Introductory Presentation Middlesex County Dental Association March 20




















- Slides: 24

Introductory Presentation Middlesex County Dental Association March 20, 2007

What is the PEARL Network? PEARL Network Background: • Practice Based Research Network (PBRN) • NIDCR awarded three 7 -yr grants to establish dental PBRNs to research “everyday” issues in the practice and delivery of oral health care. • This is the first time that the NIH has allocated funding to clinical research that directly involves dental practitioners from study concept to study completion.

PEARL NETWORK Practitioners Engaged in Applied Research and Learning

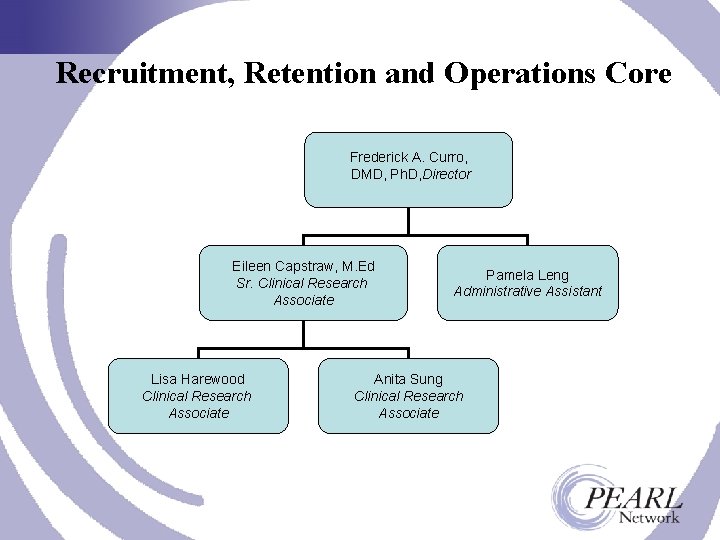
Recruitment, Retention and Operations Core Frederick A. Curro, DMD, Ph. D, Director Eileen Capstraw, M. Ed Sr. Clinical Research Associate Lisa Harewood Clinical Research Associate Pamela Leng Administrative Assistant Anita Sung Clinical Research Associate

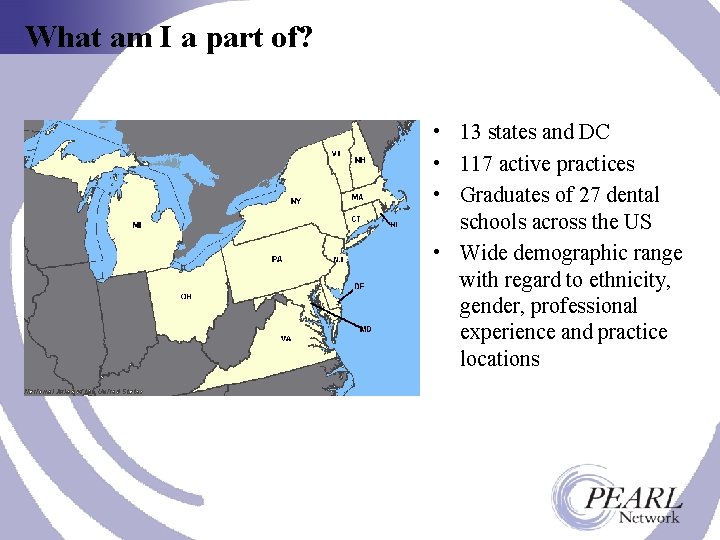
What am I a part of? • 13 states and DC • 117 active practices • Graduates of 27 dental schools across the US • Wide demographic range with regard to ethnicity, gender, professional experience and practice locations

What are the aims of the PEARL Network? 1. 2. 3. 4. 5. 6. Bridge gap between practicing dentists and academia; Recruit and maintain a minimum of 100 dental practices; Train P-Is and staff in clinical research methods; Solicit your ideas for research; Oversee and facilitate the conduct of studies; Disseminate findings to network participants, the dental profession, and the public at large to improve oral care 7. Host an Annual Research Meeting where results will be presented, new research ideas will be solicited and feedback obtained.

What are the intended benefits of the PEARL Network? 1. Be part of a unique dental fellowship that has high visibility and impact on the dental profession; 2. Deliver research-based solutions for the day-to-day problems confronted in your practice; 3. Develop new skills; 4. Earn free NYU Continuing Education (CE) Credits; 5. Attend the PEARL Network Annual Meeting; 6. Have the opportunity to publish research findings; 7. Have the opportunity to present research findings at national conferences.

Clinical Research Associate (CRA) GOAL: • To seamlessly integrate the research process into your office routine ROLE: • To provide clinical training and support to the research sites • To conduct protocol implementation and monitoring activities such as study initiation and close-out visits; source document verification and query resolutions, etc.

Responsibilities of the Practitioner-Investigator (P-I) • Is ultimately responsible for all clinical research conducted at the site • Works closely with the Practice Research Coordinator (PRC) to ensure accuracy of data entry and maintenance of Good Clinical Practice • Completes the NIH-HST and GCP tutorials on the PEARL Network website

Responsibilities of the Practice Research Coordinator (PRC) • Completes HST and GCP tutorials on the PEARL Network website • Enters study data through Electronic Data Capture (EDC) • Recruits study patients and completes the informed consent process with them • Participates in study monitoring process • Maintains GCP w/the P-I at the site

Definition • Good Clinical Practice: – A standard for the design, conduct, performance, monitoring, auditing, recording, analyses, and reporting of clinical trials that provides assurance that the data and reported results are credible and accurate, and that the rights, integrity, and confidentiality of study subjects are protected.

Your Involvement is key to the success of the PEARL Network! • The network will conduct a minimum of 16 studies over a period of seven years – Prospective observational studies – clinical trials – survey studies • Standard-of-Care studies – studies that compare the effectiveness of : • different oral health treatments, • preventive regimens and dental materials;

PRL 0602 POST-OPERATIVE HYPERSENSITIVITY (POH) IN OCCLUSAL RESTORATIONS

Protocol Review Purpose of study: • • To determine the incidence and severity of POH in patients with Class I RBC restorations measure associations between POH and: - dentin caries activity - lining and bonding materials - restorative materials and techniques - RBC restoration treatment and patientperceived Quality of Life (QOL)

PRL 0603 Trans-PBRN Case-Control Study of Osteonecrosis of the Jaw

Protocol Review Purpose of study: • • To investigate whether treatment with biophosphonates (BP) is a risk factor for osteonecrosis of the jaw (ONJ). To investigate whether dental diseases, particularly periodontal disease, and invasive dental procedures are true risk factors for ONJ, or whether these procedures are a consequence of the disease process.

PRL 0604 COMPLETE VS. PARTIAL REMOVAL OF DEEP CARIES: A COMPARISON STUDY OF TREATMENT OUTCOMES

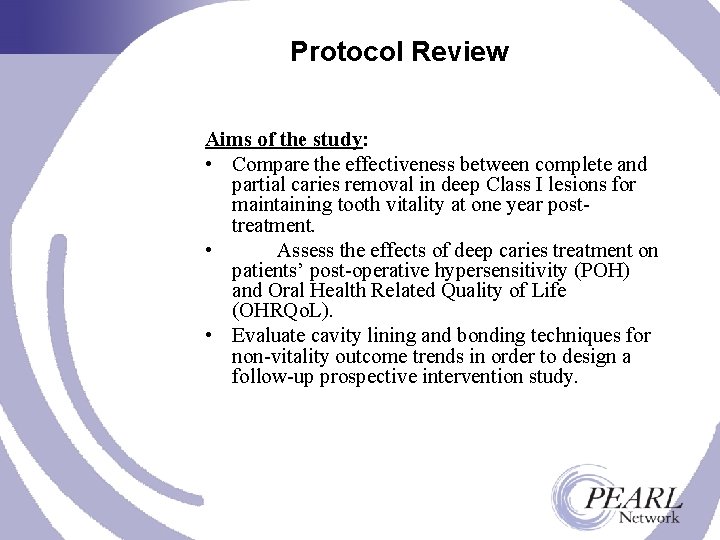
Protocol Review Aims of the study: • Compare the effectiveness between complete and partial caries removal in deep Class I lesions for maintaining tooth vitality at one year posttreatment. • Assess the effects of deep caries treatment on patients’ post-operative hypersensitivity (POH) and Oral Health Related Quality of Life (OHRQo. L). • Evaluate cavity lining and bonding techniques for non-vitality outcome trends in order to design a follow-up prospective intervention study.

PRL 0705 Outcomes for Endodontic Treatment and Restoration of Teeth in General Dental Practice

Protocol Review Aims of the study: • • • Evaluate three to five (3 -5) year outcomes of endodontic treatment and restoration of teeth in the general practice setting. Evaluate the risk factors associated with failure following endodontic treatment and restoration. Assess the effects of endodontic treatment and restoration on patients’ post-treatment hypersensitivity Quality of Life (OHRQo. L) three to five years after treatment.

Study Contact Information FOR ALL CLINICAL QUESTIONS : Director, Recruitment, Retention and Operations Core (RROC) Frederick A. Curro, DMD, Ph. D New York University College of Dentistry 421 First Avenue, Room 219 W, MC 9447 New York, NY 10010 -4086 fac 3@nyu. edu (212) 998 -9555

Study Contact Information FOR NON-CLINICAL ISSUES: Clinical Research Associates: Eileen Capstraw emc 7@nyu. edu 212 -998 -9636 Lisa Harewood lh 60@nyu. edu 212 -998 -9634 Anita Sung asung@nyu. edu 212 -998 -9459 24 Hour Help Desk (646) 265 -5405

