KF Coulometry Volumetric Coulometric Titration Volumetric Karl Fischer






- Slides: 25

KF Coulometry

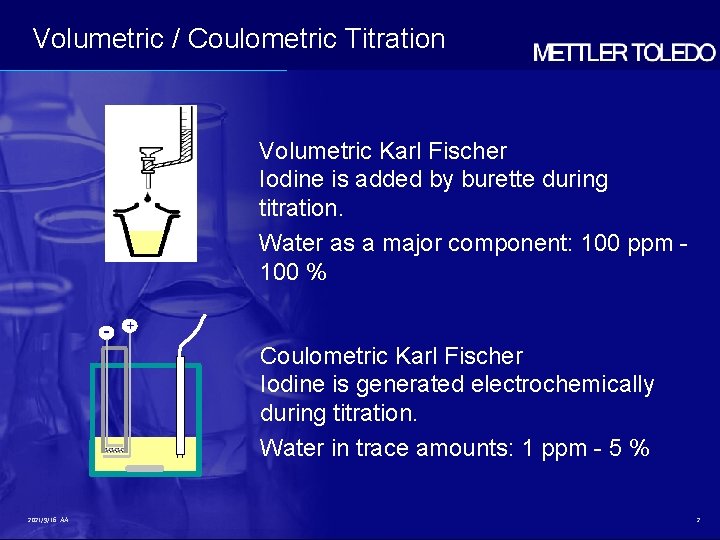
Volumetric / Coulometric Titration Volumetric Karl Fischer Iodine is added by burette during titration. Water as a major component: 100 ppm 100 % - + Coulometric Karl Fischer Iodine is generated electrochemically during titration. Water in trace amounts: 1 ppm - 5 % 2021/9/16 AA 2

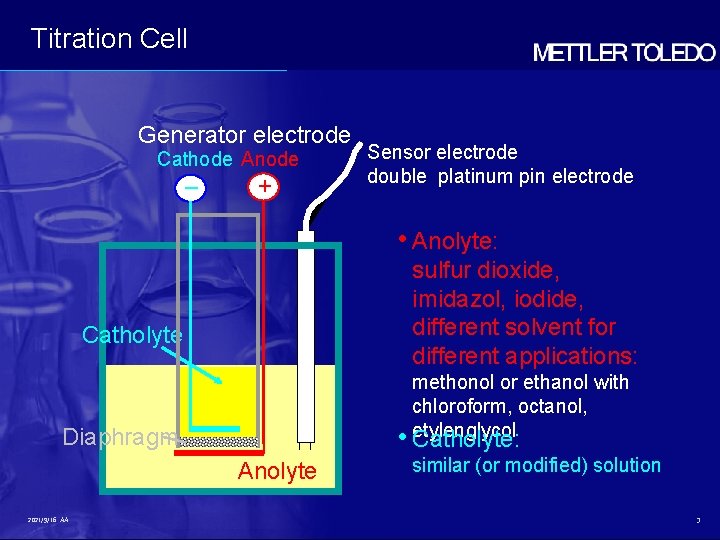
Titration Cell Generator electrode Cathode Anode – + Sensor electrode double platinum pin electrode • Anolyte: sulfur dioxide, imidazol, iodide, different solvent for different applications: Catholyte methonol or ethanol with chloroform, octanol, • etylenglycol Catholyte: Diaphragm Anolyte 2021/9/16 AA similar (or modified) solution 3

Coulometry • Based on the same reaction as volumetric Karl Fischer Titration: H 2 O + 2 I + SO 2 + RN + ROHÙ (RNH)SO 4 R + 2(RNH)I - Iodine reacts with water 1: 1 - The solvent methanol is involved in the reaction. - A suitable base keeps the p. H 5 - 7 • But iodine will be produced just in time from iodide: 2 I Anodic Oxidation I 2 + 2 e- 2021/9/16 AA 4

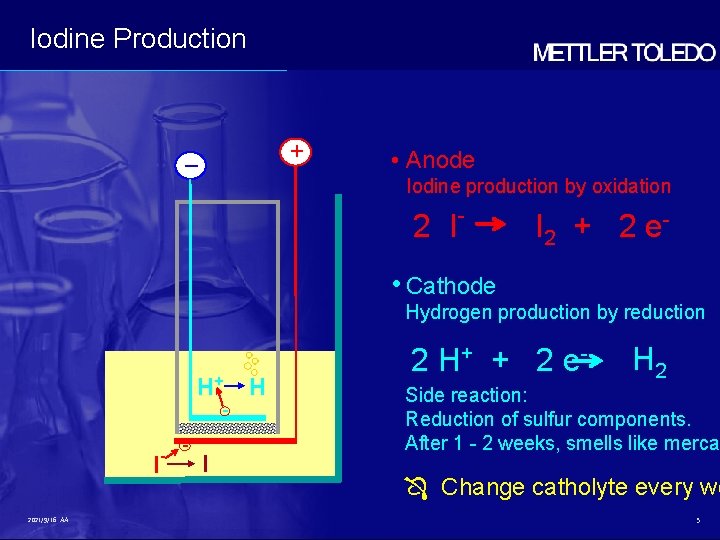
Iodine Production + – • Anode Iodine production by oxidation 2 I - I 2 + 2 e - • Cathode Hydrogen production by reduction H+ - I 2021/9/16 AA - - I H 2 H+ + 2 e - H 2 Side reaction: Reduction of sulfur components. After 1 - 2 weeks, smells like mercap Change catholyte every we 5

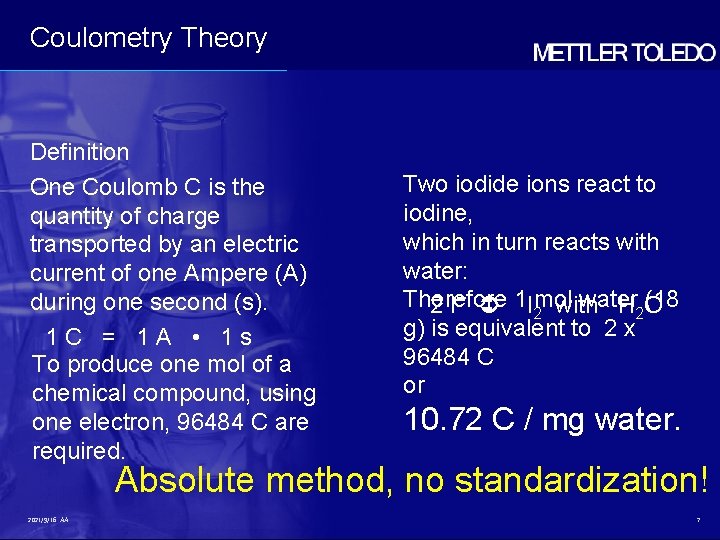
Coulometry Theory Definition One Coulomb C is the quantity of charge transported by an electric current of one Ampere (A) during one second (s). 1 C = 1 A • 1 s To produce one mol of a chemical compound, using one electron, 96484 C are required. 2021/9/16 AA Charles Augustin de Coulomb 14. 6. 1736 - 23. 8. 1806 6

Coulometry Theory Definition One Coulomb C is the quantity of charge transported by an electric current of one Ampere (A) during one second (s). 1 C = 1 A • 1 s To produce one mol of a chemical compound, using one electron, 96484 C are required. Two iodide ions react to iodine, which in turn reacts with water: Therefore water (18 2 I– 1 I 2 mol with H 2 O g) is equivalent to 2 x 96484 C or 10. 72 C / mg water. Absolute method, no standardization! 2021/9/16 AA 7

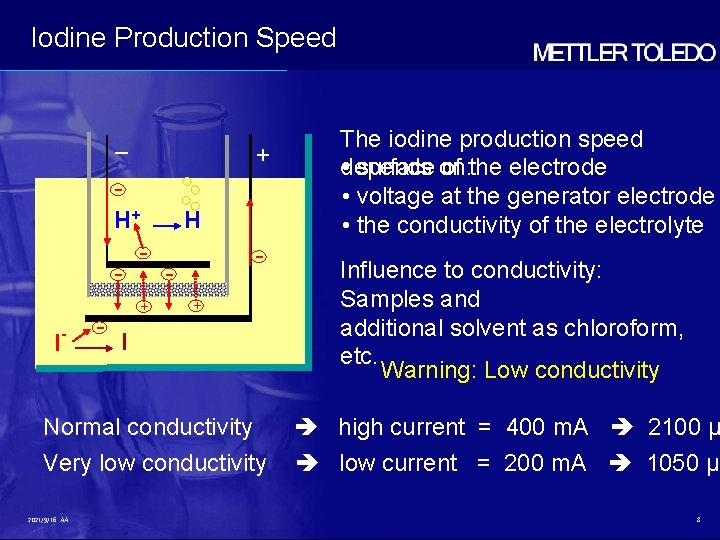
Iodine Production Speed – + H+ H I - - + I Normal conductivity Very low conductivity 2021/9/16 AA The iodine production speed • surface on: of the electrode depends • voltage at the generator electrode • the conductivity of the electrolyte Influence to conductivity: Samples and additional solvent as chloroform, etc. Warning: Low conductivity high current = 400 m. A 2100 µ low current = 200 m. A 1050 µg 8

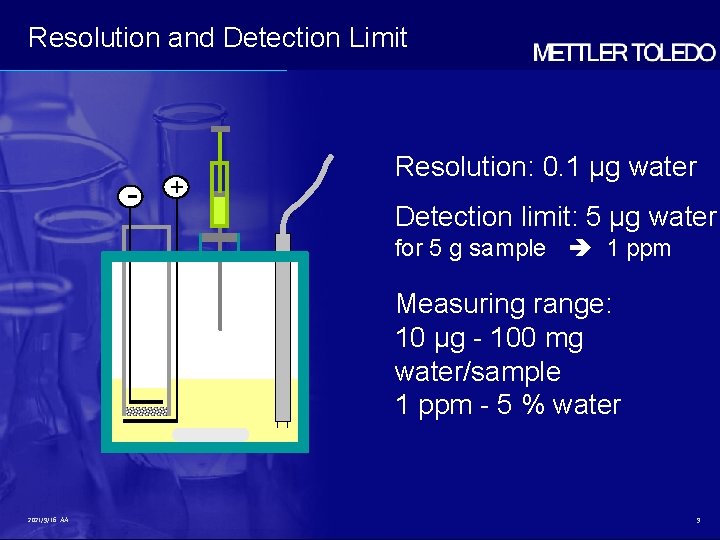
Resolution and Detection Limit - + Resolution: 0. 1 µg water Detection limit: 5 µg water for 5 g sample 1 ppm Measuring range: 10 µg - 100 mg water/sample 1 ppm - 5 % water 2021/9/16 AA 9

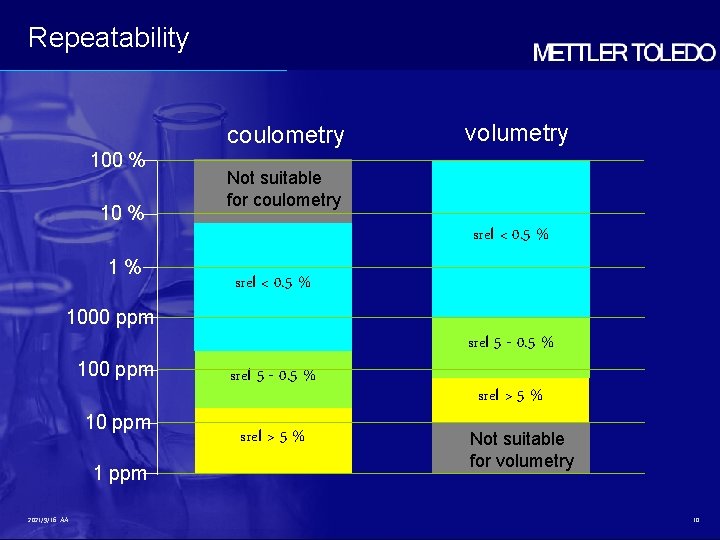
Repeatability coulometry 100 % 10 % volumetry Not suitable for coulometry srel < 0. 5 % 1% srel < 0. 5 % 1000 ppm 10 ppm 1 ppm 2021/9/16 AA srel 5 - 0. 5 % srel > 5 % Not suitable for volumetry 10

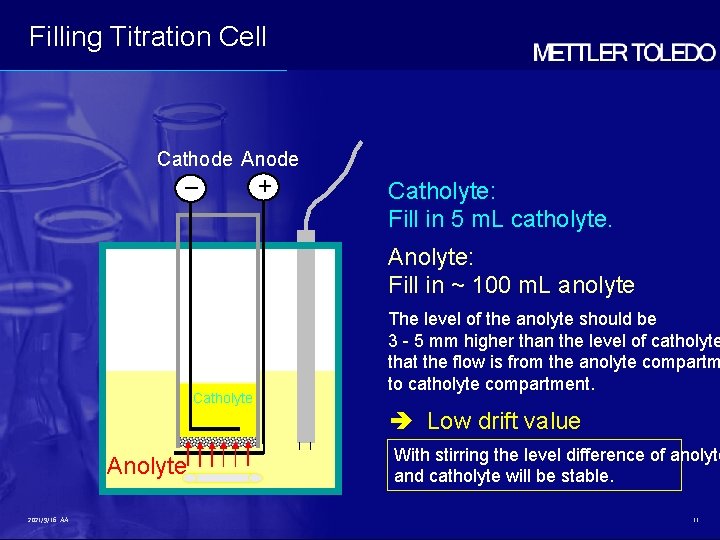
Filling Titration Cell Cathode Anode – + Catholyte: Fill in 5 m. L catholyte. Anolyte: Fill in ~ 100 m. L anolyte Catholyte The level of the anolyte should be 3 - 5 mm higher than the level of catholyte that the flow is from the anolyte compartm to catholyte compartment. Low drift value Anolyte 2021/9/16 AA With stirring the level difference of anolyte and catholyte will be stable. 11

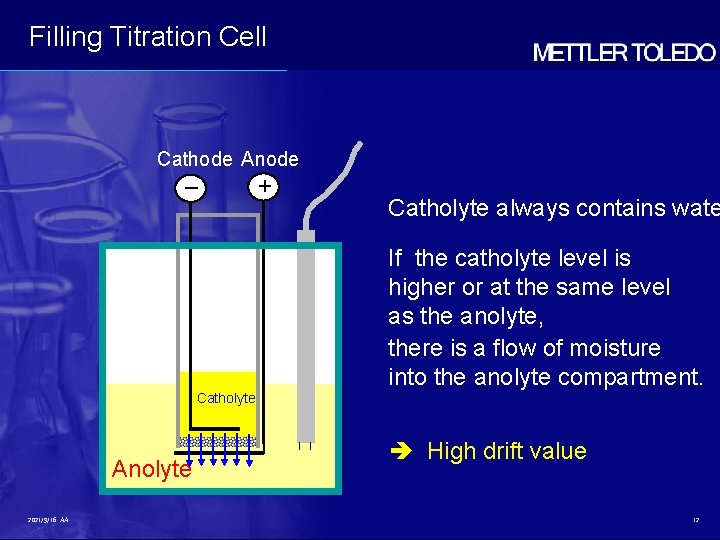
Filling Titration Cell Cathode Anode – + Catholyte always contains wate If the catholyte level is higher or at the same level as the anolyte, there is a flow of moisture into the anolyte compartment. Catholyte Anolyte 2021/9/16 AA High drift value 12

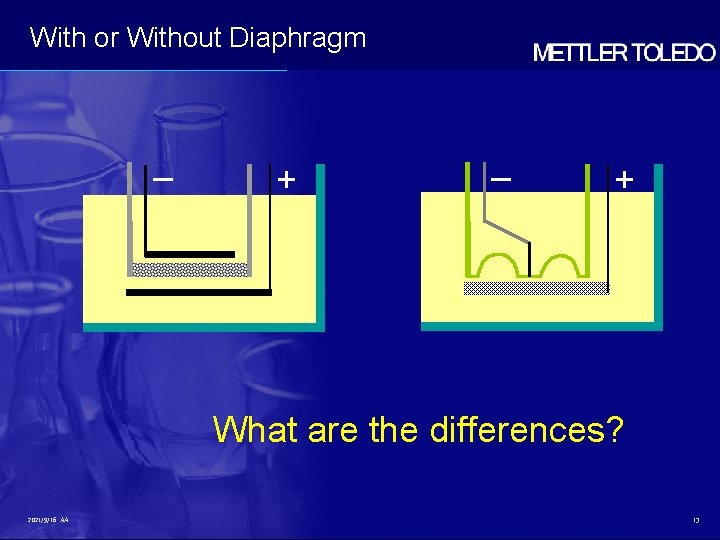
With or Without Diaphragm – + What are the differences? 2021/9/16 AA 13

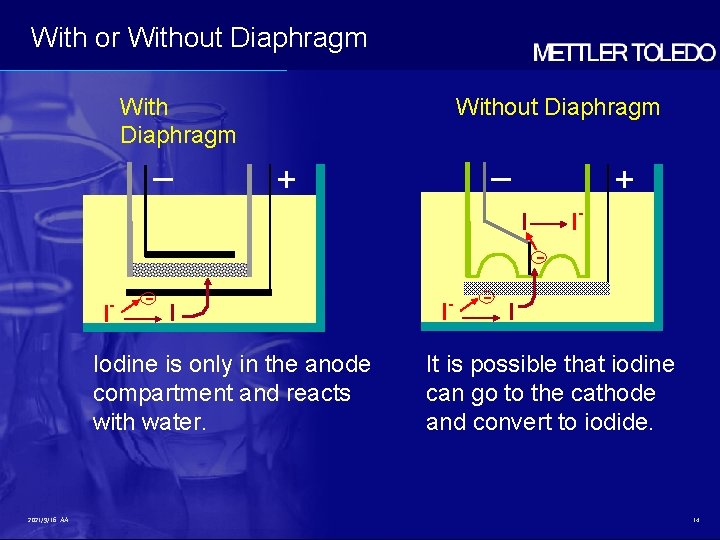
With or Without Diaphragm With Diaphragm – Without Diaphragm – + + I- I I - - I Iodine is only in the anode compartment and reacts with water. 2021/9/16 AA I - - I It is possible that iodine can go to the cathode and convert to iodide. 14

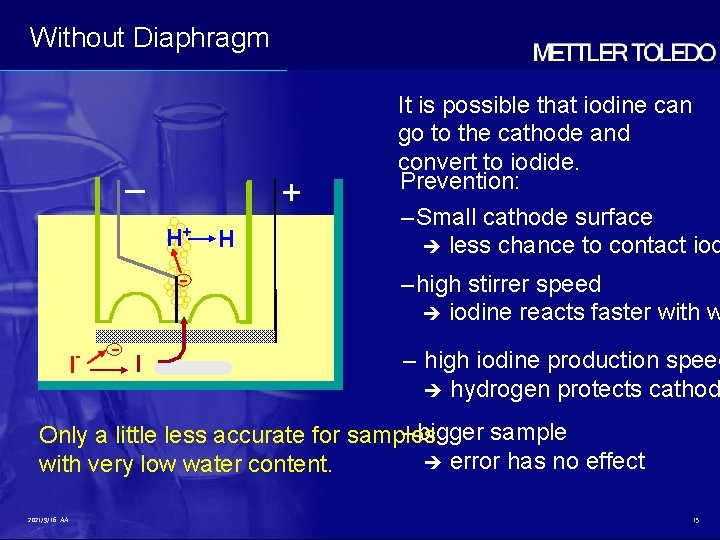
Without Diaphragm – + H+ - I - - I H It is possible that iodine can go to the cathode and convert to iodide. Prevention: – Small cathode surface less chance to contact iod – high stirrer speed iodine reacts faster with w – high iodine production speed hydrogen protects cathod – bigger sample Only a little less accurate for samples error has no effect with very low water content. 2021/9/16 AA 15

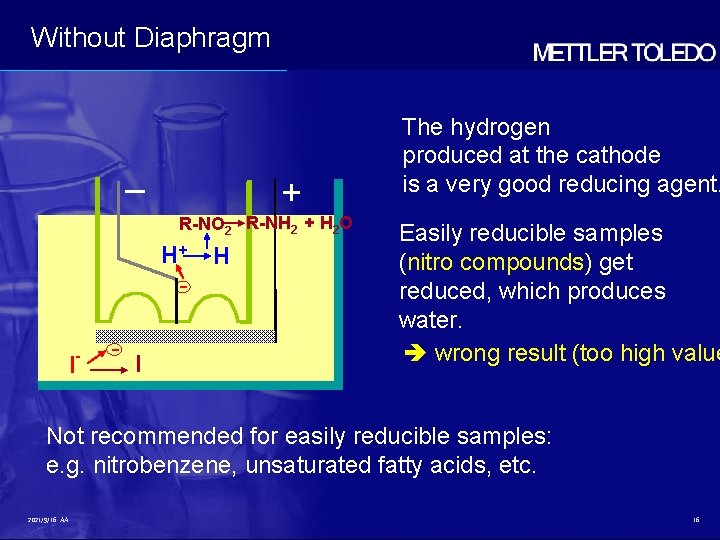
Without Diaphragm – + R-NO 2 R-NH 2 + H 2 O H+ I - - I H The hydrogen produced at the cathode is a very good reducing agent. Easily reducible samples (nitro compounds) get reduced, which produces water. wrong result (too high value Not recommended for easily reducible samples: e. g. nitrobenzene, unsaturated fatty acids, etc. 2021/9/16 AA 16

Without Diaphragm + + Titration cell easier to clean. Long-term drift value more stable. Only one reagent. Automation of emptying and refilling electrolyte. – A little bit less accuracy for very small water content (< 50 µg/sample) – Not recommended: • for easily reducible samples: nitro compounds, unsaturated fatty acids, etc. 2021/9/16 AA 17

Application With and Without Diaphragm With out diaphragm: a little bit less accuracy for very small water content (< 50 µg/sample) Examples: Transformer oil Mean n diaphragm srel 16. 3 ppm 6 19. 6 ppm 6 1. 5 % 5. 7 % 2021/9/16 AA µg water /sample with or without 34 - 40 39 - 43 with diaphragm without diaphragm 18

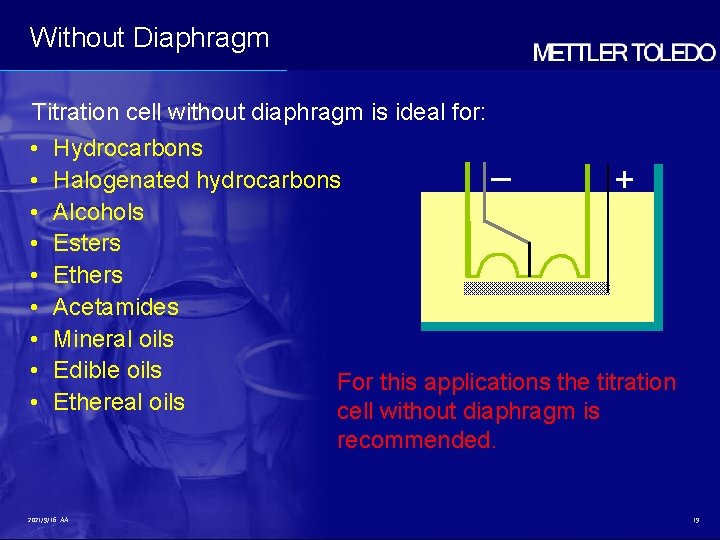
Without Diaphragm Titration cell without diaphragm is ideal for: • • • Hydrocarbons Halogenated hydrocarbons – + Alcohols Esters Ethers Acetamides Mineral oils Edible oils For this applications the titration Ethereal oils cell without diaphragm is recommended. 2021/9/16 AA 19

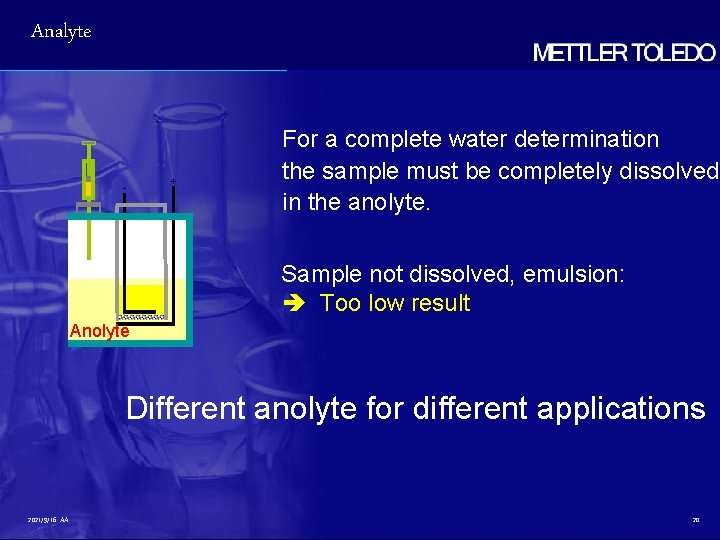
Analyte - + For a complete water determination the sample must be completely dissolved in the anolyte. Sample not dissolved, emulsion: Too low result Anolyte Different anolyte for different applications 2021/9/16 AA 20

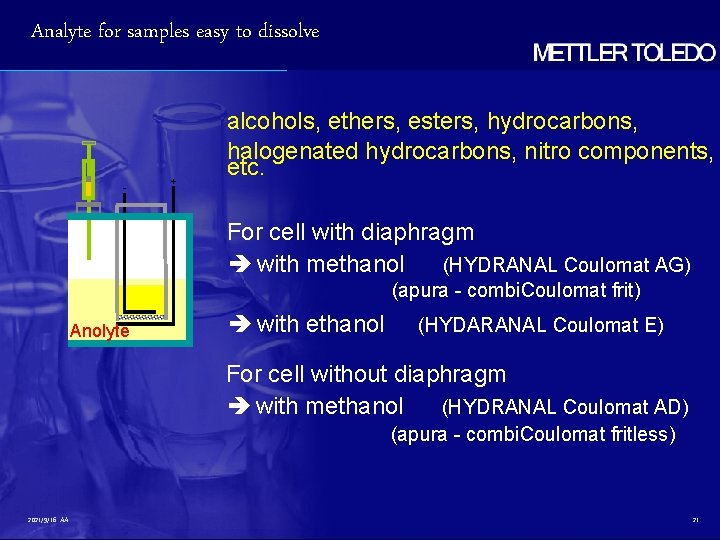
Analyte for samples easy to dissolve - + alcohols, ethers, esters, hydrocarbons, halogenated hydrocarbons, nitro components, etc. For cell with diaphragm with methanol (HYDRANAL Coulomat AG) (apura - combi. Coulomat frit) Anolyte with ethanol (HYDARANAL Coulomat E) For cell without diaphragm with methanol (HYDRANAL Coulomat AD) (apura - combi. Coulomat fritless) 2021/9/16 AA 21

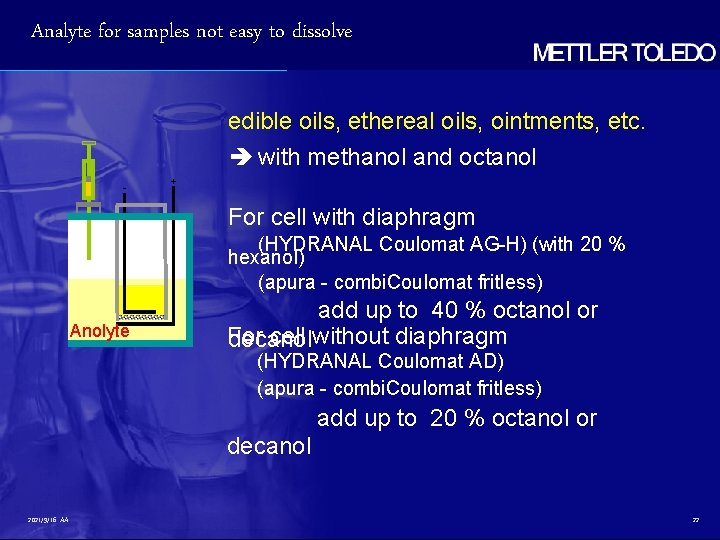
Analyte for samples not easy to dissolve edible oils, ethereal oils, ointments, etc. with methanol and octanol - + For cell with diaphragm (HYDRANAL Coulomat AG-H) (with 20 % hexanol) (apura - combi. Coulomat fritless) Anolyte add up to 40 % octanol or For cell without diaphragm decanol (HYDRANAL Coulomat AD) (apura - combi. Coulomat fritless) add up to 20 % octanol or decanol 2021/9/16 AA 22

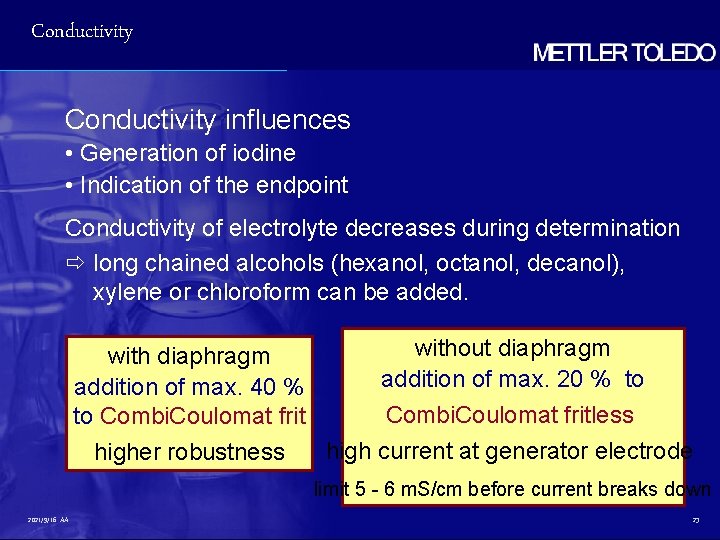
Conductivity influences • Generation of iodine • Indication of the endpoint Conductivity of electrolyte decreases during determination ð long chained alcohols (hexanol, octanol, decanol), xylene or chloroform can be added. without diaphragm with diaphragm addition of max. 20 % to addition of max. 40 % Combi. Coulomat fritless to Combi. Coulomat frit high current at generator electrode higher robustness limit 5 - 6 m. S/cm before current breaks down 2021/9/16 AA 23

Analyte for samples difficult to dissolve mineral oils, transformer oil, silicon oils, etc with methanol and chloroform - + For cell with diaphragm (HYDRANAL Coulomat A) (with 20 % chloroform) (HYDRANAL Coulomat AG) (without chloroform) (apura - combi. Coulomat frit) (without chloroform) Anolyte add chloroform (maximum 50 %) For cell without diaphragm (HYDRANAL Coulomat AD) (apura - combi. Coulomat fritless) add up to 30 % chloroform 2021/9/16 AA 24

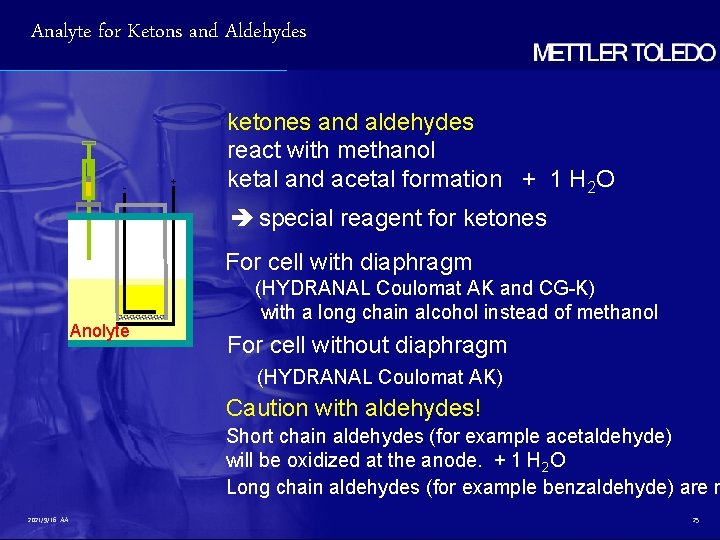
Analyte for Ketons and Aldehydes - + ketones and aldehydes react with methanol ketal and acetal formation + 1 H 2 O special reagent for ketones For cell with diaphragm Anolyte (HYDRANAL Coulomat AK and CG-K) with a long chain alcohol instead of methanol For cell without diaphragm (HYDRANAL Coulomat AK) Caution with aldehydes! Short chain aldehydes (for example acetaldehyde) will be oxidized at the anode. + 1 H 2 O Long chain aldehydes (for example benzaldehyde) are n 2021/9/16 AA 25
 Titrant vs titrate
Titrant vs titrate Karl fischer titration formula
Karl fischer titration formula Advantages and disadvantages of coulometric methods
Advantages and disadvantages of coulometric methods Coulometric method of analysis
Coulometric method of analysis Primary standard solution example
Primary standard solution example Define redox titration
Define redox titration Back titrations
Back titrations Precipitation titration questions
Precipitation titration questions Redox titration
Redox titration Overpotential
Overpotential Electrogravimetry and coulometry
Electrogravimetry and coulometry Electrogravimetry diagram
Electrogravimetry diagram Amperostatic coulometry
Amperostatic coulometry Volumetric map
Volumetric map Volumetric
Volumetric Osmometric thirst
Osmometric thirst Volumetric efficiency
Volumetric efficiency Volumetric analysis equipment
Volumetric analysis equipment Formula sae
Formula sae Pipette with frosted bands
Pipette with frosted bands Volumetric glassware and routine glassware
Volumetric glassware and routine glassware Volumetric billboards
Volumetric billboards Volumetric
Volumetric Redox volumetric analysis
Redox volumetric analysis Difference between gravimetric and volumetric analysis
Difference between gravimetric and volumetric analysis Teen power pmv
Teen power pmv