INTRODUCTION TO ORGANIC COMPOUNDS Introduction to Organic Compounds
- Slides: 15

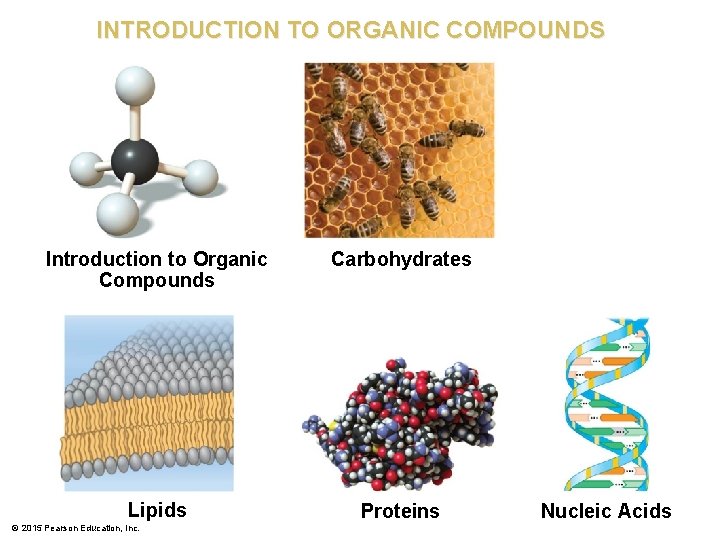
INTRODUCTION TO ORGANIC COMPOUNDS Introduction to Organic Compounds Carbohydrates Lipids Proteins © 2015 Pearson Education, Inc. Nucleic Acids

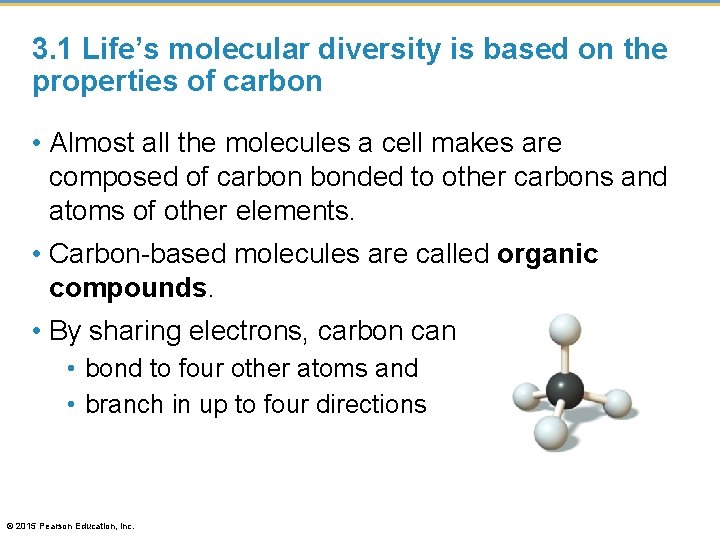
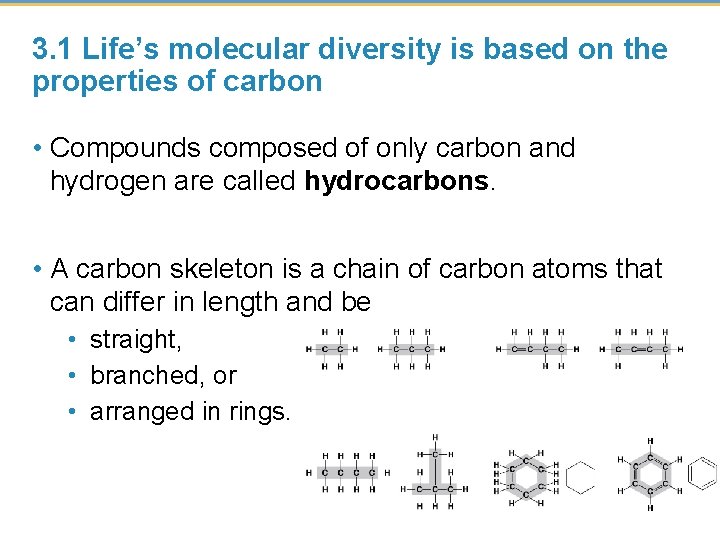
3. 1 Life’s molecular diversity is based on the properties of carbon • Almost all the molecules a cell makes are composed of carbon bonded to other carbons and atoms of other elements. • Carbon-based molecules are called organic compounds. • By sharing electrons, carbon can • bond to four other atoms and • branch in up to four directions © 2015 Pearson Education, Inc.

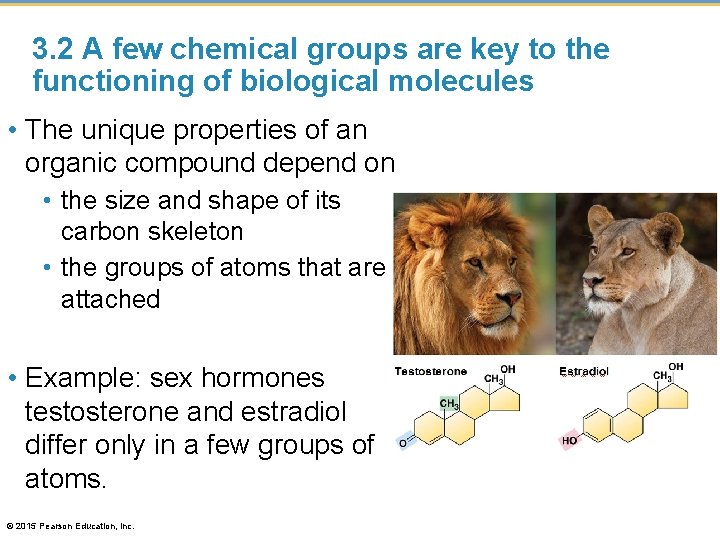
3. 2 A few chemical groups are key to the functioning of biological molecules • The unique properties of an organic compound depend on • the size and shape of its carbon skeleton • the groups of atoms that are attached • Example: sex hormones testosterone and estradiol differ only in a few groups of atoms. © 2015 Pearson Education, Inc.

3. 1 Life’s molecular diversity is based on the properties of carbon • Compounds composed of only carbon and hydrogen are called hydrocarbons. • A carbon skeleton is a chain of carbon atoms that can differ in length and be • straight, • branched, or • arranged in rings.

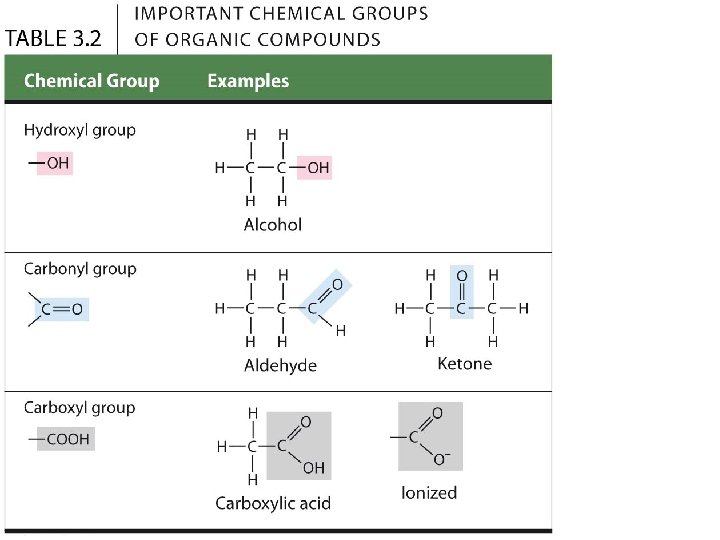
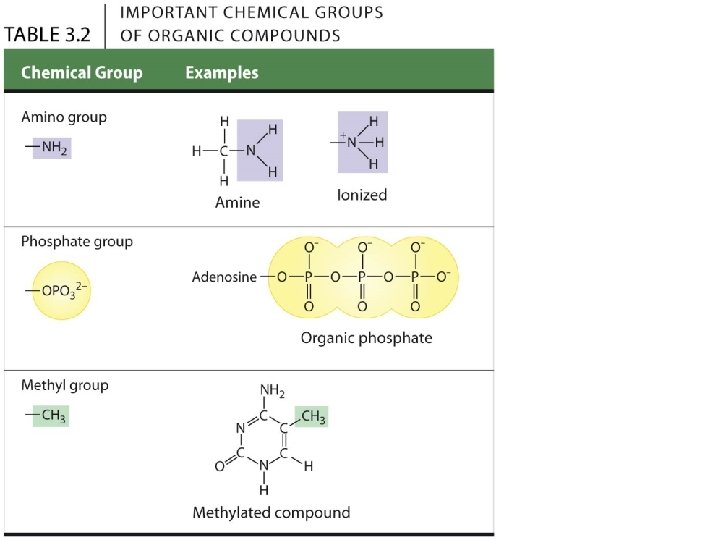
3. 2 A few chemical groups are key to the functioning of biological molecules • There are six chemical groups important in the chemistry of life. • Groups 1 -5: Functional groups • Polar - so compounds containing them are typically hydrophilic (water-loving) and soluble in water. • Group 6: Methyl group, • Nonpolar and not reactive, but still affects molecular shape and thus function. © 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

© 2015 Pearson Education, Inc.

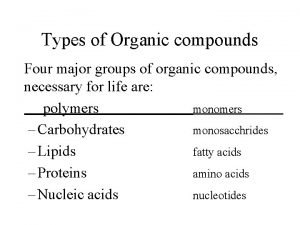
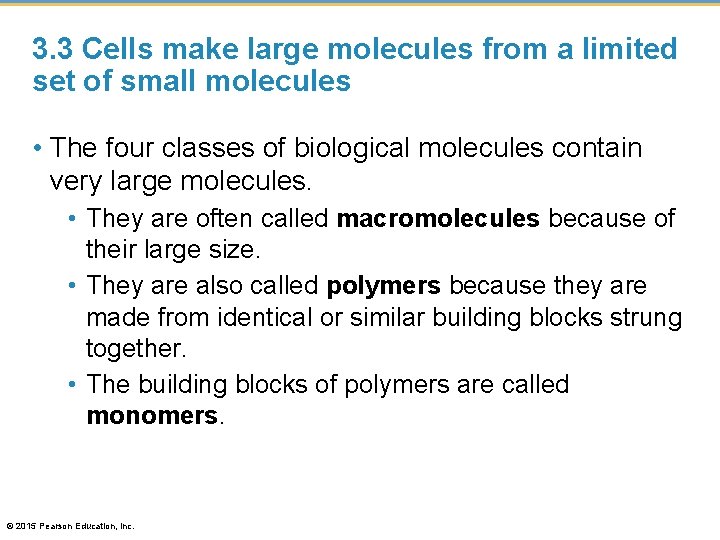
3. 3 Cells make large molecules from a limited set of small molecules • There are four classes of molecules important to organisms: 1. 2. 3. 4. carbohydrates lipids proteins nucleic acids © 2015 Pearson Education, Inc.

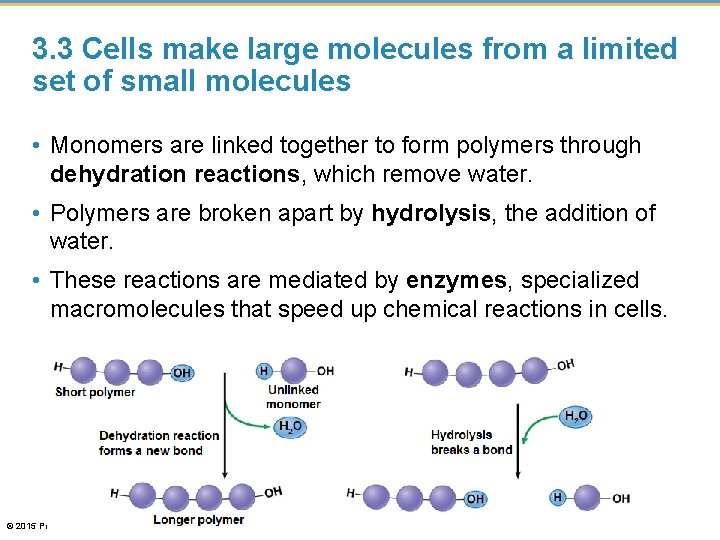
3. 3 Cells make large molecules from a limited set of small molecules • The four classes of biological molecules contain very large molecules. • They are often called macromolecules because of their large size. • They are also called polymers because they are made from identical or similar building blocks strung together. • The building blocks of polymers are called monomers. © 2015 Pearson Education, Inc.

3. 3 Cells make large molecules from a limited set of small molecules • Monomers are linked together to form polymers through dehydration reactions, which remove water. • Polymers are broken apart by hydrolysis, the addition of water. • These reactions are mediated by enzymes, specialized macromolecules that speed up chemical reactions in cells. © 2015 Pearson Education, Inc.

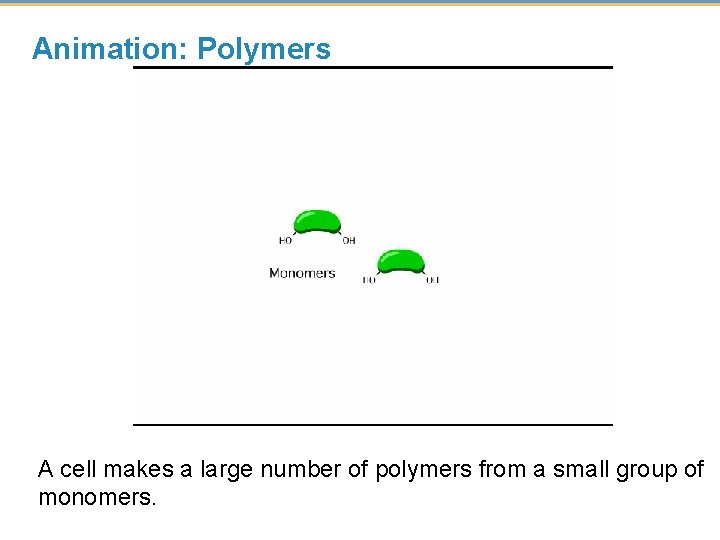
Animation: Polymers A cell makes a large number of polymers from a small group of monomers.

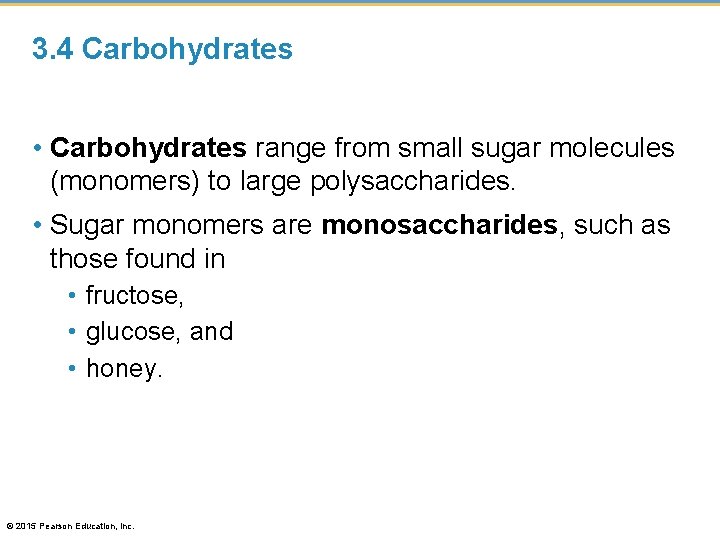
3. 4 Carbohydrates • Carbohydrates range from small sugar molecules (monomers) to large polysaccharides. • Sugar monomers are monosaccharides, such as those found in • fructose, • glucose, and • honey. © 2015 Pearson Education, Inc.

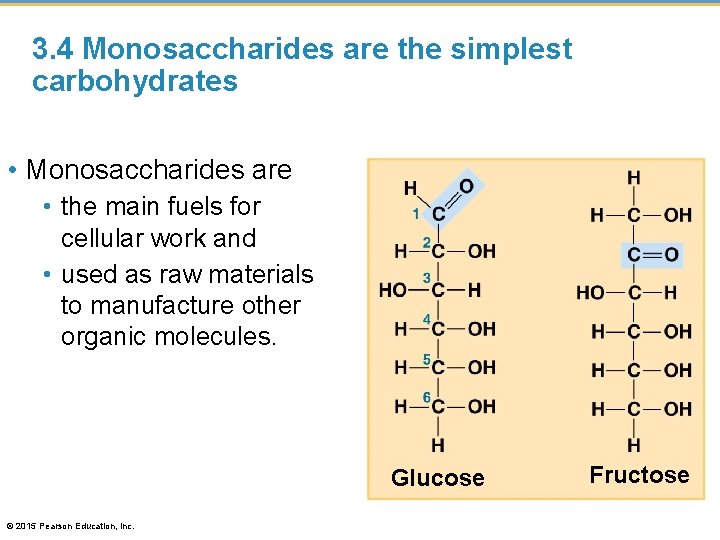
3. 4 Monosaccharides are the simplest carbohydrates • Monosaccharides are • the main fuels for cellular work and • used as raw materials to manufacture other organic molecules. Glucose © 2015 Pearson Education, Inc. Fructose

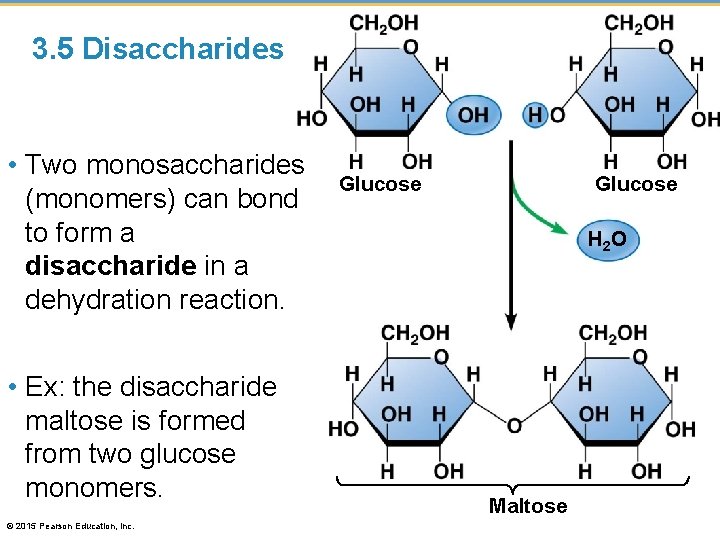
3. 5 Disaccharides • Two monosaccharides (monomers) can bond to form a disaccharide in a dehydration reaction. • Ex: the disaccharide maltose is formed from two glucose monomers. © 2015 Pearson Education, Inc. Glucose H 2 O Maltose

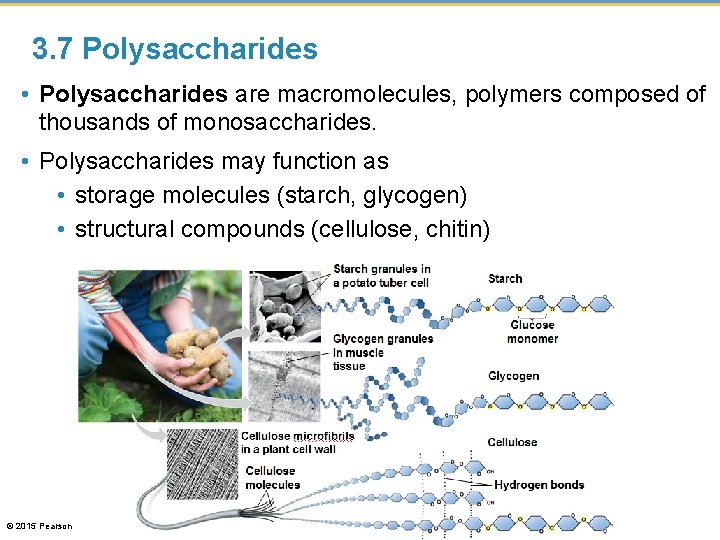
3. 7 Polysaccharides • Polysaccharides are macromolecules, polymers composed of thousands of monosaccharides. • Polysaccharides may function as • storage molecules (starch, glycogen) • structural compounds (cellulose, chitin) © 2015 Pearson Education, Inc.
 Vitamins are tasteless organic compounds
Vitamins are tasteless organic compounds Are vitamins organic compounds
Are vitamins organic compounds Types of organic compound
Types of organic compound Decomposition of organic compounds
Decomposition of organic compounds Charring test of organic and inorganic compounds
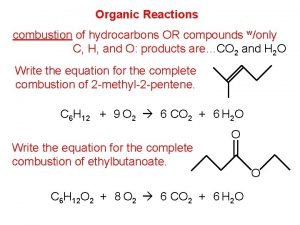
Charring test of organic and inorganic compounds Combustion reaction
Combustion reaction Hagfish slime macromolecule
Hagfish slime macromolecule All organic compounds must contain the element
All organic compounds must contain the element Organic compounds must contain:
Organic compounds must contain: Chemistry nomenclature
Chemistry nomenclature What is organic chemistry
What is organic chemistry Difference between organic and inorganic
Difference between organic and inorganic Purification and characterization of organic compounds
Purification and characterization of organic compounds Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Organic vs inorganic compounds
Organic vs inorganic compounds What is the classification of organic compounds
What is the classification of organic compounds