Future Directions Investigational Approaches to Antiretroviral Therapy Joseph














- Slides: 35

Future Directions: Investigational Approaches to Antiretroviral Therapy Joseph J. Eron, Jr, MD Professor of Medicine and Epidemiology The University of North Carolina at Chapel Hill, North Carolina Washington, DC: August 24, 2016

Slide 2 of 39 Future Directions: Investigational Approaches to Antiretroviral Therapy Joseph J. Eron, Jr, MD Professor of Medicine and Epidemiology UNC Chapel Hill August 2016

Financial Relationships With Commercial Entities �Dr Eron has received research grants awarded to his institution from Abb. Vie, Bristol-Myers Squibb, Merck & Co, Inc, and Vii. V Healthcare. He has served as a consultant to Abb. Vie, Bristol -Myers Squibb, Gilead Sciences, Inc, Glaxo. Smith. Kline, Janssen Therapeutics, and Vii. V Healthcare. (Updated 08/16/16) Slide 3 of 39

Learning Objectives After attending this presentation, participants will be able to: �Describe why new antiretroviral approaches are needed �List mechanisms of action of investigational antiretroviral drugs �Describe how these new drugs and formulations might be incorporated into clinical practice Slide 4 of 39

Slide 5 of 39 Goals of Antiretroviral Therapy • Maintain or restore the health of people living with HIV-1 (PLWHIV) through suppression of HIV-1 replication • Minimize or eliminate short and long-term adverse effects of therapy • Have therapies that are accessible to all PLWHIV • Prevent transmission of HIV-1 to others via any route of exposure

Slide 6 of 39 Published July 20, 2015 at NEJM. org

Why Do We Need New Antiretroviral Agents? • A 25 year old started on therapy today may need treatment for 6 decades! An infected infant – 8 decades • Therapy must be incredibly safe, maximally tolerated and include a range of choices – – – Renal, cardiovascular, liver and bone toxicity Safety of ART in pregnancy Therapy options for infants and children Adherence, life chaos, treatment fatigue, tolerability Aging and drug interactions (e. g. CYP 3 A 4 inhibition) • TREATMENT GAP -Not all PLWHIV in care treated – Stigma, substance use, mental health, access to clinics, transportation, clinic capacity, country stocks, availability of 3 rd line therapy • HIV-1 resistance will always be with us Slide 7 of 39

Slide 8 of 39 Continued Improvement in Currently Available ART Classes • Doravirine (NNRTI) – Fewer side effects (? ) and no food or PPI requirements – Phase III in naïve patients • Raltegravir – Once daily • Bictegravir (GS-9883; integrase inhibitor) – Single tablet, unboosted, TAF-based – Phase III in naïve and switch • Integrase-based two drug therapy – Phase III in switch (DTG or CTG plus RPV) – Phase III in naïve in development (DTG plus 3 TC) • MRK-8591 (e. Fd. A – NRTI) – Long acting oral and injectable (? )

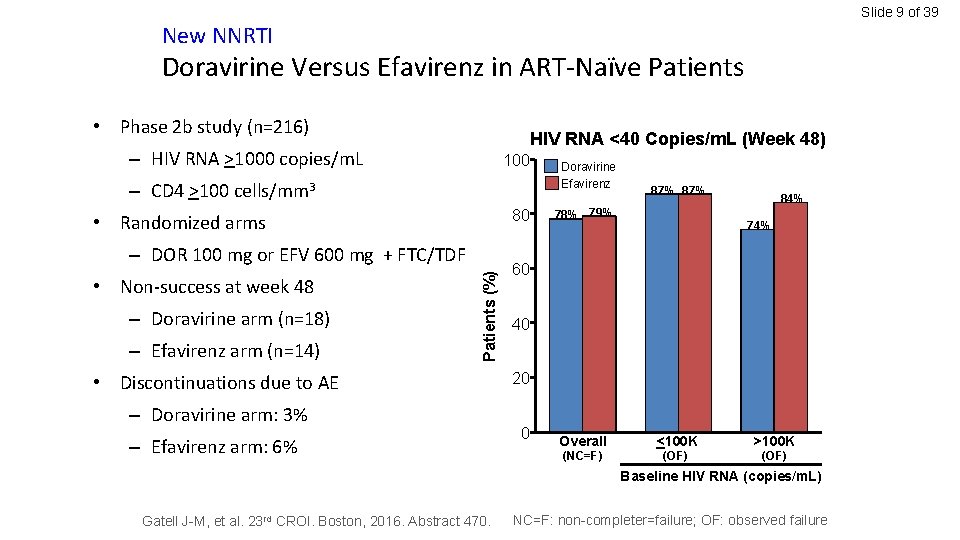
Slide 9 of 39 New NNRTI Doravirine Versus Efavirenz in ART-Naïve Patients • Phase 2 b study (n=216) HIV RNA <40 Copies/m. L (Week 48) – HIV RNA >1000 copies/m. L 100 – CD 4 >100 cells/mm 3 80 • Randomized arms • Non-success at week 48 – Doravirine arm (n=18) – Efavirenz arm (n=14) Patients (%) – DOR 100 mg or EFV 600 mg + FTC/TDF • Discontinuations due to AE – Doravirine arm: 3% – Efavirenz arm: 6% Doravirine Efavirenz 87% 78% 79% 84% 74% 60 40 20 0 Overall (NC=F) <100 K (OF) >100 K (OF) Baseline HIV RNA (copies/m. L) Gatell J-M, et al. 23 rd CROI. Boston, 2016. Abstract 470. NC=F: non-completer=failure; OF: observed failure

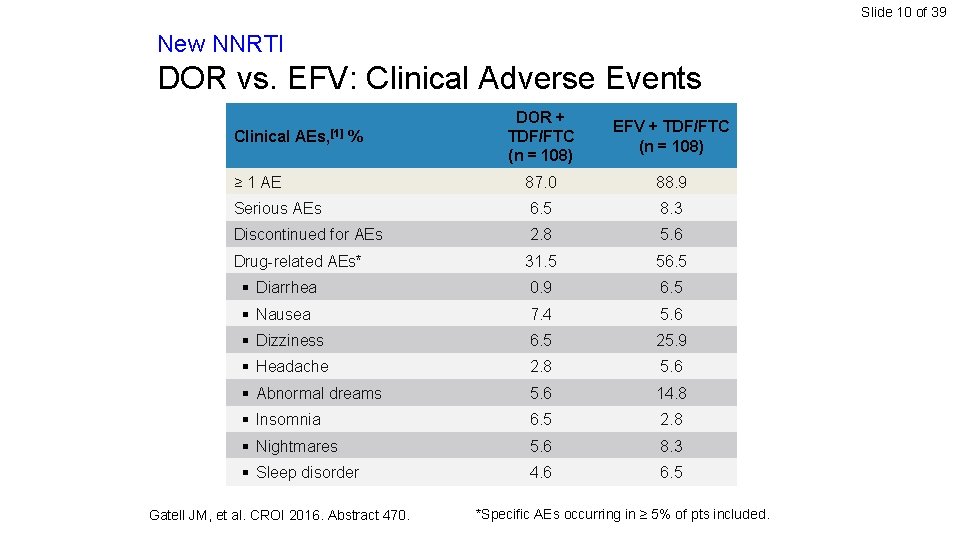
Slide 10 of 39 New NNRTI DOR vs. EFV: Clinical Adverse Events DOR + TDF/FTC (n = 108) EFV + TDF/FTC (n = 108) ≥ 1 AE 87. 0 88. 9 Serious AEs 6. 5 8. 3 Discontinued for AEs 2. 8 5. 6 Drug-related AEs* 31. 5 56. 5 § Diarrhea 0. 9 6. 5 § Nausea 7. 4 5. 6 § Dizziness 6. 5 25. 9 § Headache 2. 8 5. 6 § Abnormal dreams 5. 6 14. 8 § Insomnia 6. 5 2. 8 § Nightmares 5. 6 8. 3 § Sleep disorder 4. 6 6. 5 Clinical AEs, [1] % Gatell JM, et al. CROI 2016. Abstract 470. *Specific AEs occurring in ≥ 5% of pts included.

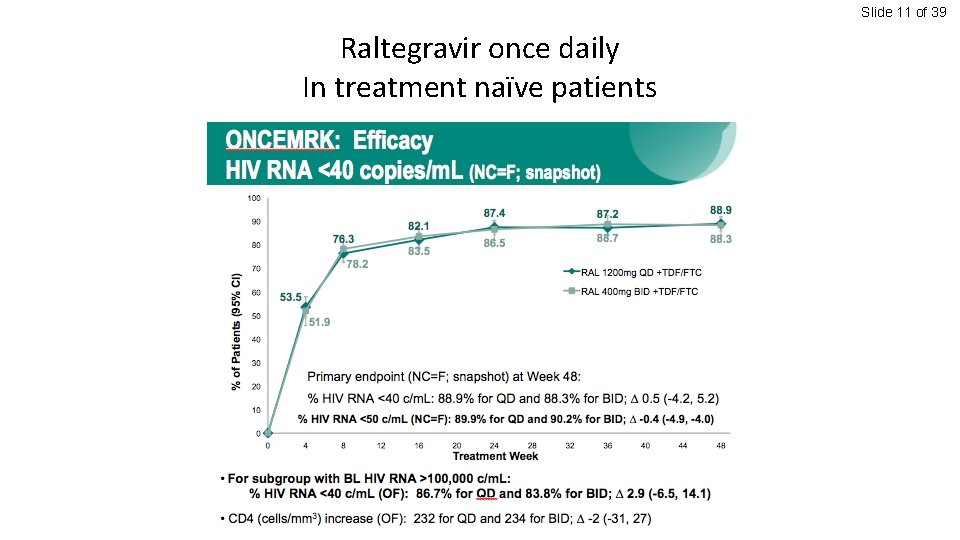
Slide 11 of 39 Raltegravir once daily In treatment naïve patients

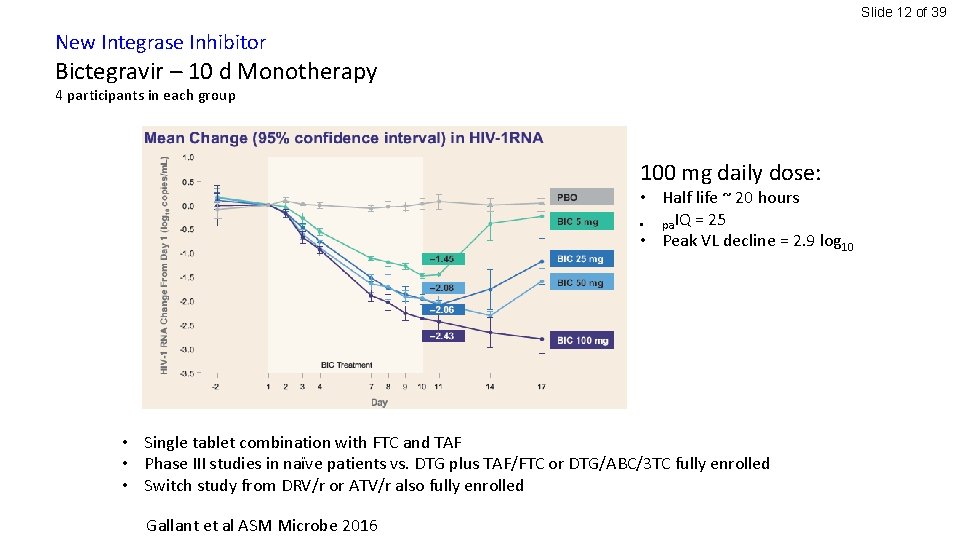
Slide 12 of 39 New Integrase Inhibitor Bictegravir – 10 d Monotherapy 4 participants in each group 100 mg daily dose: • Half life ~ 20 hours • pa. IQ = 25 • Peak VL decline = 2. 9 log 10 • Single tablet combination with FTC and TAF • Phase III studies in naïve patients vs. DTG plus TAF/FTC or DTG/ABC/3 TC fully enrolled • Switch study from DRV/r or ATV/r also fully enrolled Gallant et al ASM Microbe 2016

In. STI based 2 -drug therapy Dolutegravir plus 3 TC 48 week data: PADDLE Study PDVF = HIV RNA 99 (wk 36, 246 retest, 61 wk 48, then BDL) ACTG single arm (N = 120) underway (HIV RNA up to 500, 000) Phase III comparative trial in development Cahn et al WAC Durban 2016 Slide 13 of 39

Slide 14 of 39 Maintaining therapy for Life in all PLWHIV • Adherence – Hard to reach populations, substance use, depression, children, adolescents ……. • Life Chaos – Travel, dislocation for work or safety, surgery, drug interactions, pill fatigue, patient preference …… Long acting antiretroviral Therapy!

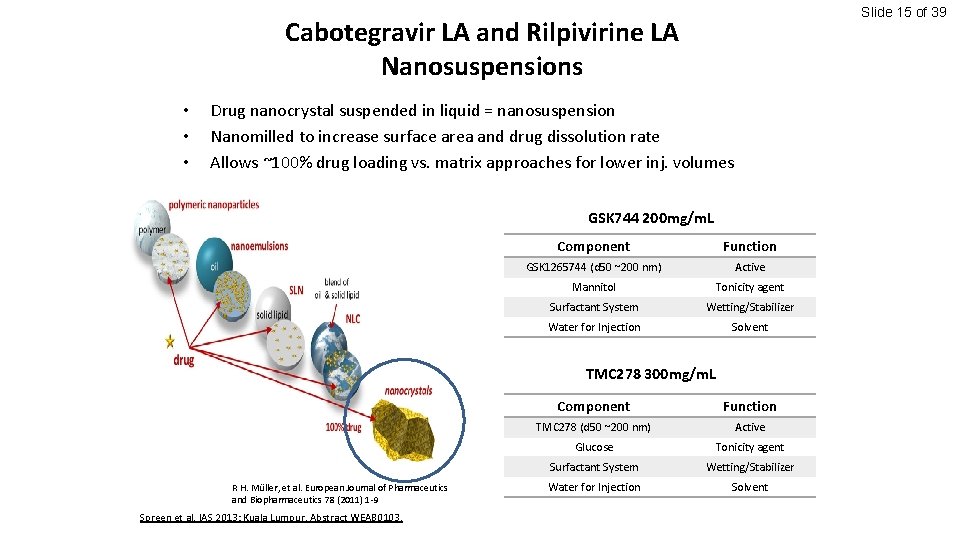
Slide 15 of 39 Cabotegravir LA and Rilpivirine LA Nanosuspensions • • • Drug nanocrystal suspended in liquid = nanosuspension Nanomilled to increase surface area and drug dissolution rate Allows ~100% drug loading vs. matrix approaches for lower inj. volumes GSK 744 200 mg/m. L Component Function GSK 1265744 (d 50 ~200 nm) Active Mannitol Tonicity agent Surfactant System Wetting/Stabilizer Water for Injection Solvent TMC 278 300 mg/m. L R H. Müller, et al. European Journal of Pharmaceutics and Biopharmaceutics 78 (2011) 1 -9 Spreen et al. IAS 2013; Kuala Lumpur. Abstract WEAB 0103. Component Function TMC 278 (d 50 ~200 nm) Active Glucose Tonicity agent Surfactant System Wetting/Stabilizer Water for Injection Solvent

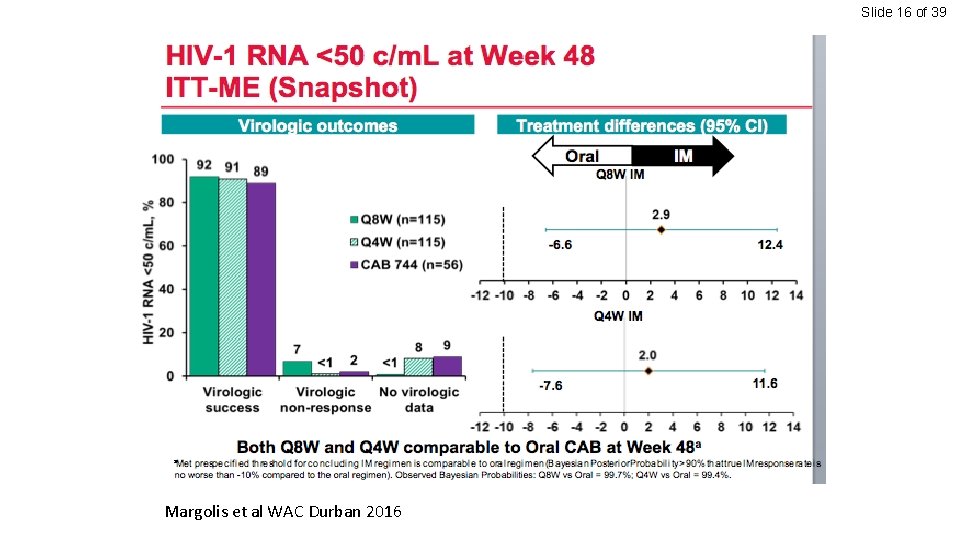
Slide 16 of 39 Margolis et al WAC Durban 2016

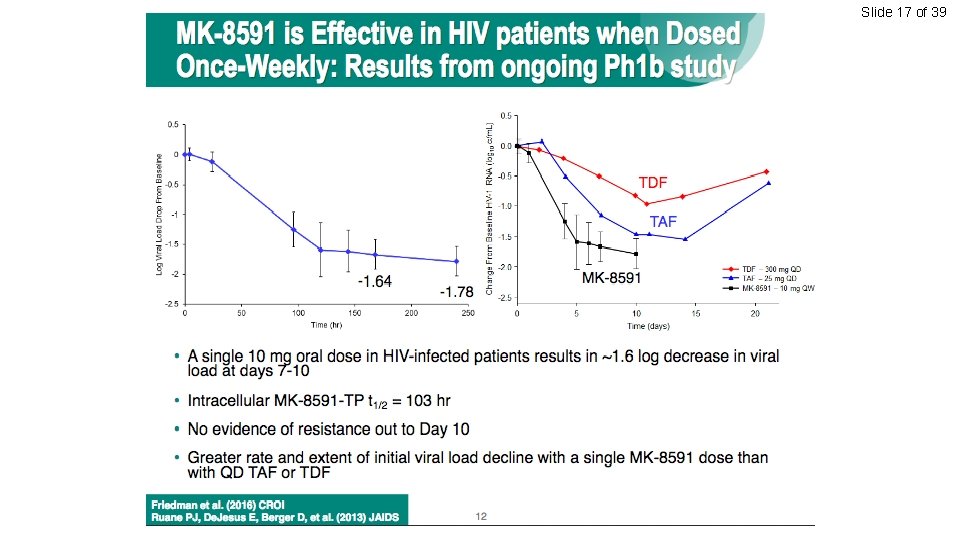
Slide 17 of 39

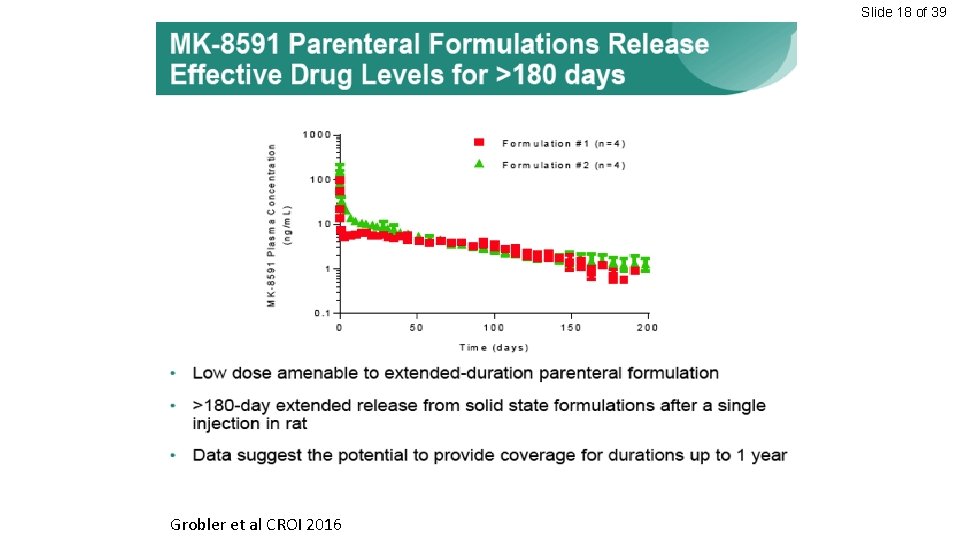
Slide 18 of 39 Grobler et al CROI 2016

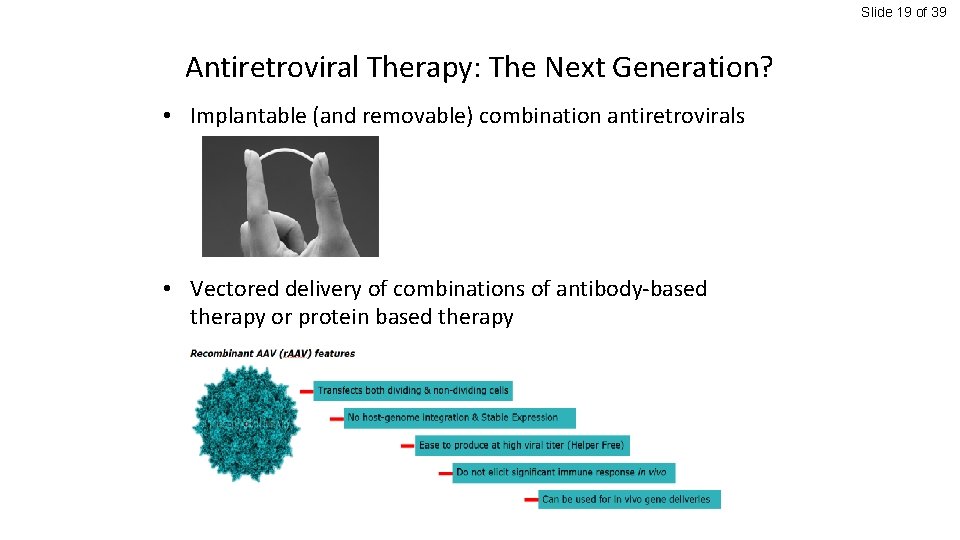
Slide 19 of 39 Antiretroviral Therapy: The Next Generation? • Implantable (and removable) combination antiretrovirals • Vectored delivery of combinations of antibody-based therapy or protein based therapy

Slide 20 of 39 RESISTANT HIV-1 WILL ALWAYS BE WITH US Four to eight decades of therapy! Previous exposure to suboptimal treatment in the developed world Limited monitoring of virologic response world-wide Transmitted drug resistance

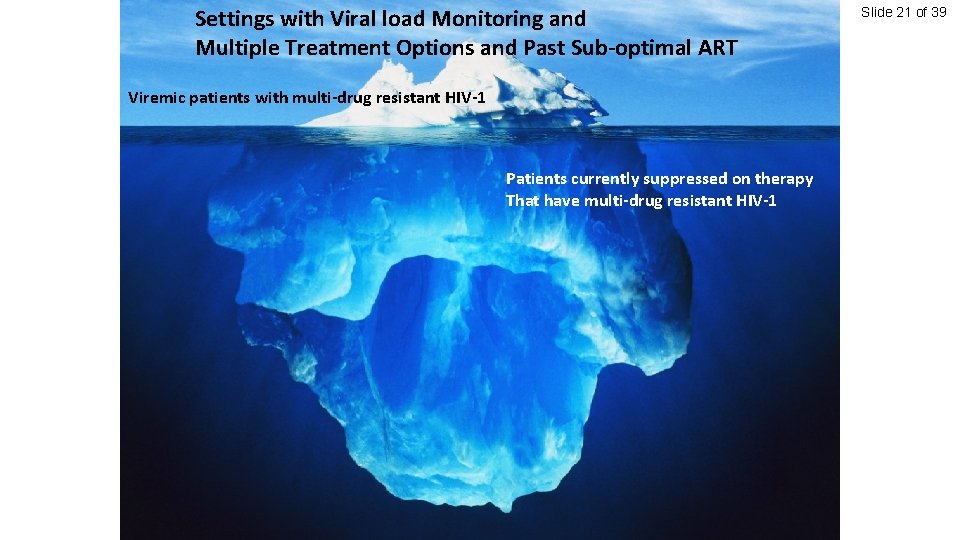
Settings with Viral load Monitoring and Multiple Treatment Options and Past Sub-optimal ART Viremic patients with multi-drug resistant HIV-1 Patients currently suppressed on therapy That have multi-drug resistant HIV-1 Slide 21 of 39

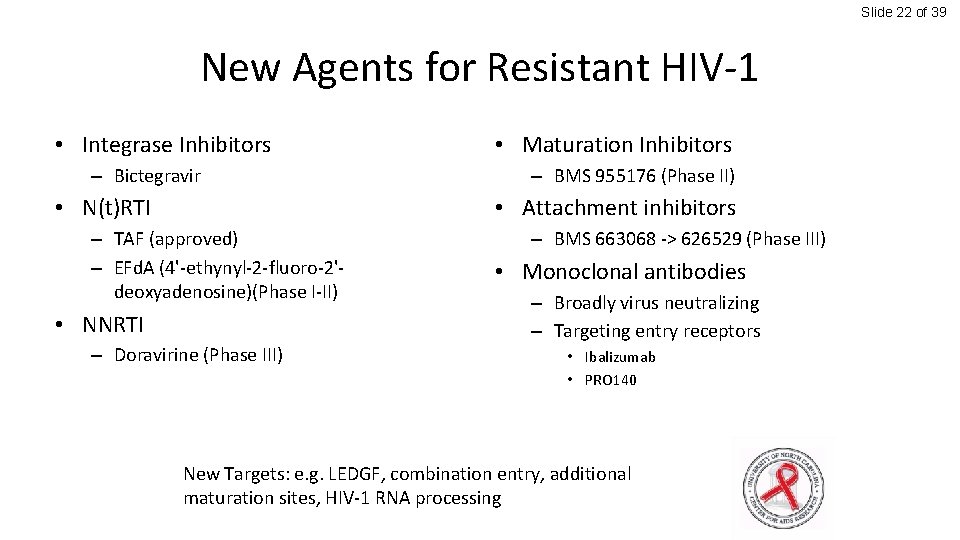
Slide 22 of 39 New Agents for Resistant HIV-1 • Integrase Inhibitors – Bictegravir • N(t)RTI • Maturation Inhibitors – BMS 955176 (Phase II) • Attachment inhibitors – TAF (approved) – EFd. A (4'-ethynyl-2 -fluoro-2'deoxyadenosine)(Phase I-II) • NNRTI – Doravirine (Phase III) – BMS 663068 -> 626529 (Phase III) • Monoclonal antibodies – Broadly virus neutralizing – Targeting entry receptors • Ibalizumab • PRO 140 New Targets: e. g. LEDGF, combination entry, additional maturation sites, HIV-1 RNA processing

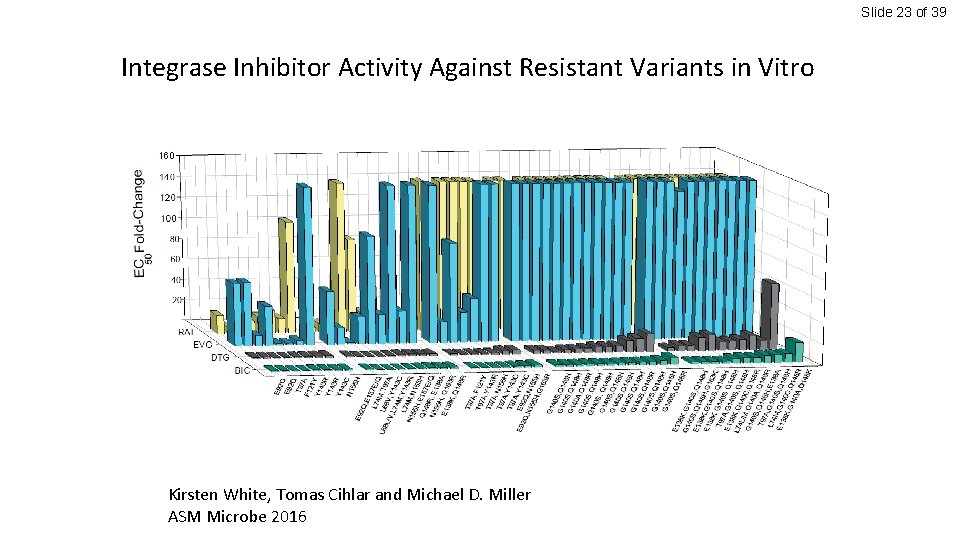
Slide 23 of 39 Integrase Inhibitor Activity Against Resistant Variants in Vitro Kirsten White, Tomas Cihlar and Michael D. Miller ASM Microbe 2016

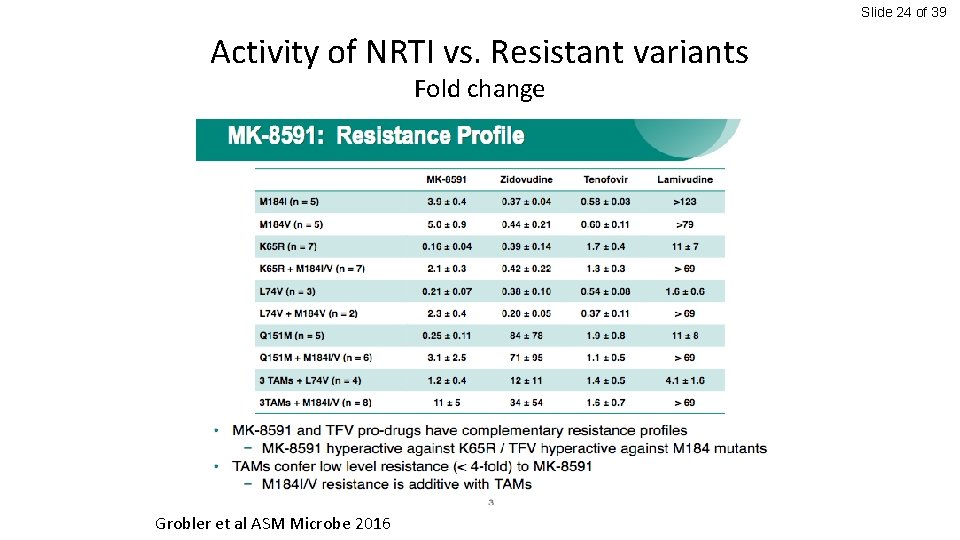
Slide 24 of 39 Activity of NRTI vs. Resistant variants Fold change Grobler et al ASM Microbe 2016

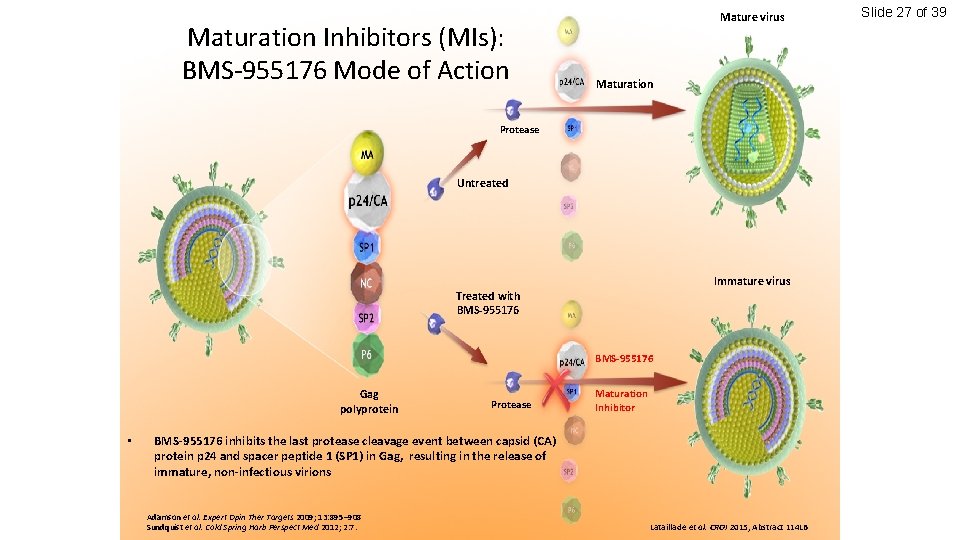
Slide 25 of 39 Maturation Inhibitors (MIs): BMS-955176 Mode of Action Gag polyprotein Lataillade et al. CROI 2015, Abstract 114 LB

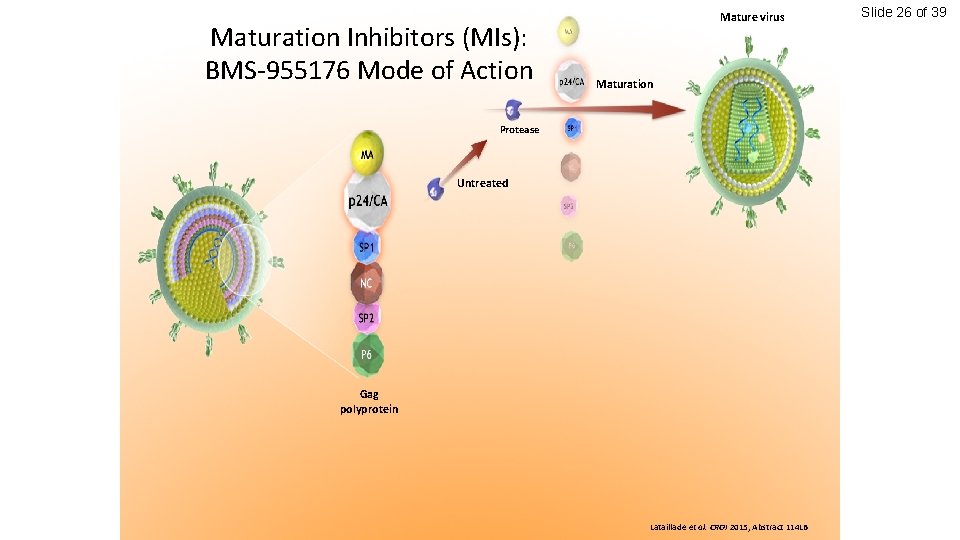
Maturation Inhibitors (MIs): BMS-955176 Mode of Action Mature virus Maturation Protease Untreated Gag polyprotein Lataillade et al. CROI 2015, Abstract 114 LB Slide 26 of 39

Maturation Inhibitors (MIs): BMS-955176 Mode of Action Mature virus Maturation Protease Untreated Immature virus Treated with BMS-955176 Gag polyprotein • Protease Maturation Inhibitor BMS-955176 inhibits the last protease cleavage event between capsid (CA) protein p 24 and spacer peptide 1 (SP 1) in Gag, resulting in the release of immature, non-infectious virions Adamson et al. Expert Opin Ther Targets 2009; 13: 895– 908 Sundquist et al. Cold Spring Harb Perspect Med 2012; 2: 7. Lataillade et al. CROI 2015, Abstract 114 LB Slide 27 of 39

Slide 28 of 39 BMS-955176: Median Change in HIV-1 RNA over Time 1 Median change in HIV-1 RNA from baseline, log 10 copies/m. L Placebo Dosing period 0. 8 0. 6 5 mg 0. 4 0. 2 10 mg 0 -0. 2 20 mg -0. 4 -0. 6 -0. 8 40 mg -1 -1. 2 80 mg -1. 4 -1. 6 Study days 120 mg -1. 8 1 • 2 3 4 5 6 7 8 9 10 11 12 13 14//17//19// 24 25 Median change in HIV-1 RNA from baseline to Day 11 reached ~-1. 4 log 10 c/m. L See Abstract 425, 464 Lataillade et al. CROI 2015, Abstract 114 LB

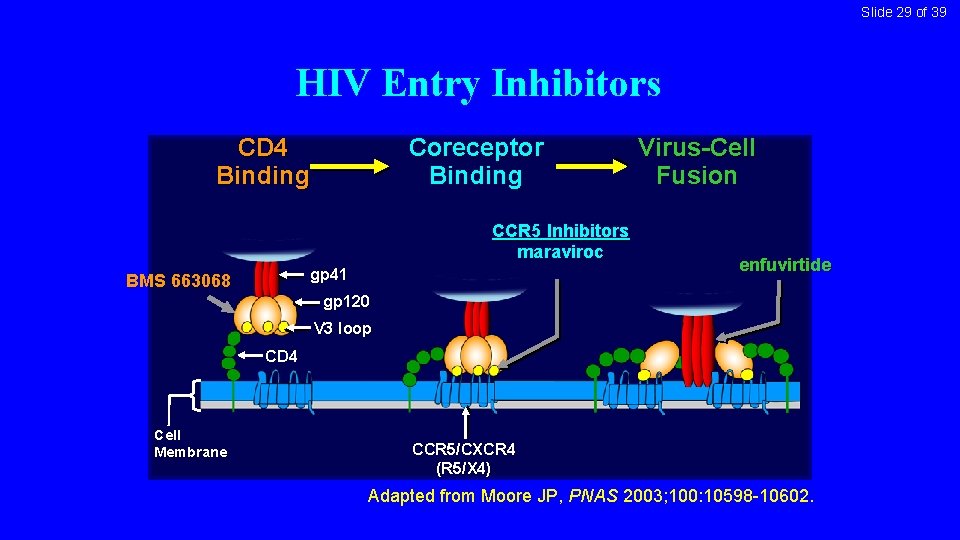
Slide 29 of 39 HIV Entry Inhibitors CD 4 Binding Coreceptor Binding CCR 5 Inhibitors maraviroc gp 41 BMS 663068 Virus-Cell Fusion enfuvirtide gp 120 V 3 loop CD 4 Cell Membrane CCR 5/CXCR 4 (R 5/X 4) Adapted from Moore JP, PNAS 2003; 100: 10598 -10602.

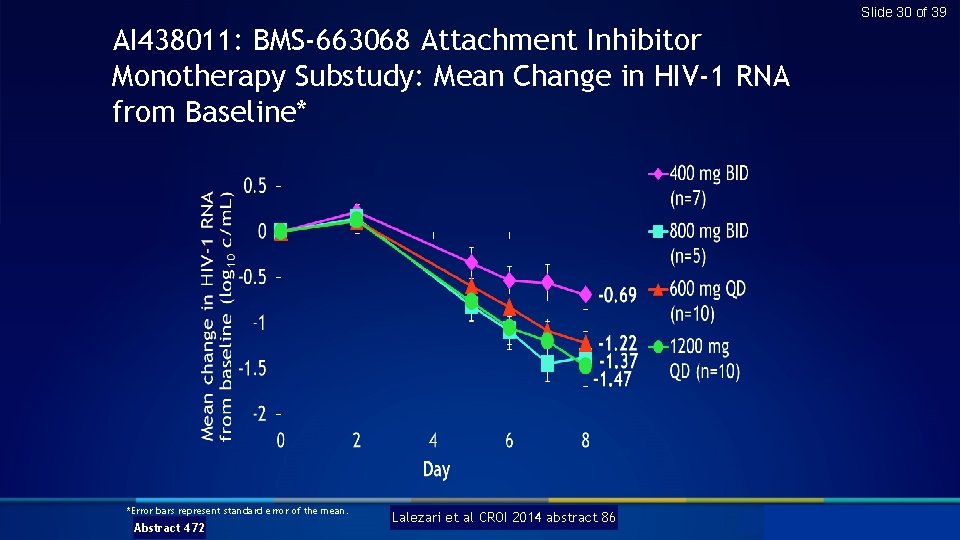
Slide 30 of 39 AI 438011: BMS-663068 Attachment Inhibitor Monotherapy Substudy: Mean Change in HIV-1 RNA from Baseline* *Error bars represent standard error of the mean. Abstract 472 Lalezari et al CROI 2014 abstract 86

Slide 31 of 39 Attachment Inhibitor – Clinical Development BMS-663068 • HIV-1 variants have a range of susceptibility – In Phase IIB study 6% had a BMS-626529 IC 50 >100 n. M at screening • Phase IIB study in participants with limited resistance – Attachment Inhibitor (over a range of doses) plus RAL and TDF had similar activity over 48 weeks to ATV/r plus RAL plus TDF • Phase III study: highly ARV-experienced pts with MDR HIV – If at least one fully active ARV then • BMS-663068 600 mg or placebo BID for 8 days with no change in background ART followed by BMS-663068 600 mg BID for 48 weeks or longer with optimized background – If no fully active ARV then • BMS-663068 600 mg BID for 48 weeks or longer with optimized background therapy

Slide 32 of 39 BROADLY NEUTRALIZING ANTIBODIES Can they be harnessed as therapy?

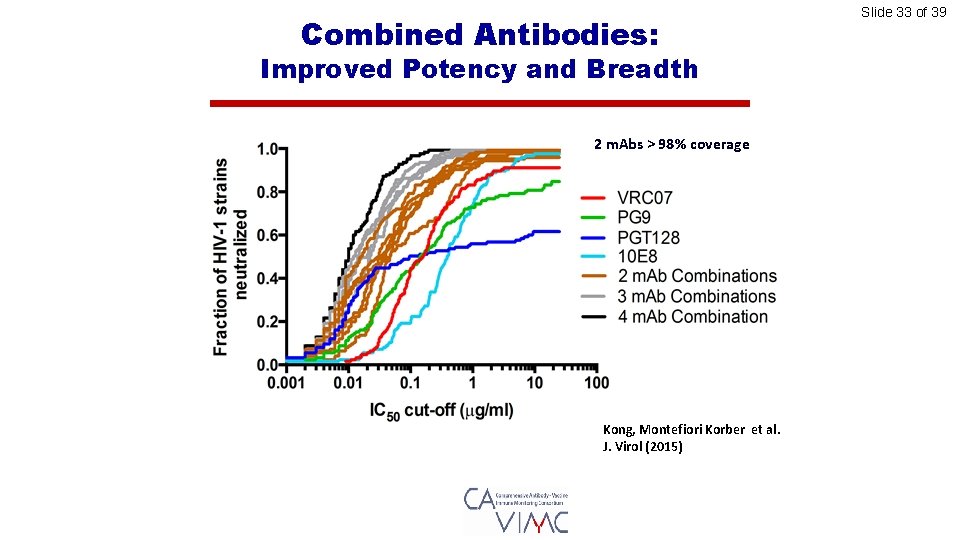
Combined Antibodies: Improved Potency and Breadth 2 m. Abs > 98% coverage Kong, Montefiori Korber et al. J. Virol (2015) Slide 33 of 39

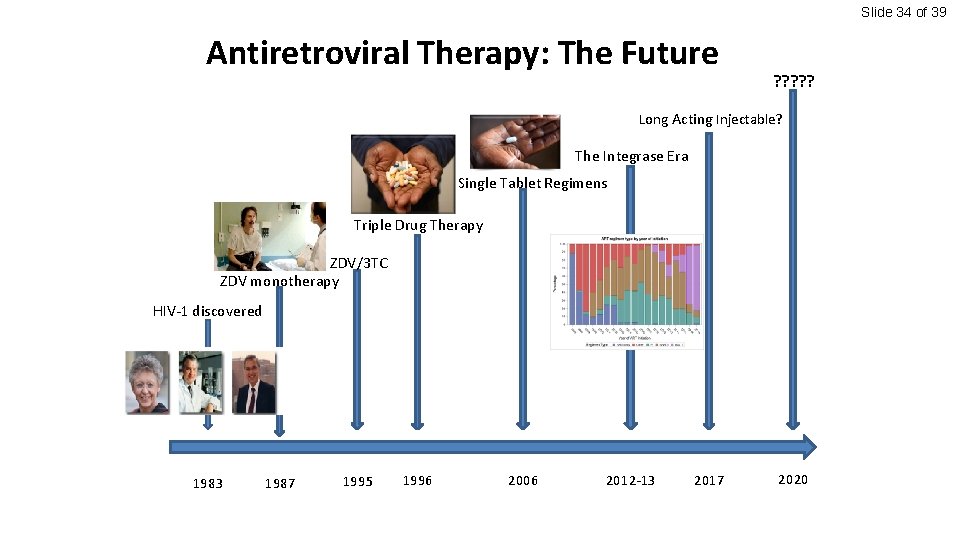
Slide 34 of 39 Antiretroviral Therapy: The Future ? ? ? Long Acting Injectable? The Integrase Era Single Tablet Regimens Triple Drug Therapy ZDV/3 TC ZDV monotherapy HIV-1 discovered 1983 1987 1995 1996 2006 2012 -13 2017 2020

Future Directions: Investigational Approaches to Antiretroviral Therapy Joseph J. Eron, Jr, MD Professor of Medicine and Epidemiology The University of North Carolina at Chapel Hill, North Carolina Washington, DC: August 24, 2016
 Investigational product manufacturing
Investigational product manufacturing Antiretroviral
Antiretroviral Antiretroviral
Antiretroviral Future perfect continuous tense sentences
Future perfect continuous tense sentences Future perfect simple continuous
Future perfect simple continuous Joseph pratt group therapy
Joseph pratt group therapy Joseph pratt group therapy
Joseph pratt group therapy Future directions in erp
Future directions in erp Humanistic therapy aims to
Humanistic therapy aims to Psychodynamic and humanistic therapies have in common
Psychodynamic and humanistic therapies have in common Bioness integrated therapy system price
Bioness integrated therapy system price Future perfect future continuous exercises
Future perfect future continuous exercises Future plans and finished future actions
Future plans and finished future actions What is past future continuous tense
What is past future continuous tense Future nurse programme
Future nurse programme Future perfect x
Future perfect x Future perfect presentation
Future perfect presentation Future plan present continuous
Future plan present continuous Nulti i prvi kondicional
Nulti i prvi kondicional Present and past tense
Present and past tense Tense
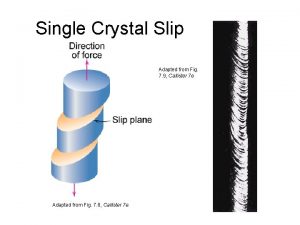
Tense Slip in single crystals
Slip in single crystals Turnbull direction
Turnbull direction New directions hse
New directions hse A straight path that goes without end in both directions
A straight path that goes without end in both directions Mad gab examples
Mad gab examples Geography skills handbook
Geography skills handbook New directions for institutional research
New directions for institutional research System architecture directions for networked sensors
System architecture directions for networked sensors But these aren't cheap labour
But these aren't cheap labour Four directions teachings
Four directions teachings Intermediate directions
Intermediate directions Angular movement
Angular movement Directions
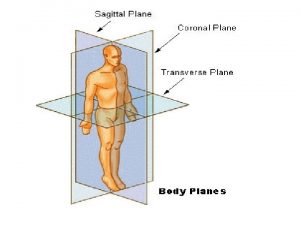
Directions Directional terms anatomy
Directional terms anatomy What directions do latitude lines run
What directions do latitude lines run