Investigational Approaches to Antiretroviral Therapy Eric S Daar





![Virologic Outcome[1] Ibalizumab + OBR � 9 pts reported 17 serious AEs – Day Virologic Outcome[1] Ibalizumab + OBR � 9 pts reported 17 serious AEs – Day](https://slidetodoc.com/presentation_image_h2/ca4e6c6ba6fa2a8370989006fad381b0/image-30.jpg)

- Slides: 33

Investigational Approaches to Antiretroviral Therapy Eric S. Daar, MD Professor of Medicine University of California Los Angeles, California FORMATTED: 04/26/17 Chicago, Illinois: May 10, 2017 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

Financial Relationships With Commercial Entities �Dr Daar has received research and grant support from Gilead Sciences, Inc, Merck, and Vii. V Healthcare. He has also received consulting fees from Bristol-Myers Squibb, Gilead Sciences, Inc, Janssen Therapeutics, Merck, Teva, and Vii. V Healthcare. (Updated 04/26/17) Slide 2 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

Learning Objectives After attending this presentation, learners will be able to: �Summarize treatments being investigated for HIV- infected persons with viremia �Describe treatments being investigated for those with virologic suppression on antiretroviral therapy Slide 3 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

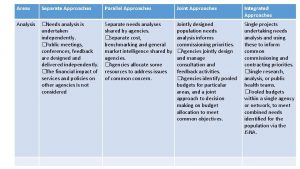
Investigational Treatment Strategies Slide 4 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

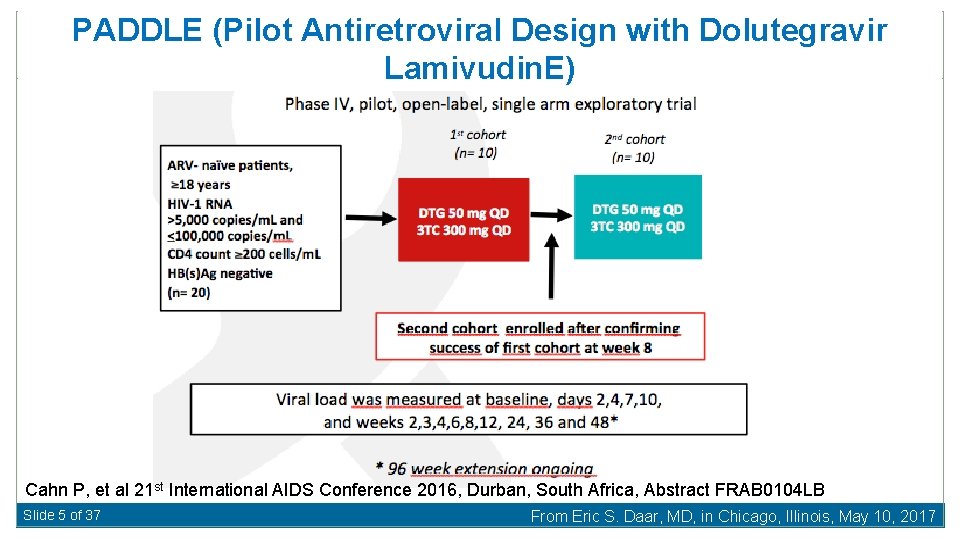
PADDLE (Pilot Antiretroviral Design with Dolutegravir Lamivudin. E) Cahn P, et al 21 st International AIDS Conference 2016, Durban, South Africa, Abstract FRAB 0104 LB Slide 5 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

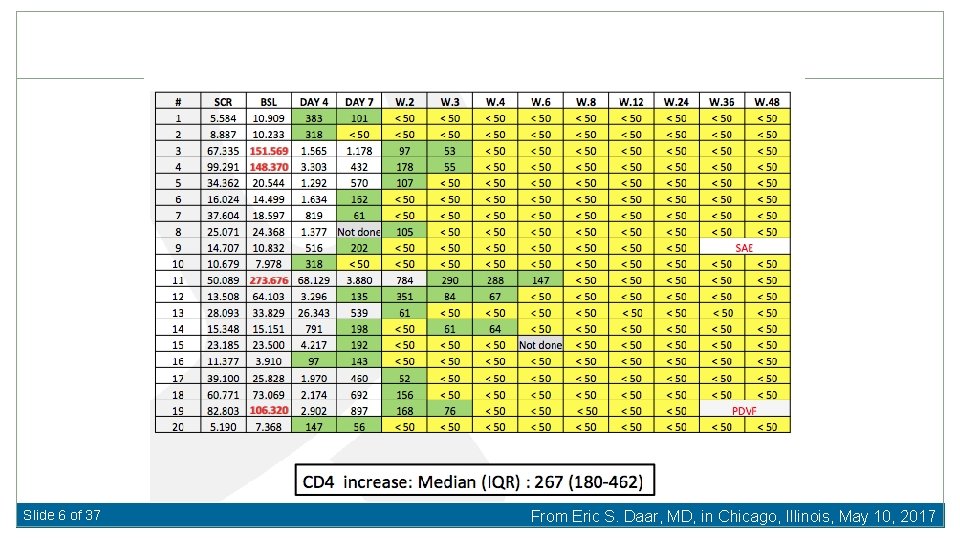
Slide 6 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

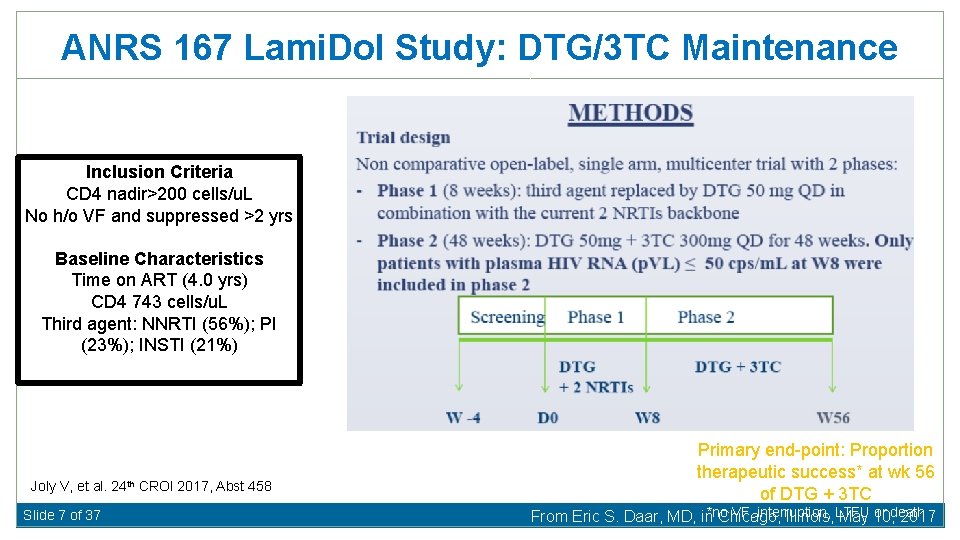
ANRS 167 Lami. Dol Study: DTG/3 TC Maintenance Inclusion Criteria CD 4 nadir>200 cells/u. L No h/o VF and suppressed >2 yrs Baseline Characteristics Time on ART (4. 0 yrs) CD 4 743 cells/u. L Third agent: NNRTI (56%); PI (23%); INSTI (21%) Joly V, et al. 24 th CROI 2017, Abst 458 Slide 7 of 37 Primary end-point: Proportion therapeutic success* at wk 56 of DTG + 3 TC VF, interruption, or death From Eric S. Daar, MD, in*no Chicago, Illinois, LTFU May 10, 2017

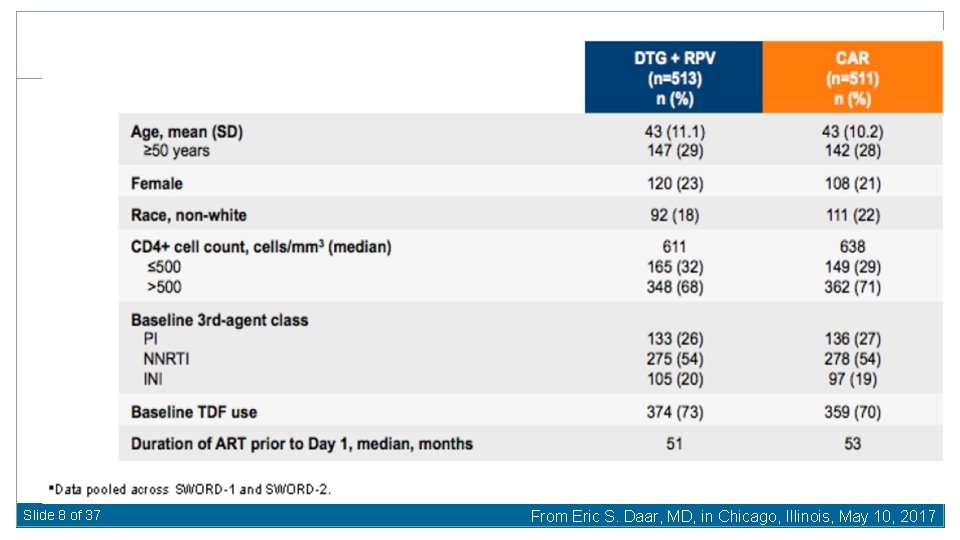
Slide 8 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

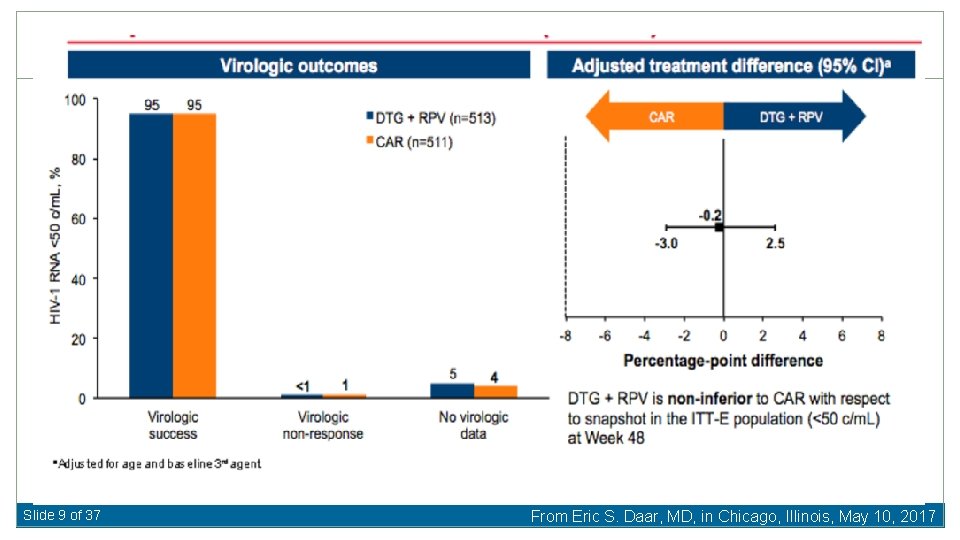
Slide 9 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

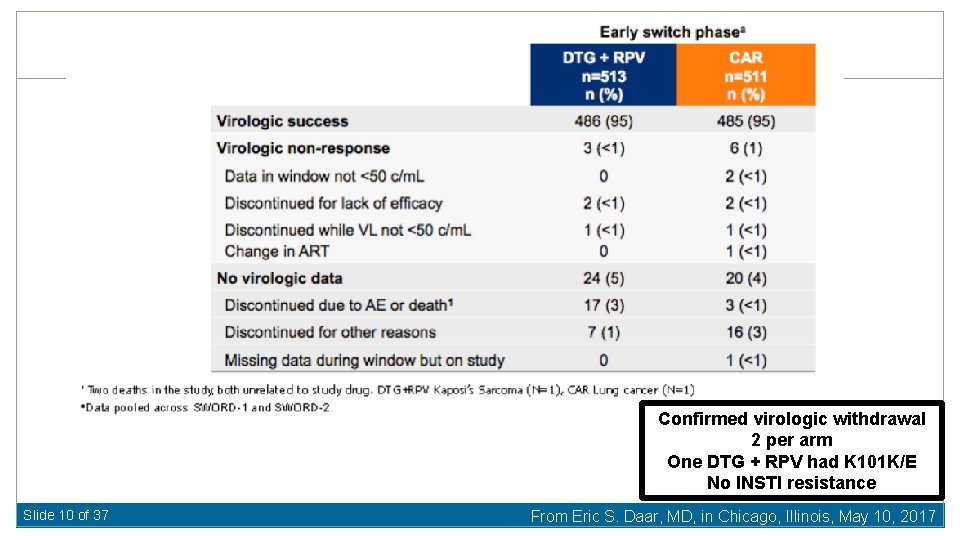
Confirmed virologic withdrawal 2 per arm One DTG + RPV had K 101 K/E No INSTI resistance Slide 10 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

Investigational Drugs/Formulations Slide 11 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

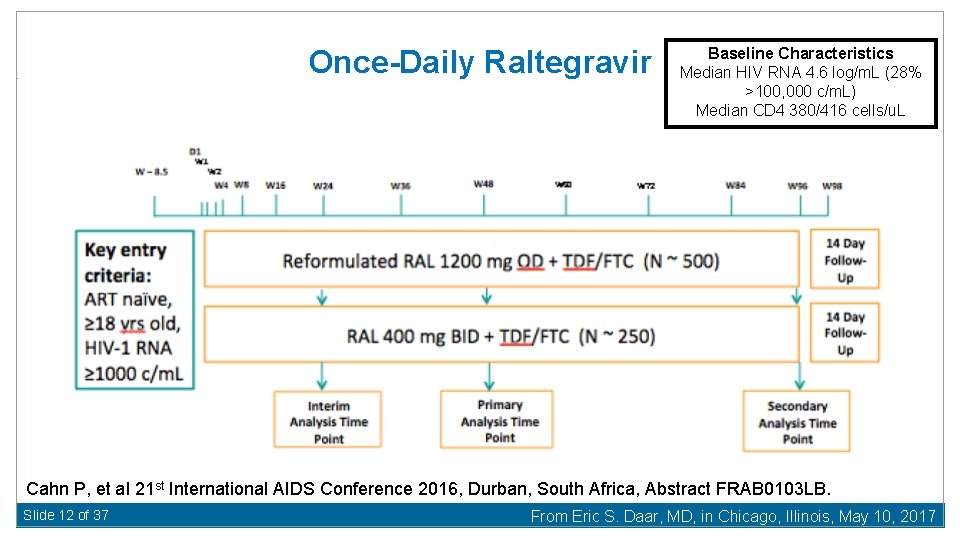
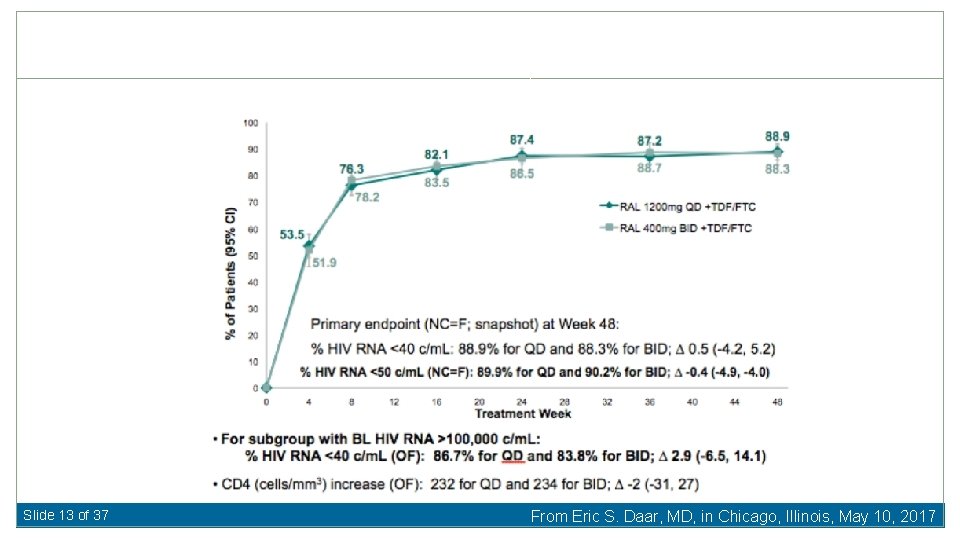
Once-Daily Raltegravir Baseline Characteristics Median HIV RNA 4. 6 log/m. L (28% >100, 000 c/m. L) Median CD 4 380/416 cells/u. L Cahn P, et al 21 st International AIDS Conference 2016, Durban, South Africa, Abstract FRAB 0103 LB. Slide 12 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

Slide 13 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

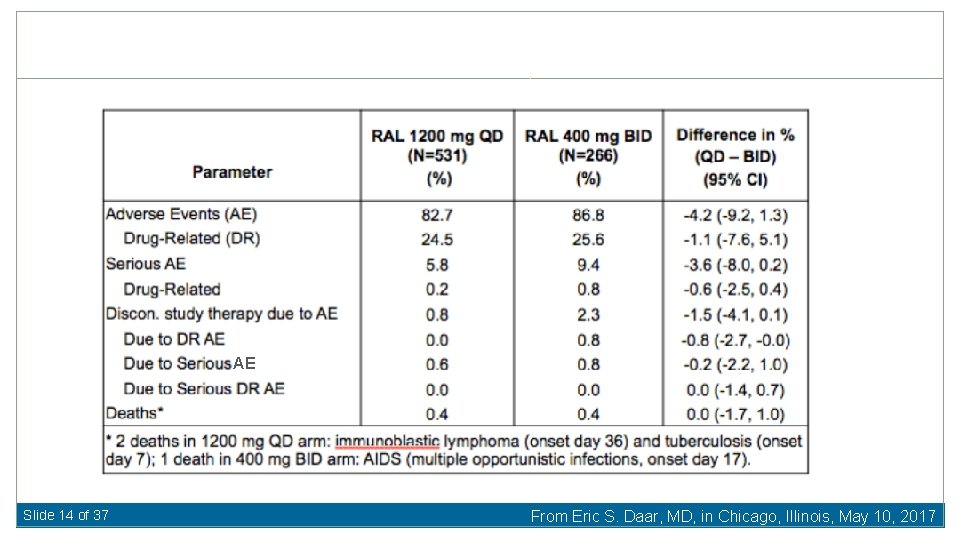
AE Slide 14 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

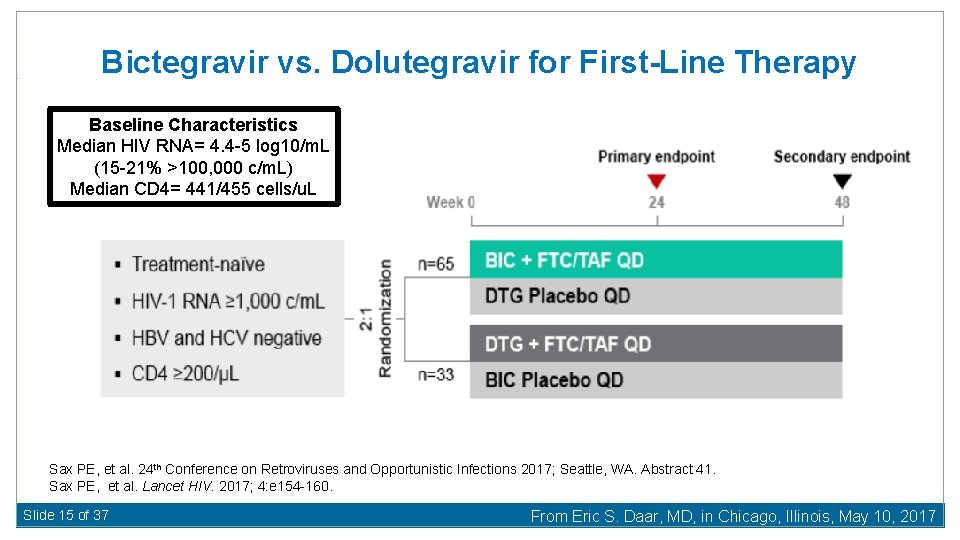
Bictegravir vs. Dolutegravir for First-Line Therapy Baseline Characteristics Median HIV RNA= 4. 4 -5 log 10/m. L (15 -21% >100, 000 c/m. L) Median CD 4= 441/455 cells/u. L Sax PE, et al. 24 th Conference on Retroviruses and Opportunistic Infections 2017; Seattle, WA. Abstract 41. Sax PE, et al. Lancet HIV. 2017; 4: e 154 -160. Slide 15 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

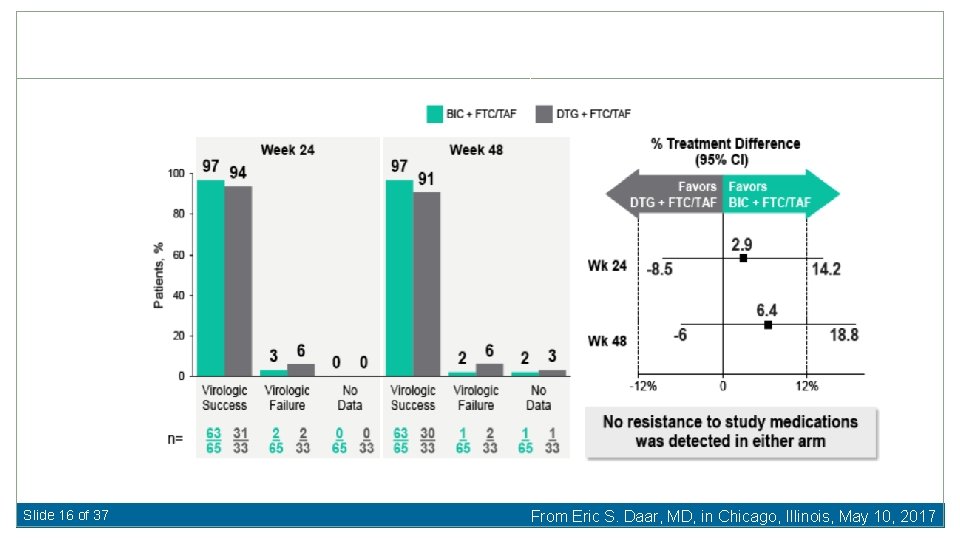
Slide 16 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

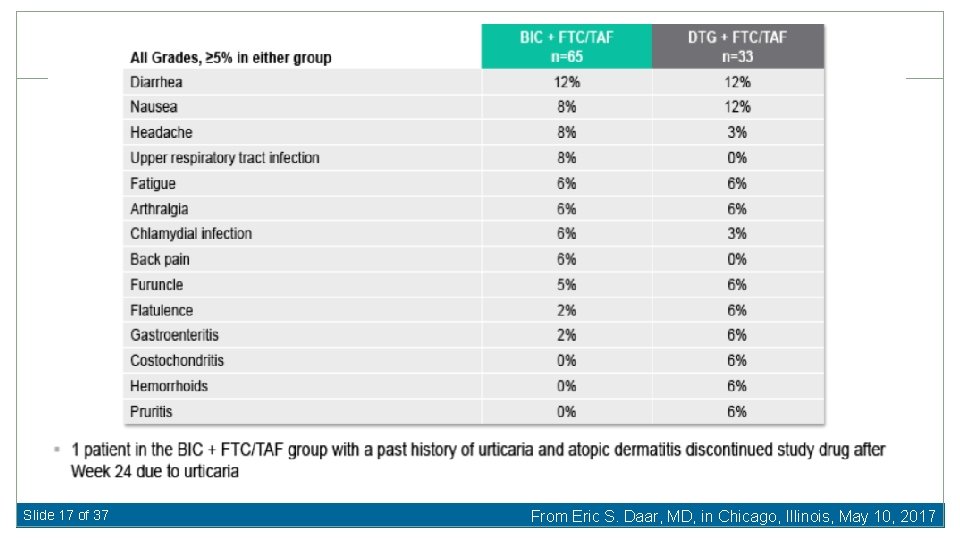
Slide 17 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

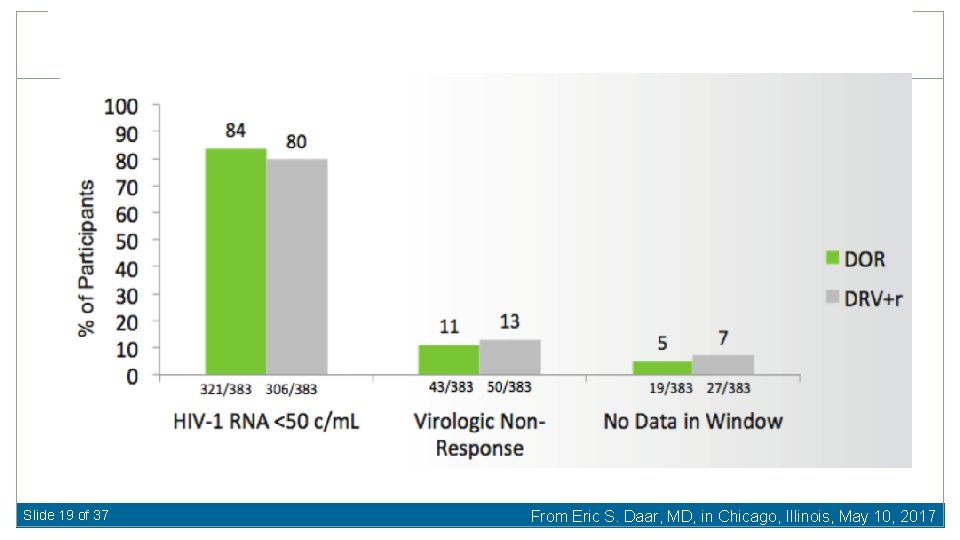
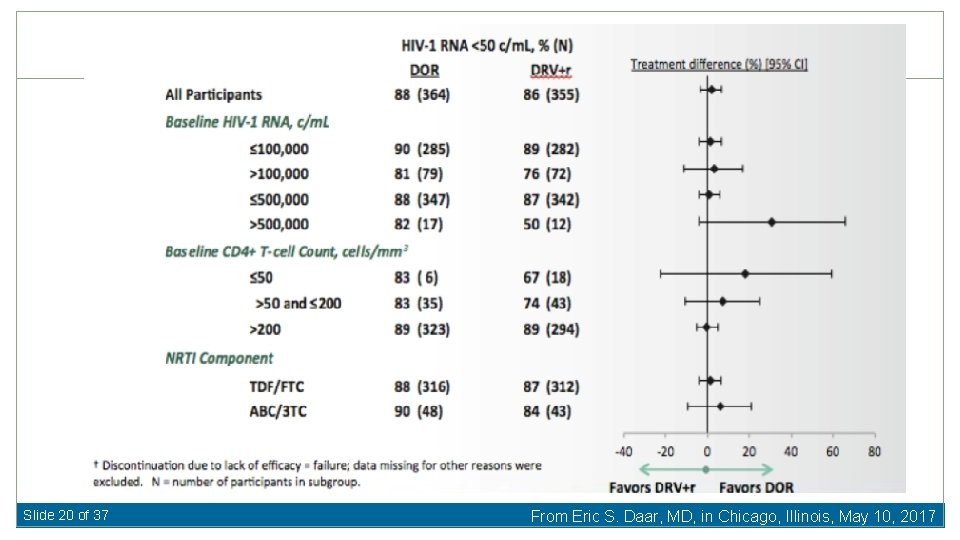
Doravirine vs. Darunavir/ritonavir for First-Line Therapy Baseline Characteristics Median HIV RNA 4. 4 log 10 c/m. L (19 -22% >100, 000 c/m. L) Median CD 4 412 -433 cells/u. L 87 -88% TDF/FTC Molin J-M, et al. 24 th CROI 2017, Abst 45 LB Slide 18 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

Slide 19 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

Slide 20 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

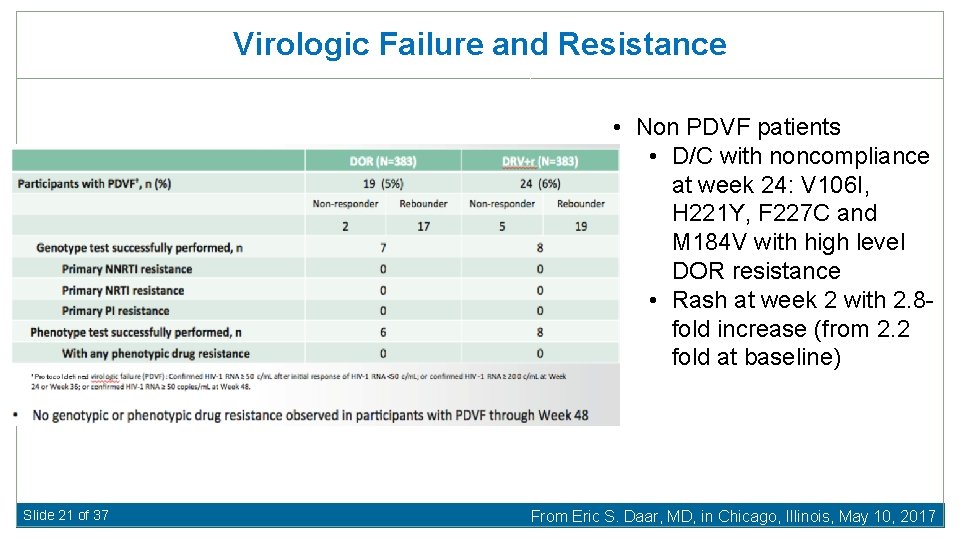
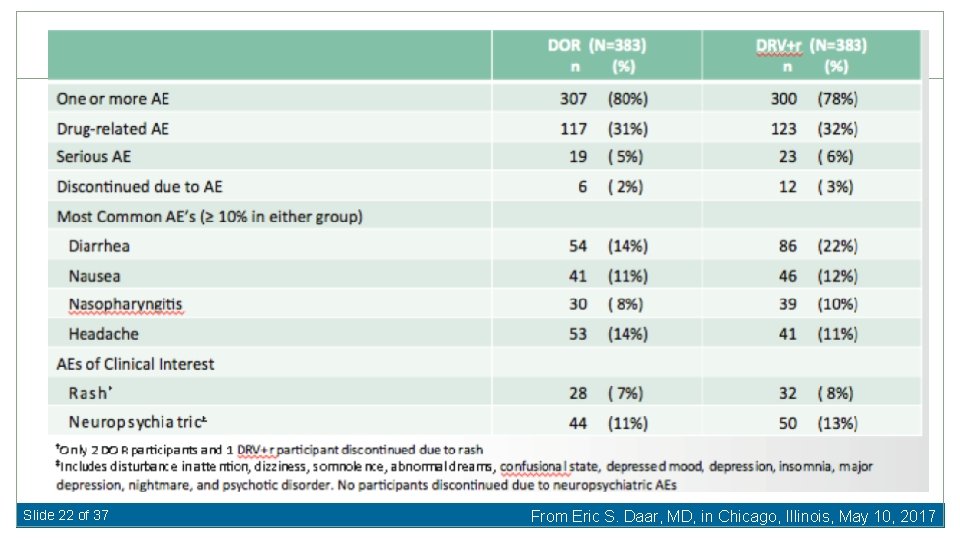
Virologic Failure and Resistance • Non PDVF patients • D/C with noncompliance at week 24: V 106 I, H 221 Y, F 227 C and M 184 V with high level DOR resistance • Rash at week 2 with 2. 8 fold increase (from 2. 2 fold at baseline) Slide 21 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

Slide 22 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

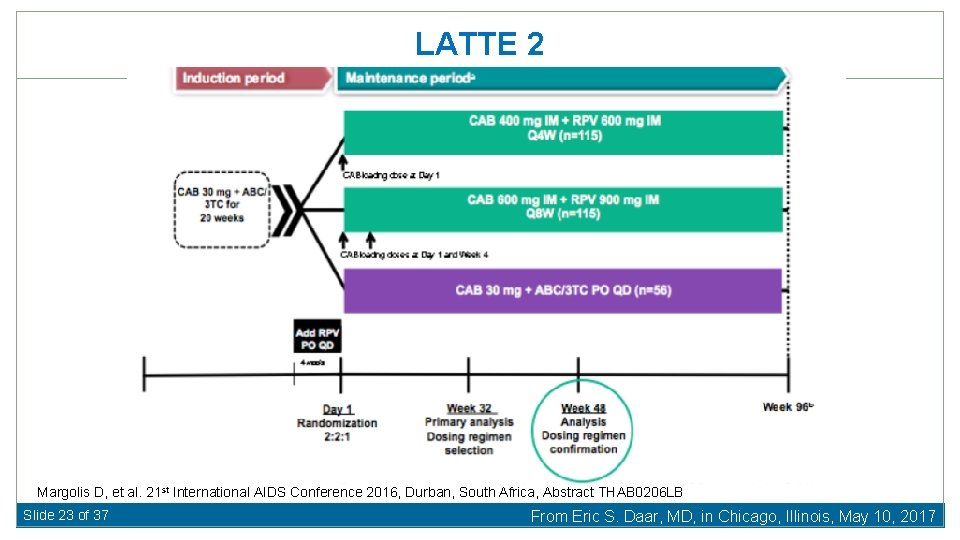
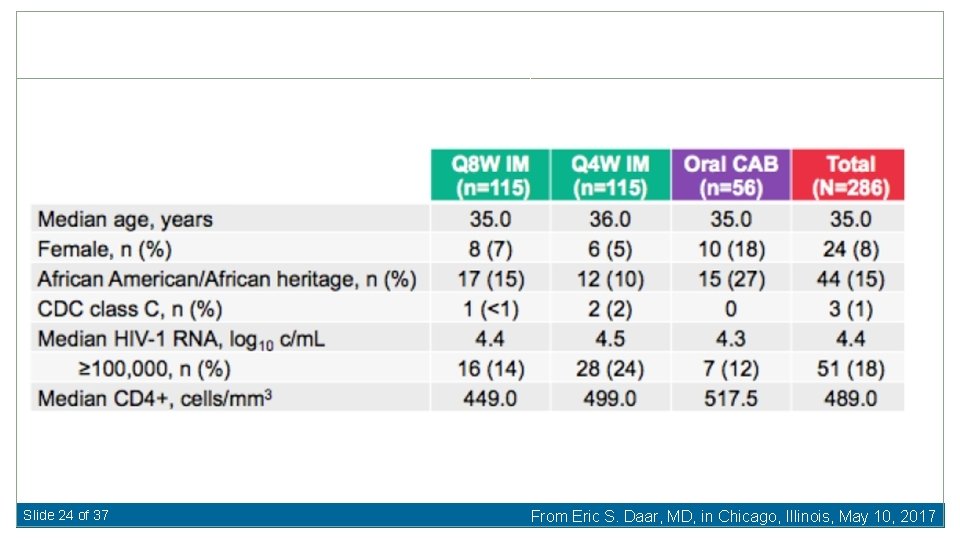
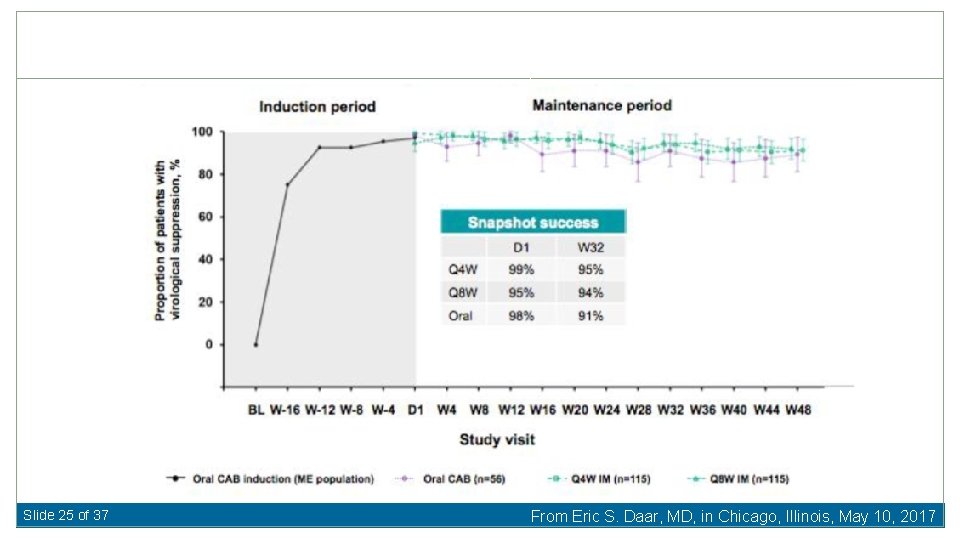
LATTE 2 Margolis D, et al. 21 st International AIDS Conference 2016, Durban, South Africa, Abstract THAB 0206 LB Slide 23 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

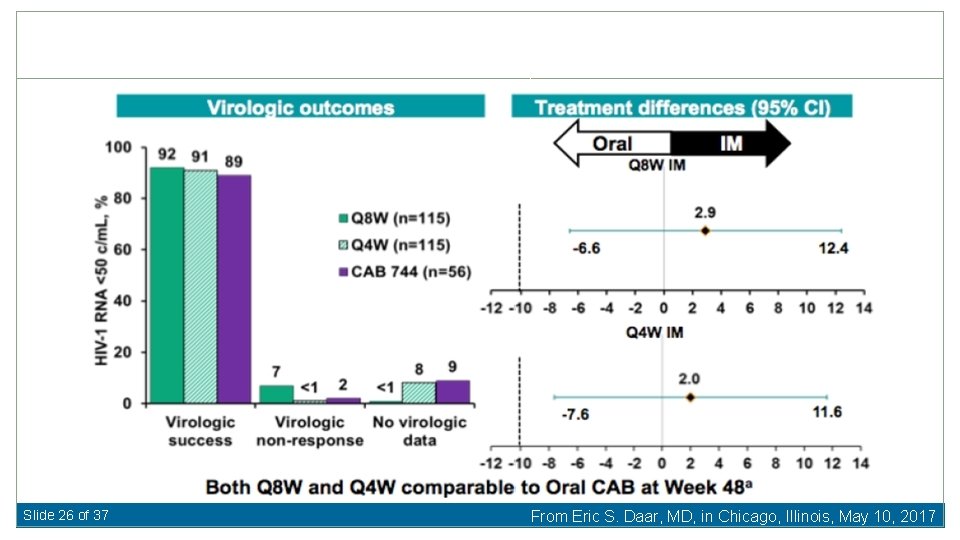
Slide 24 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

Slide 25 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

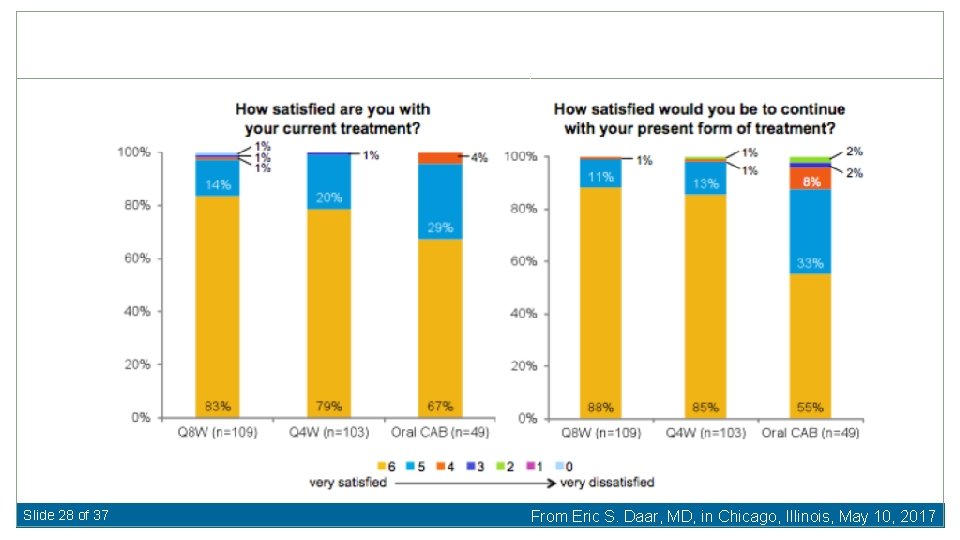
Slide 26 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

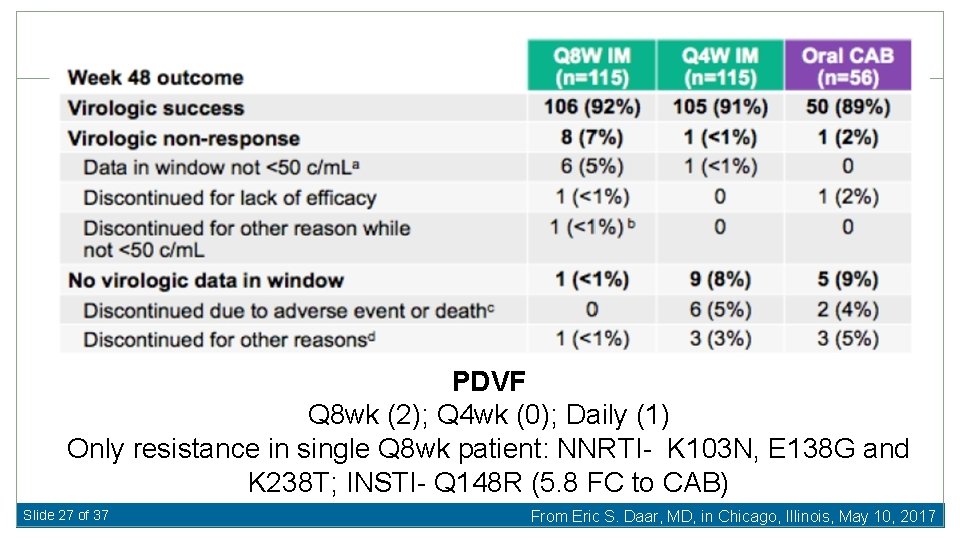
PDVF Q 8 wk (2); Q 4 wk (0); Daily (1) Only resistance in single Q 8 wk patient: NNRTI- K 103 N, E 138 G and K 238 T; INSTI- Q 148 R (5. 8 FC to CAB) Slide 27 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

Slide 28 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

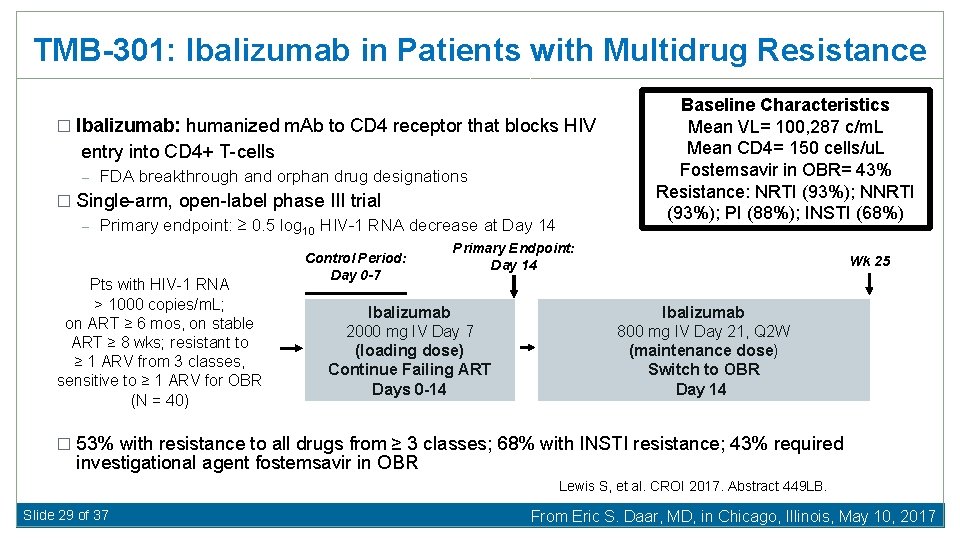
TMB-301: Ibalizumab in Patients with Multidrug Resistance � Ibalizumab: humanized m. Ab to CD 4 receptor that blocks HIV entry into CD 4+ T-cells – FDA breakthrough and orphan drug designations � Single-arm, open-label phase III trial – Primary endpoint: ≥ 0. 5 log 10 HIV-1 RNA decrease at Day 14 Pts with HIV-1 RNA > 1000 copies/m. L; on ART ≥ 6 mos, on stable ART ≥ 8 wks; resistant to ≥ 1 ARV from 3 classes, sensitive to ≥ 1 ARV for OBR (N = 40) Control Period: Day 0 -7 Baseline Characteristics Mean VL= 100, 287 c/m. L Mean CD 4= 150 cells/u. L Fostemsavir in OBR= 43% Resistance: NRTI (93%); NNRTI (93%); PI (88%); INSTI (68%) Primary Endpoint: Day 14 Ibalizumab 2000 mg IV Day 7 (loading dose) Continue Failing ART Days 0 -14 Wk 25 Ibalizumab 800 mg IV Day 21, Q 2 W (maintenance dose) Switch to OBR Day 14 � 53% with resistance to all drugs from ≥ 3 classes; 68% with INSTI resistance; 43% required investigational agent fostemsavir in OBR Lewis S, et al. CROI 2017. Abstract 449 LB. Slide 29 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017
![Virologic Outcome1 Ibalizumab OBR 9 pts reported 17 serious AEs Day Virologic Outcome[1] Ibalizumab + OBR � 9 pts reported 17 serious AEs – Day](https://slidetodoc.com/presentation_image_h2/ca4e6c6ba6fa2a8370989006fad381b0/image-30.jpg)
Virologic Outcome[1] Ibalizumab + OBR � 9 pts reported 17 serious AEs – Day 14 ≥ 0. 5 log 10 HIV-1 RNA decrease, % 83* ≥ 1. 0 log 10 HIV-1 RNA decrease, % 60 Mean HIV-1 RNA decrease, log 10 1. 1 – ≥ 1. 0 log 10 HIV-1 RNA decrease, % 55 – ≥ 2. 0 log 10 HIV-1 RNA decrease, % 48 – HIV-1 RNA < 50 copies/m. L, % 43 HIV-1 RNA < 200 copies/m. L, % 50 Mean HIV-1 RNA decrease from BL, log 10 1. 6 � 9 other pts discontinued Wk 24 *Primary endpoint; P <. 0001 vs 3% at end of control period. 1. Lewis S, et al. CROI 2017. Abstract 449 LB. 2. Clinical. Trials. gov. NCT 02707861. Slide 30 of 37 1 drug-related SAE (IRIS) resulted in discontinuation Death (n = 4; liver failure, Kaposi sarcoma; end-stage AIDS, lymphoma) Consent withdrawal (n = 3) Lost to follow-up (n = 2) � No cases of anti-ibalizumab antibodies � Phase III TMB-311 study ongoing; extension for pts from TMB-301; accepting new pts[2] From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

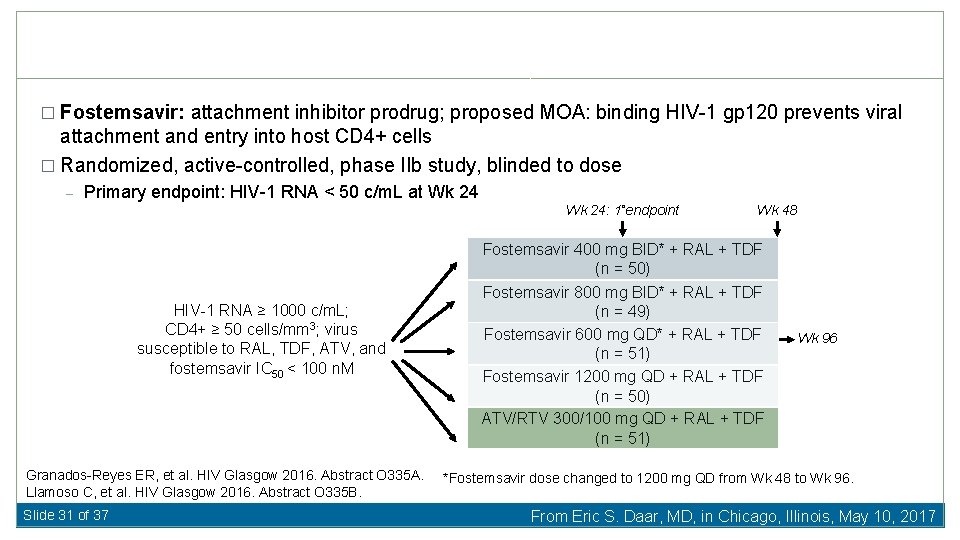
� Fostemsavir: attachment inhibitor prodrug; proposed MOA: binding HIV-1 gp 120 prevents viral attachment and entry into host CD 4+ cells � Randomized, active-controlled, phase IIb study, blinded to dose – Primary endpoint: HIV-1 RNA < 50 c/m. L at Wk 24: 1 endpoint HIV-infected pts with exposure HIV-1 RNA ≥ 1000 c/m. L; CD 4+ ≥ 50 cells/mm 3; virus susceptible to RAL, TDF, ATV, and fostemsavir IC 50 < 100 n. M (N = 254) Granados-Reyes ER, et al. HIV Glasgow 2016. Abstract O 335 A. Llamoso C, et al. HIV Glasgow 2016. Abstract O 335 B. Slide 31 of 37 Wk 48 Fostemsavir 400 mg BID* + RAL + TDF (n = 50) Fostemsavir 800 mg BID* + RAL + TDF (n = 49) Fostemsavir 600 mg QD* + RAL + TDF (n = 51) Fostemsavir 1200 mg QD + RAL + TDF (n = 50) ATV/RTV 300/100 mg QD + RAL + TDF (n = 51) Wk 96 *Fostemsavir dose changed to 1200 mg QD from Wk 48 to Wk 96. From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

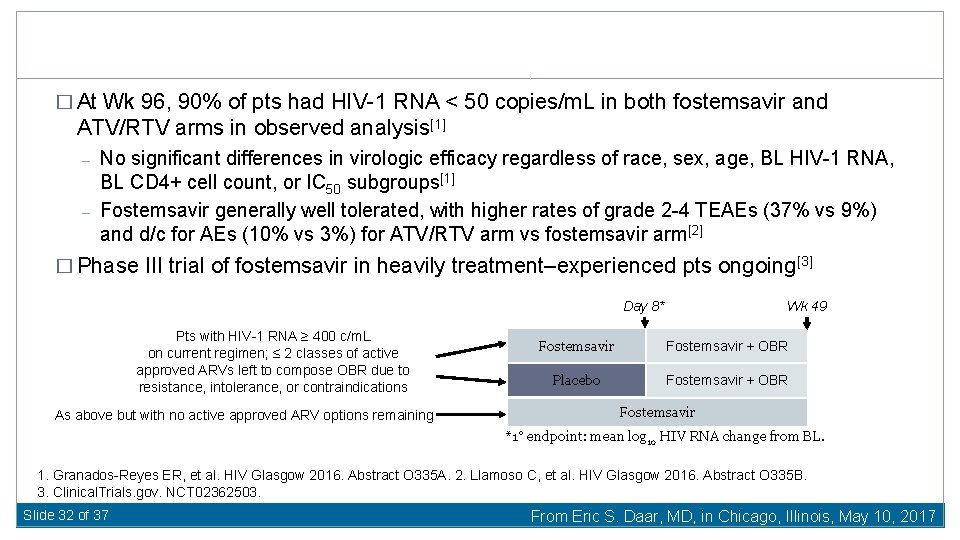
� At Wk 96, 90% of pts had HIV-1 RNA < 50 copies/m. L in both fostemsavir and ATV/RTV arms in observed analysis[1] – – No significant differences in virologic efficacy regardless of race, sex, age, BL HIV-1 RNA, BL CD 4+ cell count, or IC 50 subgroups[1] Fostemsavir generally well tolerated, with higher rates of grade 2 -4 TEAEs (37% vs 9%) and d/c for AEs (10% vs 3%) for ATV/RTV arm vs fostemsavir arm[2] � Phase III trial of fostemsavir in heavily treatment–experienced pts ongoing[3] Day 8* Pts with HIV-1 RNA ≥ 400 c/m. L on current regimen; ≤ 2 classes of active approved ARVs left to compose OBR due to resistance, intolerance, or contraindications As above but with no active approved ARV options remaining Wk 49 Fostemsavir + OBR Placebo Fostemsavir + OBR Fostemsavir *1° endpoint: mean log 10 HIV RNA change from BL. 1. Granados-Reyes ER, et al. HIV Glasgow 2016. Abstract O 335 A. 2. Llamoso C, et al. HIV Glasgow 2016. Abstract O 335 B. 3. Clinical. Trials. gov. NCT 02362503. Slide 32 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017

Thank you for your Attention Slide 33 of 37 From Eric S. Daar, MD, in Chicago, Illinois, May 10, 2017
 Investigational product manufacturing
Investigational product manufacturing Antiretroviral
Antiretroviral Antiretroviral
Antiretroviral Karen daar
Karen daar Karen daar
Karen daar Karen daar
Karen daar Bioness integrated therapy system occupational therapy
Bioness integrated therapy system occupational therapy Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Both psychoanalysis and humanistic therapy stress
Both psychoanalysis and humanistic therapy stress Corpus approaches to discourse analysis
Corpus approaches to discourse analysis User support requirements
User support requirements Basic approaches to leadership
Basic approaches to leadership Approaches to message authentication
Approaches to message authentication Humanistic theory carl rogers
Humanistic theory carl rogers Single species approaches to protecting biodiversity
Single species approaches to protecting biodiversity Approach research meaning
Approach research meaning Self-help approach in community development examples
Self-help approach in community development examples What is virtue ethics
What is virtue ethics General pricing approaches
General pricing approaches Approaches to the study of comparative politics
Approaches to the study of comparative politics Approaches meets masters
Approaches meets masters Trends shaping hrm
Trends shaping hrm Contoh pendekatan penilaian hasil belajar
Contoh pendekatan penilaian hasil belajar A ray of light approaches a jar of honey
A ray of light approaches a jar of honey Two approaches in corporate governance
Two approaches in corporate governance Direct/expository approach
Direct/expository approach Activity planning software project management
Activity planning software project management The structural approach
The structural approach Ib approaches to teaching and learning
Ib approaches to teaching and learning A comparison of approaches to large-scale data analysis
A comparison of approaches to large-scale data analysis Theory x and theory y
Theory x and theory y Applying critical approaches to literary analysis
Applying critical approaches to literary analysis Marketing research approaches to demand estimation
Marketing research approaches to demand estimation Approaches to formulation of accounting theory
Approaches to formulation of accounting theory