ISolubility Product AcidBase Volumetric Analysis Dr Prem D

I-Solubility Product: Acid-Base Volumetric Analysis Dr. Prem D. Sattsangi Copyright © 2009
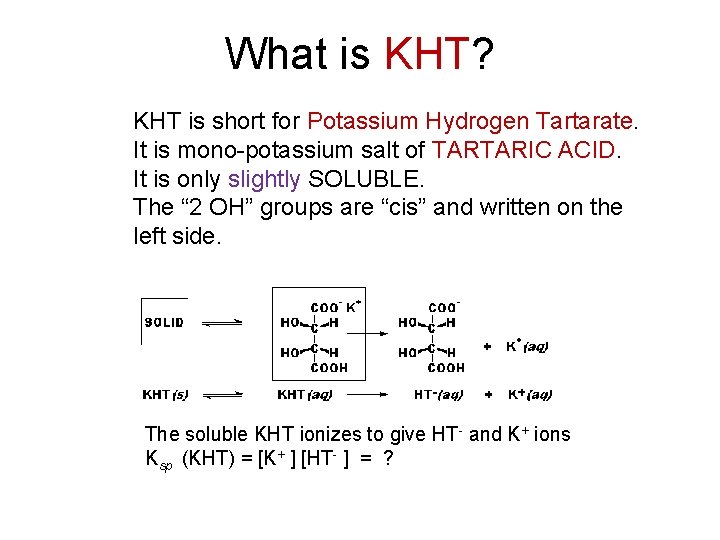
What is KHT? KHT is short for Potassium Hydrogen Tartarate. It is mono-potassium salt of TARTARIC ACID. It is only slightly SOLUBLE. The “ 2 OH” groups are “cis” and written on the left side. The soluble KHT ionizes to give HT- and K+ ions Ksp (KHT) = [K+ ] [HT- ] = ?
#1 -A Solubility Product Experiment Record Room temperature at the top of each experiment 1. Solubility of KHT in presence of a common ion, [K+] 2. Solubility of KHT in distilled water 3. Determination of an Unknown Potassium concentration
![#2 Section-1. Solubility of KHT in presence of a common ion, [K+] Each student #2 Section-1. Solubility of KHT in presence of a common ion, [K+] Each student](http://slidetodoc.com/presentation_image/0239576ca58c130def34c8ef726bb09c/image-4.jpg)
#2 Section-1. Solubility of KHT in presence of a common ion, [K+] Each student will be assigned a concentration of KNO 3 from 0. 040 M, 0. 060, 0. 080 M …. to 0. 160 M. PROCEDURE: 1. Calculate mass of KNO 3 needed to prepare 100 m. L of 0. 190 M solution. [n = M x V; Atomic masses (K = 39. 09, N = 14. 01, O = 16. 00)]. (= 1. 92 g) FW KNO 3 = 101. 1 1. 0. 190 mol x 0. 100 L x 101. 1 g = 1. 92 g 1 L 1 mol H. it I.
![#2 b-Preparing solution Weigh on the table top balance. [+/- 0. 03 g]. Add #2 b-Preparing solution Weigh on the table top balance. [+/- 0. 03 g]. Add](http://slidetodoc.com/presentation_image/0239576ca58c130def34c8ef726bb09c/image-5.jpg)
#2 b-Preparing solution Weigh on the table top balance. [+/- 0. 03 g]. Add it to the 100 m. L Volumetric flask and make it up to the mark using STOCK-KHT saturated solution. POUR SLOWLY through Funnel. AVOID OVER FILLING Stopper securely. Shake by inversion rotation occasionally. Allow time to equilibrate Proceed with Section 2 of the experiment.

#3 Section-2. Solubility of KHT in distilled water, Filtration of saturated KHT PROCEDURE: Set up a filtration apparatus. Moisten the filter paper with Saturated KHT solution. Place a 150/250 m. L beaker underneath the funnel. Do not fill the filter paper to the top. Leave ~ 1 cm empty. Allow to filter. As filtration proceeds, add more solution. Filter all of it.
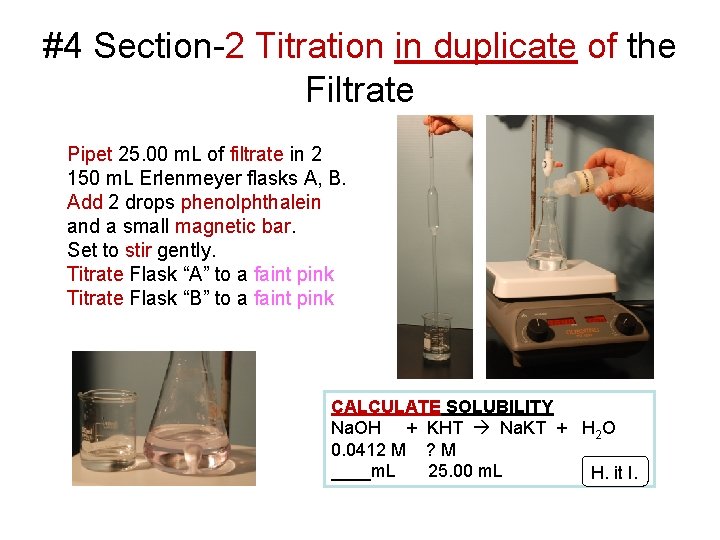
#4 Section-2 Titration in duplicate of the Filtrate Pipet 25. 00 m. L of filtrate in 2 150 m. L Erlenmeyer flasks A, B. Add 2 drops phenolphthalein and a small magnetic bar. Set to stir gently. Titrate Flask “A” to a faint pink Titrate Flask “B” to a faint pink CALCULATE SOLUBILITY Na. OH + KHT Na. KT + H 2 O 0. 0412 M ? M ____m. L 25. 00 m. L H. it I.
![#5 Section-1 Solubility of KHT Filtration of [K+] in KHT(satd. ), Titration, and Calculation #5 Section-1 Solubility of KHT Filtration of [K+] in KHT(satd. ), Titration, and Calculation](http://slidetodoc.com/presentation_image/0239576ca58c130def34c8ef726bb09c/image-8.jpg)
#5 Section-1 Solubility of KHT Filtration of [K+] in KHT(satd. ), Titration, and Calculation These are exactly the same as done before for Section 1. For the assigned [K+], write, Na. OH (m. L) and [HT-] on the BLACK BOARD. A manual plot of the graph is due on the next turn. This graph will be used by you to figure out the [K+] in the U. K. CALCULATE SOLUBILITY Na. OH + KHT Na. KT + H 2 O 0. 0412 M ? M ____m. L 25. 00 m. L
![#6 Section-1. Solubility of KHT in Presence of [K+] added Data: [KHT] 0. 0436 #6 Section-1. Solubility of KHT in Presence of [K+] added Data: [KHT] 0. 0436](http://slidetodoc.com/presentation_image/0239576ca58c130def34c8ef726bb09c/image-9.jpg)
#6 Section-1. Solubility of KHT in Presence of [K+] added Data: [KHT] 0. 0436 0. 0382 0. 0362 0. 0313 0. 0252 0. 0205 Solubilty of KHT in presence of [K+] 0. 05 Solubility of KHT [KHT] [K+] 0. 04 0. 06 0. 08 0. 12 0. 14 0. 045 0. 04 0. 035 Series 1 0. 03 Linear(Series 0) 0. 025 0. 02 0. 015 0. 03 Graph will be used for U. K. 0. 08 [K+] added 0. 13 If the solubility of KHT in presence of (U. K. ) = 0. 0315 M; [K+] added = 0. 081 M
![#7 Section-3. Determination of an Unknown [K+] Filtration, Titration, and Calculation A U. K. #7 Section-3. Determination of an Unknown [K+] Filtration, Titration, and Calculation A U. K.](http://slidetodoc.com/presentation_image/0239576ca58c130def34c8ef726bb09c/image-10.jpg)
#7 Section-3. Determination of an Unknown [K+] Filtration, Titration, and Calculation A U. K. solution containing KNO 3 and KHT (saturated) will be supplied in a 125 m. L Erlenmeyer. Filtration, Titration in duplicate, and Calculation are exactly the same as done before for Section 1. RESULT: Extrapolate the Solubility of KHT on the graph to find the [K+] value for the U. K. A Report is due on the following Lab period.
- Slides: 10