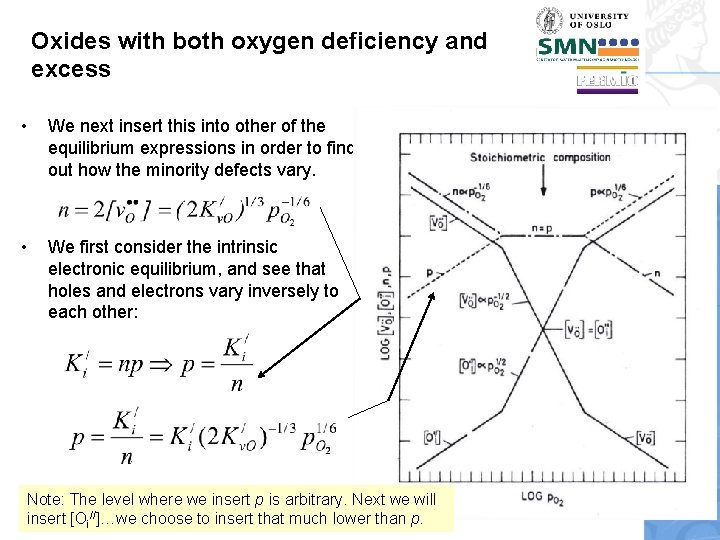
Oxides with both oxygen deficiency and excess • We next insert this into other of the equilibrium expressions in order to find out how the minority defects vary. • We first consider the intrinsic electronic equilibrium, and see that holes and electrons vary inversely to each other: Note: The level where we insert p is arbitrary. Next we will insert [Oi//]…we choose to insert that much lower than p.
![Oxides with both oxygen deficiency and excess Next we will solve for Oi Oxides with both oxygen deficiency and excess • Next we will solve for [Oi//].](https://slidetodoc.com/presentation_image_h2/cfbc6ea8ff18b01a4e989ac4b263add2/image-48.jpg)
Oxides with both oxygen deficiency and excess • Next we will solve for [Oi//]. For this we can use the anti-Frenkel equilibrium and insert [v. O. . ] from or we use the equilibrium for oxygen excess and insert p from • In any case we obtain [Oi//] p. O 21/6

Oxides with both oxygen deficiency and excess • • • The first minority defect to take over as dominating is in our case holes taking over for oxygen vacancies. The new pair of dominating defects and the new electroneutrality condition are Inserting this into the reduction and oxidation equilibria (involving electronic defects and point defects) yields new dependencies for the point defects.

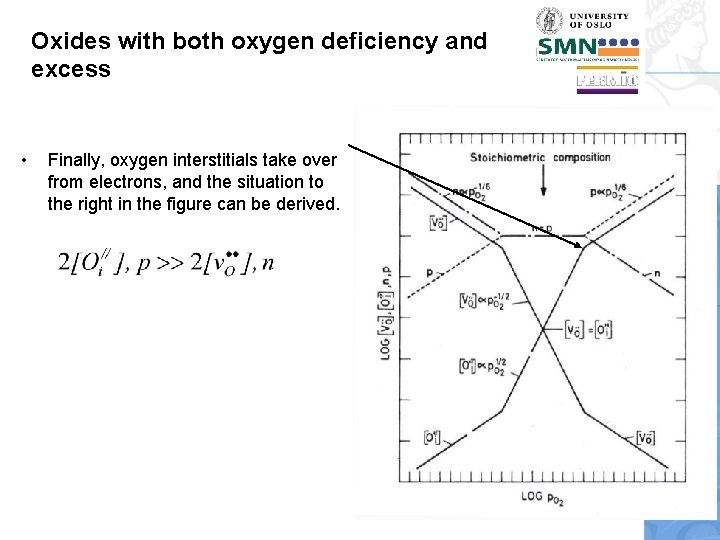
Oxides with both oxygen deficiency and excess • Finally, oxygen interstitials take over from electrons, and the situation to the right in the figure can be derived.

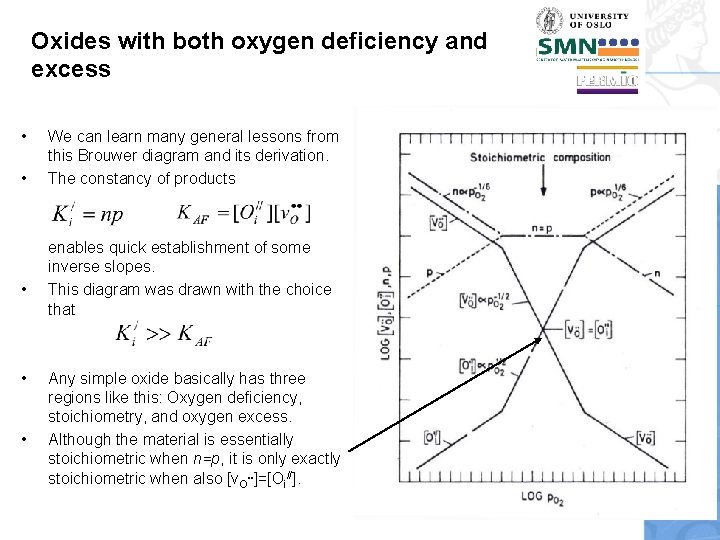
Oxides with both oxygen deficiency and excess • • • We can learn many general lessons from this Brouwer diagram and its derivation. The constancy of products enables quick establishment of some inverse slopes. This diagram was drawn with the choice that Any simple oxide basically has three regions like this: Oxygen deficiency, stoichiometry, and oxygen excess. Although the material is essentially stoichiometric when n=p, it is only exactly stoichiometric when also [v. O. . ]=[Oi//].

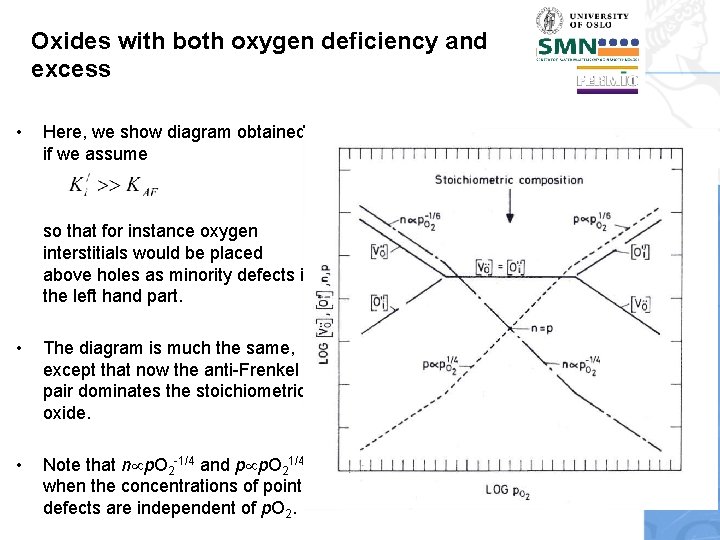
Oxides with both oxygen deficiency and excess • Here, we show diagram obtained if we assume so that for instance oxygen interstitials would be placed above holes as minority defects in the left hand part. • The diagram is much the same, except that now the anti-Frenkel pair dominates the stoichiometric oxide. • Note that n p. O 2 -1/4 and p p. O 21/4 when the concentrations of point defects are independent of p. O 2.

Concluding remarks • We have worked on the relations between ΔG, ΔS, ΔH, ΔG 0, ΔS 0, ΔH 0 and K for reactions. – You may have obtained variable understanding… • You have learnt to and must be able to write equilibrium coefficient expressions for a given reaction – Precise or with simplifications are both OK… • • • You have learnt to choose a simple electroneutrality and insert it into K to obtain expressions for the dominating defects. You have learnt typical ways to plot T- and p. O 2 -dependencies; van ‘t Hoff and Brouwer diagrams. You have learnt to insert the resulting expression for a majority defect into an equilibrium coefficient expression for a minority defect, in order to find how the minority defect behaves. You have learnt to plot the majority and minority defects in Brouwer diagrams that represent schematically the behaviour of all defects in regions of simplified limiting electroneutralities. By this, you have learnt the main quantitative tools in defect chemistry.
 Endoflagello
Endoflagello Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Reduction half reaction
Reduction half reaction Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Frenkel defect
Frenkel defect Unacceptable weld profile
Unacceptable weld profile Cuts and washes defects in casting
Cuts and washes defects in casting Difference between schottky defect and frenkel defect
Difference between schottky defect and frenkel defect Firmness of the yolk and freedom from yolk defects
Firmness of the yolk and freedom from yolk defects Execution defects
Execution defects Twinning tablet defects
Twinning tablet defects Secondary education commission is also known as
Secondary education commission is also known as Drop core
Drop core Chain sling defects
Chain sling defects Brown classification of maxillary defects
Brown classification of maxillary defects Ctc en ctq
Ctc en ctq Ppm
Ppm Planer defect
Planer defect Physical defects in personality development
Physical defects in personality development Furcation entrance
Furcation entrance Spina bifida
Spina bifida Cup shake wood
Cup shake wood Delamination injection molding
Delamination injection molding Types of concrete defects
Types of concrete defects Butter defects
Butter defects Point defects
Point defects Classification of casting defects
Classification of casting defects Birth defects causes
Birth defects causes Endocardial cushion defects
Endocardial cushion defects Rind galls in wood
Rind galls in wood Radial shakes
Radial shakes Thin film coating
Thin film coating Hassan fareed physics
Hassan fareed physics Defect amplification model
Defect amplification model Fault is manifestation of
Fault is manifestation of Slip defect
Slip defect Learning from defects
Learning from defects Surface defects examples
Surface defects examples Gravure cylinder defects
Gravure cylinder defects Apps.searo.who.int.login-database
Apps.searo.who.int.login-database Types of extrusion
Types of extrusion Core defects of diabetes
Core defects of diabetes Wire drawing defects
Wire drawing defects Crystal defects
Crystal defects Upset forging advantages and disadvantages
Upset forging advantages and disadvantages Neural tube defects
Neural tube defects Respiratory chain defects
Respiratory chain defects Spina bifida occulta images
Spina bifida occulta images Forging shape factor
Forging shape factor Neural tube defects
Neural tube defects What are the main defects in montessori method
What are the main defects in montessori method Defects in cream
Defects in cream