June 4 2020 Stakeholder Engagement on ICH E





















- Slides: 29

June 4, 2020 Stakeholder Engagement on ICH E 6 Guideline for Good Clinical Practice – Day 1 M. Khair El. Zarrad, E 6 Rapporteur Food & Drug Administration Pamela Tenaerts, Moderator Clinical Trials Transformation Initiative

Welcome • This web conference is being recorded and will be posted to the FDA & CTTI websites • All participants are muted upon entry • Questions can be entered in the chat box during the webinar • Questions and comments will be collated analyzed to inform the ongoing work to update the ICH E 6 GCP guideline • This web conference includes Closed Captioning • At the bottom of your screen, click • Closed Caption > Show Subtitles • For best viewing, click on show speaker/slides side by side

Agenda – Day 1 • Welcome, Opening Remarks • Session I: ICH Process & Updating ICH E 6 GCP Guidelines • Session II: CTTI ICH E 6 Survey and Stakeholder Input • Session III: Perspectives from EWG Members • BREAK • Session IV: Perspectives from Clinical Investigators • Session V: Perspectives from Patient Organizations • Closing Remarks, Plans for Day 2

Session I: ICH Process & Updating ICH E 6 GCP Guidelines Theresa M. Mullin Food & Drug Administration M. Khair El. Zarrad Food & Drug Administration Fergus Sweeney European Commission

International Harmonization of Regulatory Requirements for Human Drugs Overview of ICH* Theresa Mullin, Ph. D. ** Associate Director for Strategic Initiatives FDA Center for Drug Evaluation and Research ** Chair, ICH Management Committee *International Council for Harmonisation Of Technical Requirements For Pharmaceuticals for Human Use (ICH)

ICH Background • Since 1990, a unique harmonisation effort to: • Improve efficiency of new drug development and registration process • Prevent duplication of clinical trials in humans and minimize use of animal testing ---without compromising safety and effectiveness • Accomplished through the development and implementation of harmonised technical regulatory Guidelines • Keys to success: • Involvement of both regulators and industry • Science-based, well-managed, consensus driven • Limited number of players with comparable regulatory and technical expertise/capability • Commitment of regulators to implement products of harmonisation 6

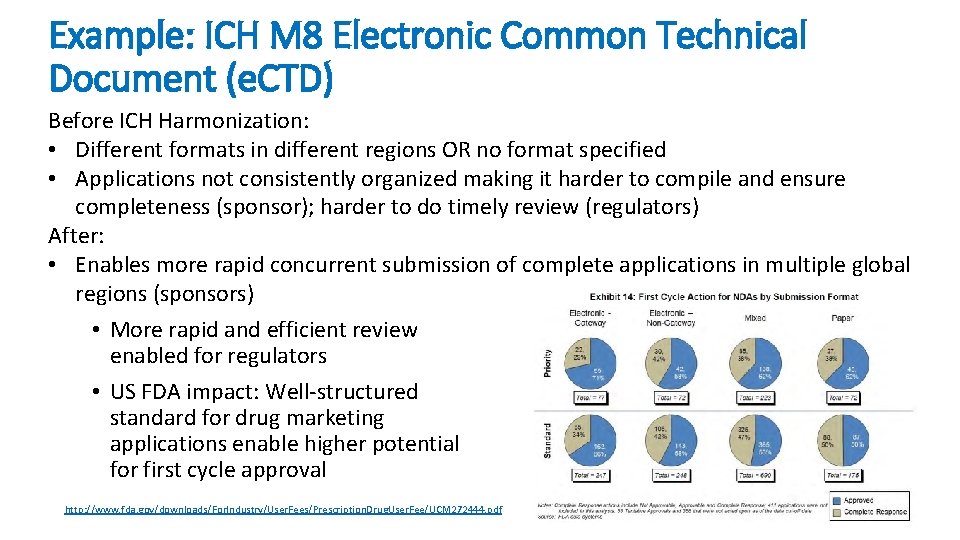
Example: ICH M 8 Electronic Common Technical Document (e. CTD) Before ICH Harmonization: • Different formats in different regions OR no format specified • Applications not consistently organized making it harder to compile and ensure completeness (sponsor); harder to do timely review (regulators) After: • Enables more rapid concurrent submission of complete applications in multiple global regions (sponsors) • More rapid and efficient review enabled for regulators • US FDA impact: Well-structured standard for drug marketing applications enable higher potential for first cycle approval http: //www. fda. gov/downloads/For. Industry/User. Fees/Prescription. Drug. User. Fee/UCM 272444. pdf

Example: ICH E 6 Good Clinical Practice Before ICH Harmonization: • Different requirements for conduct & oversight of trials across regions • Foreign clinical data (collected in one region) often not accepted in another region • Repeat clinical trials were a fact of life in drug development if a sponsor wished to market a drug in more than one region After: • Repeat of clinical trials obviated; foreign clinical trial data submitted in support of an application in any ICH region (potential need for bridging study) • Continued growth and success of multiregional clinical studies made possible, with clear harmonized requirements for investigators, sponsors, IRBs, data collection, training, documentation, etc. • Earlier submission across regions, enabling potential for near-simultaneous launch & earlier access to new medicines across regions

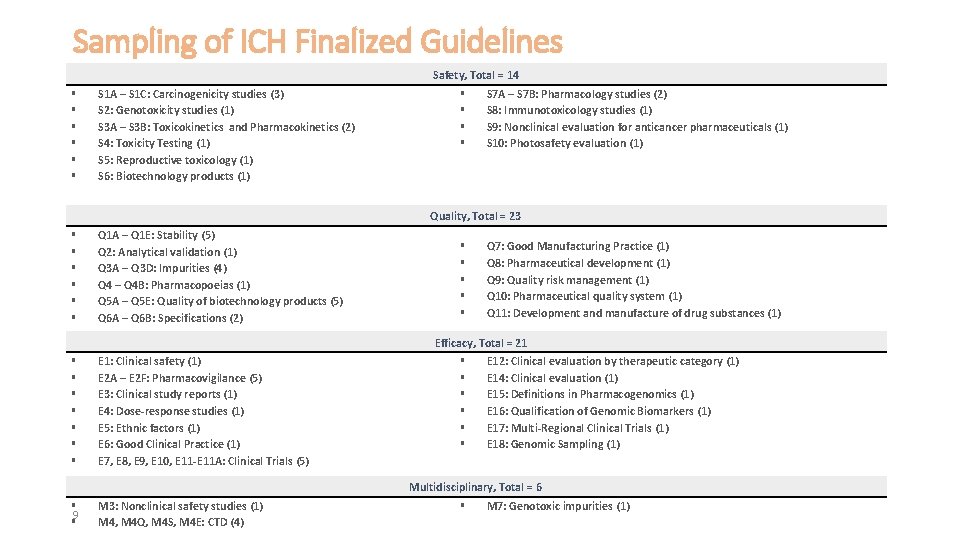
Sampling of ICH Finalized Guidelines Safety, Total = 14 S 1 A – S 1 C: Carcinogenicity studies (3) S 2: Genotoxicity studies (1) S 3 A – S 3 B: Toxicokinetics and Pharmacokinetics (2) S 4: Toxicity Testing (1) S 5: Reproductive toxicology (1) S 6: Biotechnology products (1) S 7 A – S 7 B: Pharmacology studies (2) S 8: Immunotoxicology studies (1) S 9: Nonclinical evaluation for anticancer pharmaceuticals (1) S 10: Photosafety evaluation (1) Quality, Total = 23 Q 1 A – Q 1 E: Stability (5) Q 2: Analytical validation (1) Q 3 A – Q 3 D: Impurities (4) Q 4 – Q 4 B: Pharmacopoeias (1) Q 5 A – Q 5 E: Quality of biotechnology products (5) Q 6 A – Q 6 B: Specifications (2) Q 7: Good Manufacturing Practice (1) Q 8: Pharmaceutical development (1) Q 9: Quality risk management (1) Q 10: Pharmaceutical quality system (1) Q 11: Development and manufacture of drug substances (1) Efficacy, Total = 21 E 1: Clinical safety (1) E 2 A – E 2 F: Pharmacovigilance (5) E 3: Clinical study reports (1) E 4: Dose-response studies (1) E 5: Ethnic factors (1) E 6: Good Clinical Practice (1) E 7, E 8, E 9, E 10, E 11 -E 11 A: Clinical Trials (5) E 12: Clinical evaluation by therapeutic category (1) E 14: Clinical evaluation (1) E 15: Definitions in Pharmacogenomics (1) E 16: Qualification of Genomic Biomarkers (1) E 17: Multi-Regional Clinical Trials (1) E 18: Genomic Sampling (1) Multidisciplinary, Total = 6 9 M 3: Nonclinical safety studies (1) M 4, M 4 Q, M 4 S, M 4 E: CTD (4) M 7: Genotoxic impurities (1)

New Topic Selection Process • Any ICH Member and Observer can submit proposals for New Topics for harmonisation • A subcommittee of the MC collects and prioritizes New Topic proposals for Assembly review • The ICH Assembly reviews topic proposals once per year and selects new topics for harmonisation during an Assembly meeting

Initiation of ICH Harmonization for a New Topic The ICH Assembly endorses a New Topic. The ICH Assembly endorses the Concept Paper Outline. An informal WG is established to develop a final Concept Paper and Business Plan. The MC approves the final Concept Paper and Business Plan and a formal EWG/IWG is established.

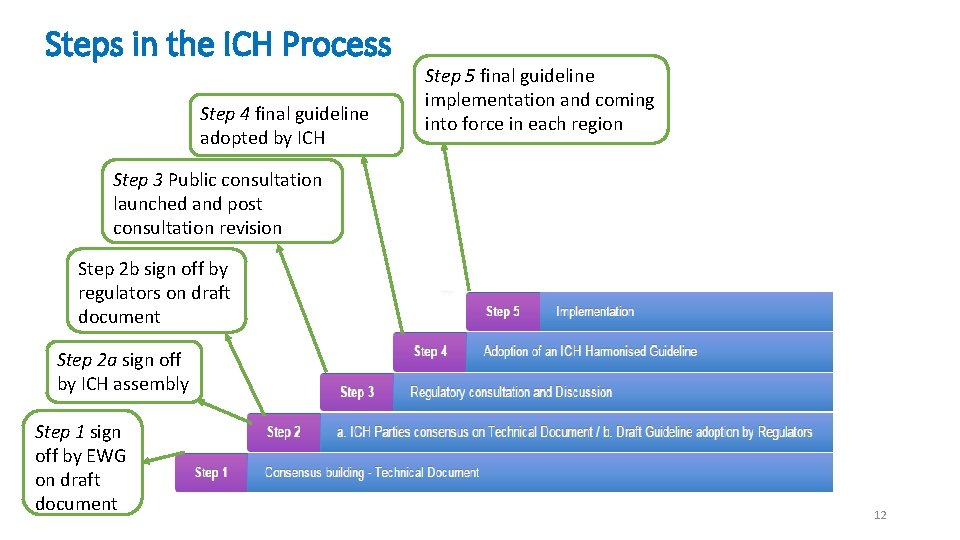
Steps in the ICH Process Step 4 final guideline adopted by ICH Step 5 final guideline implementation and coming into force in each region Step 3 Public consultation launched and post consultation revision Step 2 b sign off by regulators on draft document Step 2 a sign off by ICH assembly Step 1 sign off by EWG on draft document 12

ICH Reform Goals for Reform 1. Focus global pharmaceutical regulatory harmonisation work in one venue. 2. Create a venue that gives to all key pharmaceutical regulatory authorities and industry stakeholders the opportunity to be more actively involved in pharmaceutical harmonisation work. 3. Maintain efficient and well-managed operations and harmonisation work processes. The ICH Association, established in October 2015, is a non-profit legal entity under Swiss law with the aim to focus global pharmaceutical regulatory harmonisation work in one venue. http: //www. ich. org/about/articles-procedures. html 13

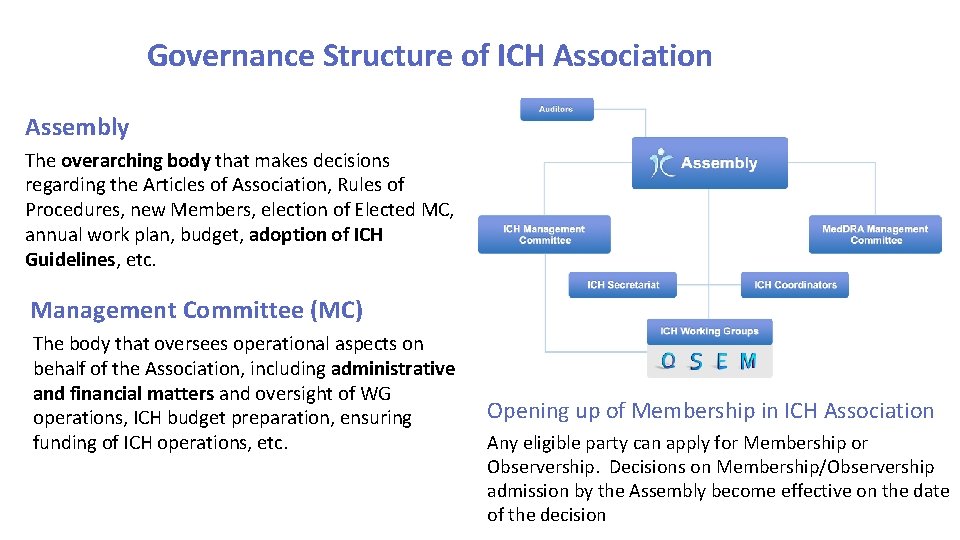
Governance Structure of ICH Association Assembly The overarching body that makes decisions regarding the Articles of Association, Rules of Procedures, new Members, election of Elected MC, annual work plan, budget, adoption of ICH Guidelines, etc. Management Committee (MC) The body that oversees operational aspects on behalf of the Association, including administrative and financial matters and oversight of WG operations, ICH budget preparation, ensuring funding of ICH operations, etc. Opening up of Membership in ICH Association Any eligible party can apply for Membership or Observership. Decisions on Membership/Observership admission by the Assembly become effective on the date of the decision 14

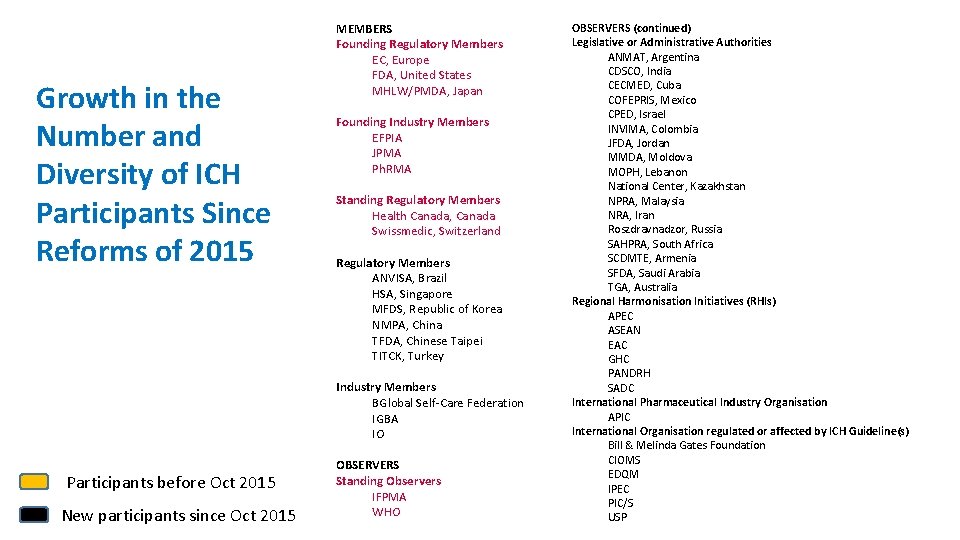
Growth in the Number and Diversity of ICH Participants Since Reforms of 2015 MEMBERS Founding Regulatory Members EC, Europe FDA, United States MHLW/PMDA, Japan Founding Industry Members EFPIA JPMA Ph. RMA Standing Regulatory Members Health Canada, Canada Swissmedic, Switzerland Regulatory Members ANVISA, Brazil HSA, Singapore MFDS, Republic of Korea NMPA, China TFDA, Chinese Taipei TITCK, Turkey Industry Members BGlobal Self-Care Federation IGBA IO Participants before Oct 2015 New participants since Oct 2015 OBSERVERS Standing Observers IFPMA WHO OBSERVERS (continued) Legislative or Administrative Authorities ANMAT, Argentina CDSCO, India CECMED, Cuba COFEPRIS, Mexico CPED, Israel INVIMA, Colombia JFDA, Jordan MMDA, Moldova MOPH, Lebanon National Center, Kazakhstan NPRA, Malaysia NRA, Iran Roszdravnadzor, Russia SAHPRA, South Africa SCDMTE, Armenia SFDA, Saudi Arabia TGA, Australia Regional Harmonisation Initiatives (RHIs) APEC ASEAN EAC GHC PANDRH SADC International Pharmaceutical Industry Organisation APIC International Organisation regulated or affected by ICH Guideline(s) Bill & Melinda Gates Foundation CIOMS EDQM IPEC PIC/S USP 15

ICH as of May 29, 2020 • ICH comprises of 49 Members and Observers: • 17 Members • 32 Observers • ICH comprises of 34 active working groups • ICH directly involves 1082 persons: • 95 Representatives of Members/Observers in the ICH Assembly, the ICH Management Committee (MC) and the Med. DRA MC • 759 Experts in WGs • 237 Persons serving in support roles 16

Opportunities and Rewards of ICH Reform 1. Enabling growth in the number and diversity of participants • Encouraging participation and expanding portfolio (e. g. , generic drugs) 2. Preserving the technical expertise and efficiency • Continuing assessment and enhancements as needed to maintain efficiency, e. g. , manage size of Expert Working Groups 3. Increasing ICH focus on strategy • More formal structure and approach enables longer planning horizon and management continuity • Shift from “where are there existing gaps? ” to “what are the emerging needs/opportunities? ” PLUS “where are existing gaps? ” • Reflection papers help identify opportunities, e. g. , GCP Renovation 17

Thank you

ICH E 6 Good Clinical Practice Harmonizing Regulations for Clinical Trial Conduct M. Khair El. Zarrad Deputy Director Office of Medical Policy Center for Drug Evaluation and Research

ICH E 6(R 3) Expert Working Group (EWG) Members

ICH E 6: An Important Global Standard Global Clinical Trials Evolving Evidence Generation Paradigm Increasingly Digital World Innovative Clinical Trial Designs

ICH E 6: An Important Global Standard for Clinical Trial Conduct • E 6: Good Clinical Practice (GCP) – finalized in 1996 o Describes the responsibilities and expectations of stakeholders in the conduct of clinical trials. o GCP covers aspects of monitoring, reporting, and archiving of clinical trials o Addenda for essential documents and investigator brochures • E 6(R 2) – finalized in 2016 o Addendum to encourage implementation of improved and more efficient approaches to GCP, while continuing to ensure human subject protections o Updated standards for electronic records E 8 clinical trial design principles E 6 GCP clinical trial conduct principles

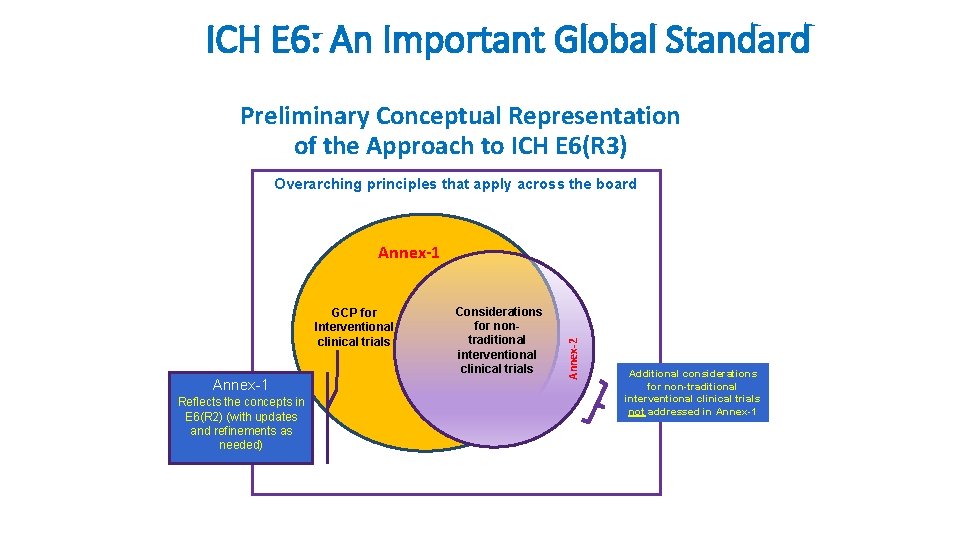
ICH E 6: An Important Global Standard Preliminary Conceptual Representation of the Approach to ICH E 6(R 3) Overarching principles that apply across the board GCP for Interventional clinical trials Annex-1 Reflects the concepts in E 6(R 2) (with updates and refinements as needed) Considerations for nontraditional interventional clinical trials Annex-2 Annex-1 Additional considerations for non-traditional interventional clinical trials not addressed in Annex-1

Overview of ICH E 6(R 3) Revisions • Annex 1 – Interventional Clinical • Annex 2 – Non-traditional Trials Interventional Clinical Trials • Considers principles as they relate to the use of unapproved or approved drugs in a controlled setting with prospective allocation of treatment to participants and collection of trial data – Considers principles as they relate to the use of nontraditional clinical trial designs such as pragmatic clinical trials and decentralized clinical trials, as well as those trials that incorporate real-world data sources 24

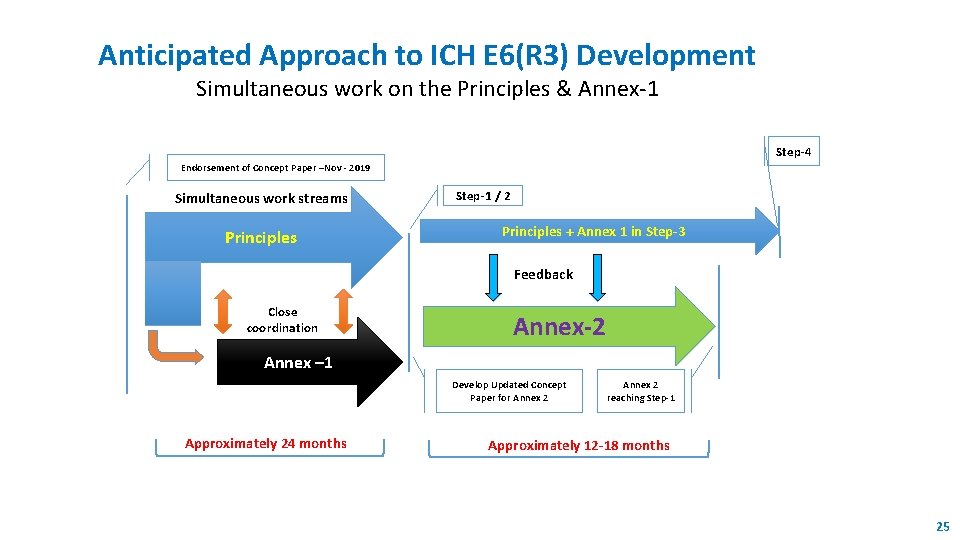
Anticipated Approach to ICH E 6(R 3) Development Simultaneous work on the Principles & Annex-1 Step-4 Endorsement of Concept Paper –Nov - 2019 Simultaneous work streams Principles Step-1 / 2 Principles + Annex 1 in Step-3 Feedback Close coordination Annex-2 Annex – 1 Develop Updated Concept Paper for Annex 2 Approximately 24 months Annex 2 reaching Step-1 Approximately 12 -18 months 25

Engagement • Many stakeholders are impacted by ICH E 6 GCP guidelines • E 6 stakeholder outreach approaches are approved by ICH and are ongoing • The knowledge gained by learning from stakeholder experiences and viewpoints will further enrich EWG discussions ICH E 6 Summary Engagement Plan - https: //admin. ich. org/sites/default/files/2020 -05/E 6 R 3_Public. Engagemen. Summary_2020_0421. pdf

ICH E 6(R 3) • Comprehensive principles that remain relevant as technology evolve and clinical trial design advances • Leveraging and facilitating an increasingly digital ecosystem • Risk-based approach and proportionality • Thoughtful process throughout clinical trial conception, design, conduct and analyses

Thank you!

Stakeholder Engagement for ICH E 6 Fergus Sweeney Regulatory Chair European Commission