Kinetic Theory of Gases Chemistry Mrs Coyle Part




- Slides: 16

Kinetic Theory of Gases Chemistry Mrs. Coyle

Part I- Kinetic Molecular Theory and Pressure


Gas Phase

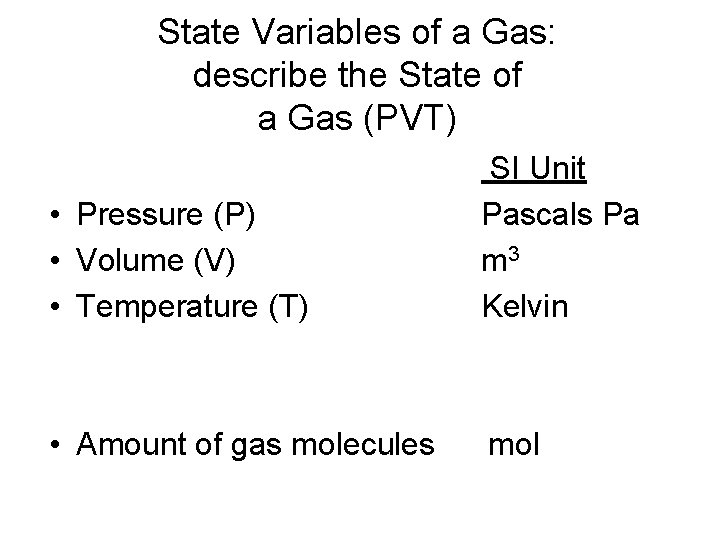
State Variables of a Gas: describe the State of a Gas (PVT) • Pressure (P) • Volume (V) • Temperature (T) SI Unit Pascals Pa m 3 Kelvin • Amount of gas molecules mol

Standard Temperature and Pressure (STP) • 1 atm =760 mm. Hg (torr) = 101. 3 k. Pa • 273 K =0 0 C

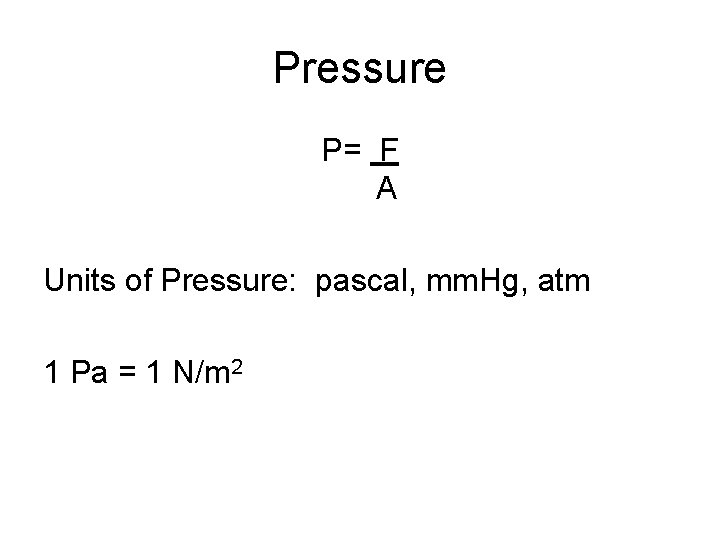
Pressure P= F A Units of Pressure: pascal, mm. Hg, atm 1 Pa = 1 N/m 2

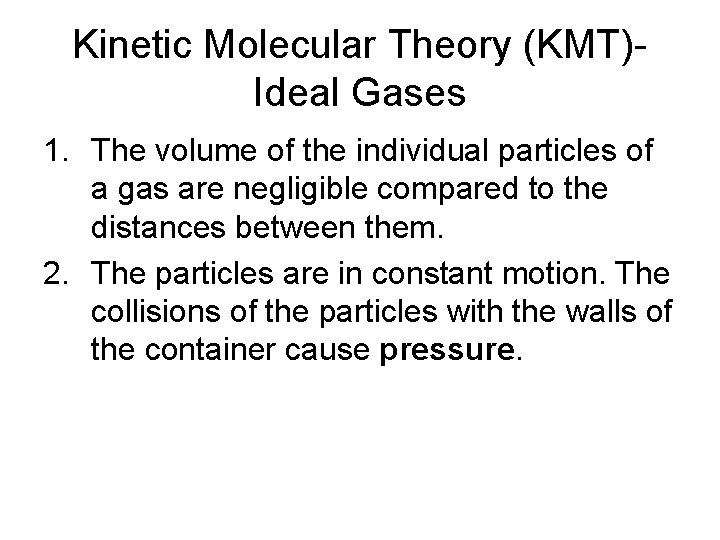
Kinetic Molecular Theory (KMT)Ideal Gases 1. The volume of the individual particles of a gas are negligible compared to the distances between them. 2. The particles are in constant motion. The collisions of the particles with the walls of the container cause pressure.

KMT Continued 3. The particles are assumed not to exert attracting or repelling forces on each other. The collisions are perfectly elastic. No kinetic energy is lost before and after the collision. 4. The average kinetic energy of a gas is directly proportional to the Kelvin temperature.

Simulation • http: //www 2. biglobe. ne. jp/~norimari/scienc e/Java. App/Mole/e-gas. html

Part II • Temperature and KE • Measuring Pressure: Barometer, Manometer

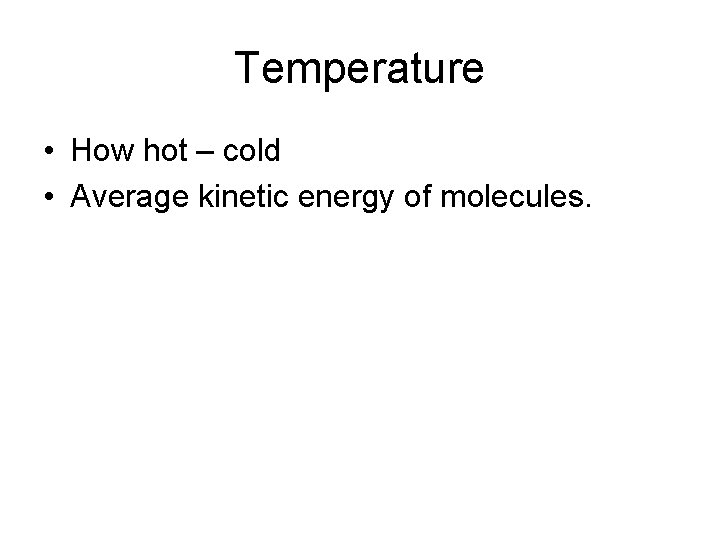
Temperature • How hot – cold • Average kinetic energy of molecules.

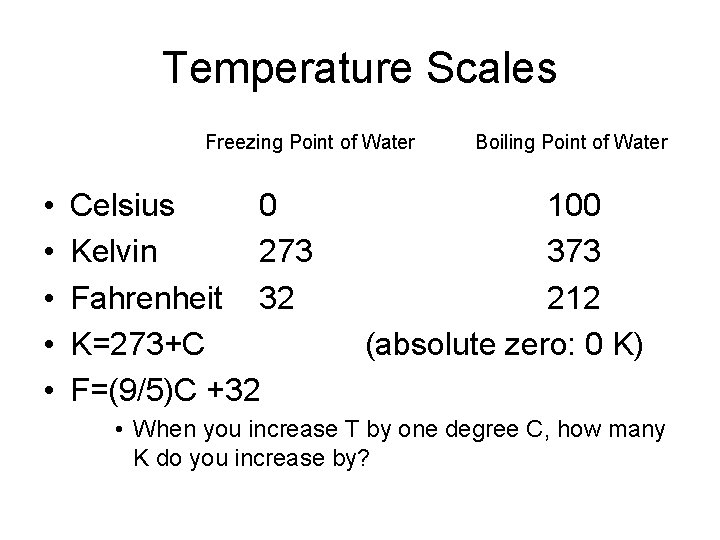
Temperature Scales Freezing Point of Water • • • Celsius 0 Kelvin 273 Fahrenheit 32 K=273+C F=(9/5)C +32 Boiling Point of Water 100 373 212 (absolute zero: 0 K) • When you increase T by one degree C, how many K do you increase by?

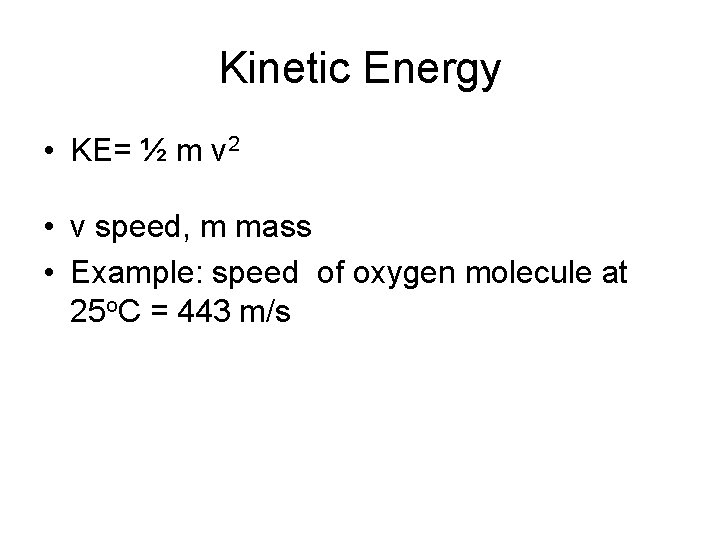
Kinetic Energy • KE= ½ m v 2 • v speed, m mass • Example: speed of oxygen molecule at 25 o. C = 443 m/s

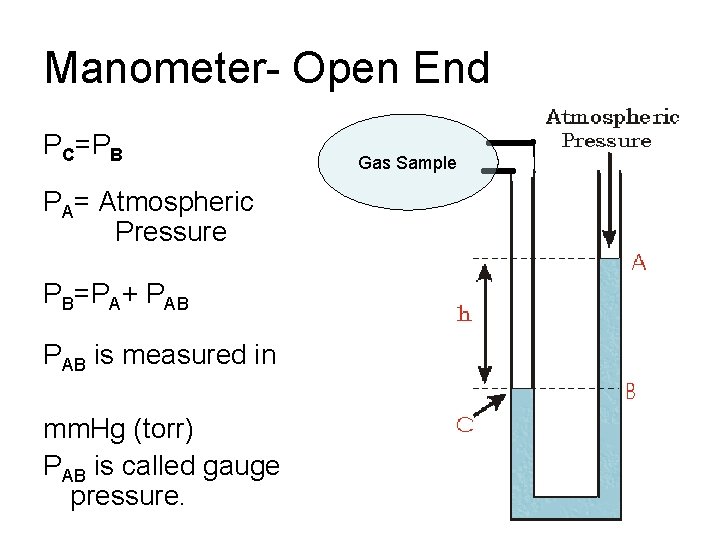
Manometer- Open End PC=PB PA= Atmospheric Pressure PB=PA+ PAB is measured in mm. Hg (torr) PAB is called gauge pressure. Gas Sample

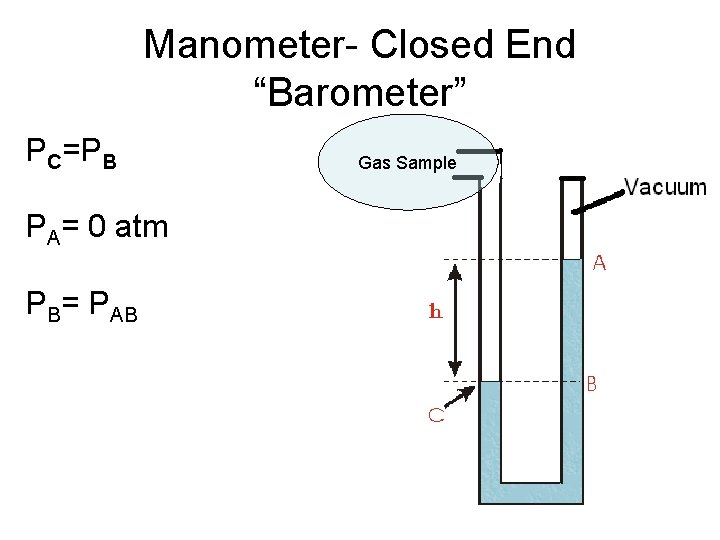
Manometer- Closed End “Barometer” PC=PB PA= 0 atm PB= PAB Gas Sample
 Kinetic theory for ideal gases
Kinetic theory for ideal gases Kenetic particle theory
Kenetic particle theory Kinetic theory of gases
Kinetic theory of gases Basic postulates of kinetic theory of gases
Basic postulates of kinetic theory of gases Kinetic theory
Kinetic theory Kinetic molecular theory
Kinetic molecular theory Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases General gas equation is
General gas equation is They are mrs garcia and mrs castro
They are mrs garcia and mrs castro They are mrs garcia and mrs castro
They are mrs garcia and mrs castro Mrs. darling was ___________ of mrs. s.
Mrs. darling was ___________ of mrs. s. Law of combining volumes
Law of combining volumes Coyle view
Coyle view Coyle method
Coyle method Coyle method
Coyle method Caitlin coyle
Caitlin coyle Do coyle
Do coyle