Introduction to Organic Chemistry and Alkanes Comparison of



- Slides: 13

Introduction to Organic Chemistry and Alkanes

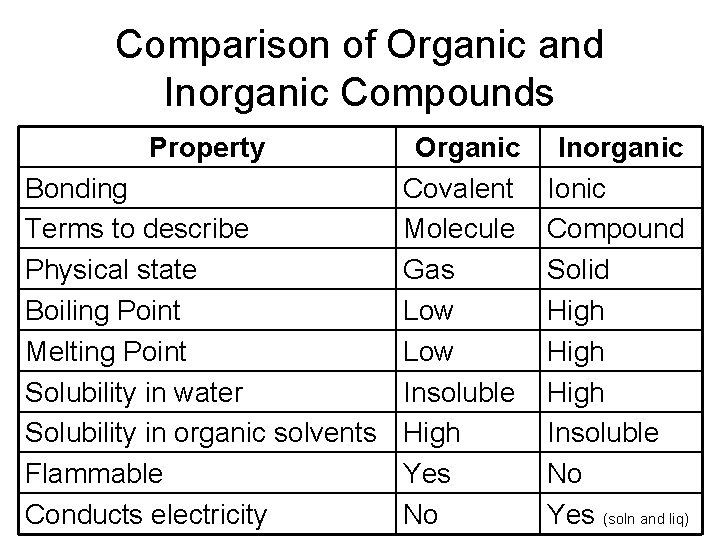
Comparison of Organic and Inorganic Compounds Property Organic Bonding Covalent Terms to describe Molecule Physical state Gas Boiling Point Low Melting Point Low Solubility in water Insoluble Solubility in organic solvents High Flammable Yes Conducts electricity No Inorganic Ionic Compound Solid High Insoluble No Yes (soln and liq)

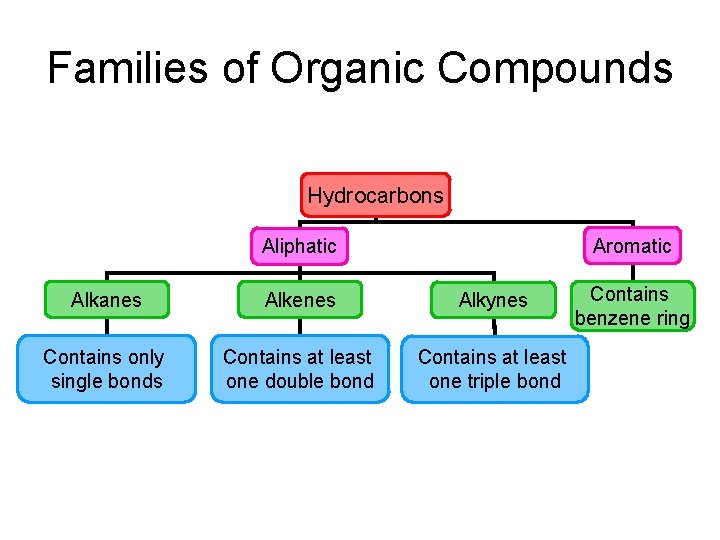
Families of Organic Compounds Hydrocarbons Aromatic Aliphatic Alkanes Alkenes Alkynes Contains only single bonds Contains at least one double bond Contains at least one triple bond Contains benzene ring

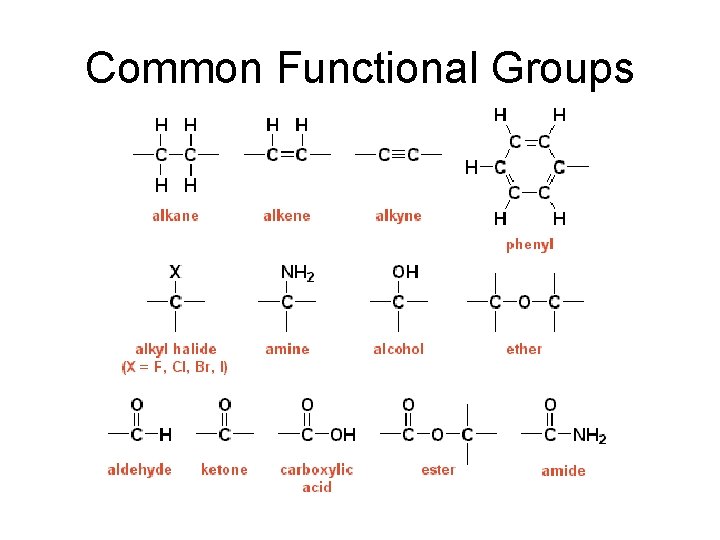
Common Functional Groups

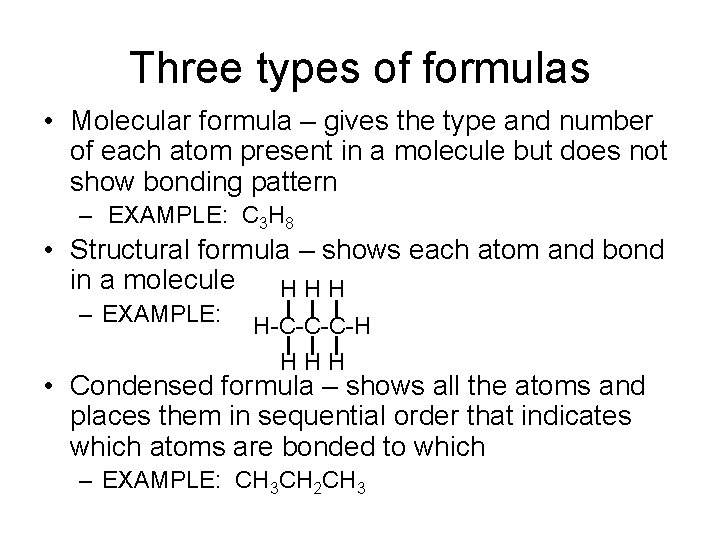
Three types of formulas • Molecular formula – gives the type and number of each atom present in a molecule but does not show bonding pattern – EXAMPLE: C 3 H 8 • Structural formula – shows each atom and bond in a molecule HHH – EXAMPLE: H-C-C-C-H HHH • Condensed formula – shows all the atoms and places them in sequential order that indicates which atoms are bonded to which – EXAMPLE: CH 3 CH 2 CH 3

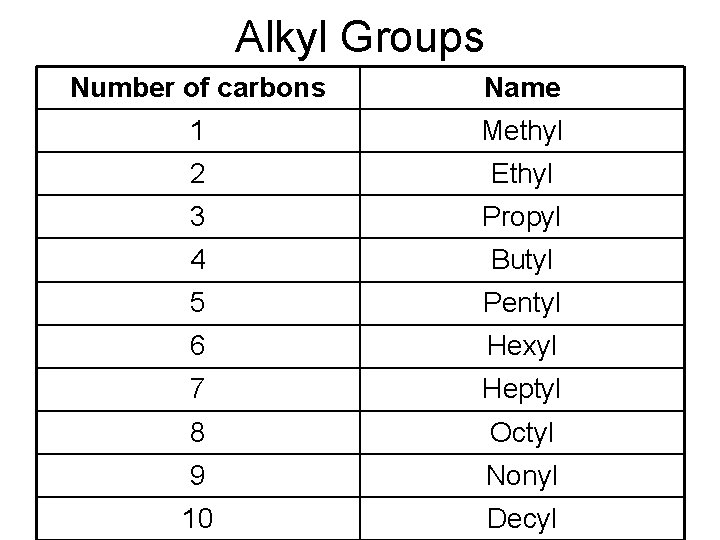
Alkyl Groups Number of carbons Name 1 Methyl 2 Ethyl 3 Propyl 4 Butyl 5 Pentyl 6 Hexyl 7 Heptyl 8 Octyl 9 Nonyl 10 Decyl

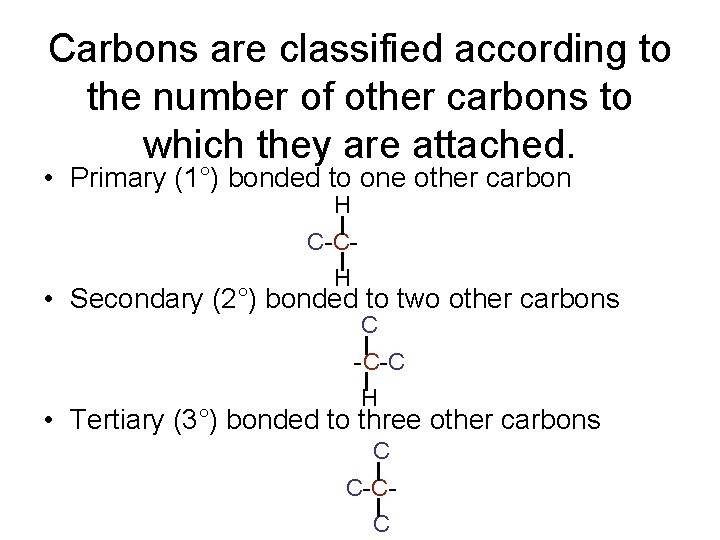
Carbons are classified according to the number of other carbons to which they are attached. • Primary (1°) bonded to one other carbon H C-CH • Secondary (2°) bonded to two other carbons C -C-C H • Tertiary (3°) bonded to three other carbons C C-CC

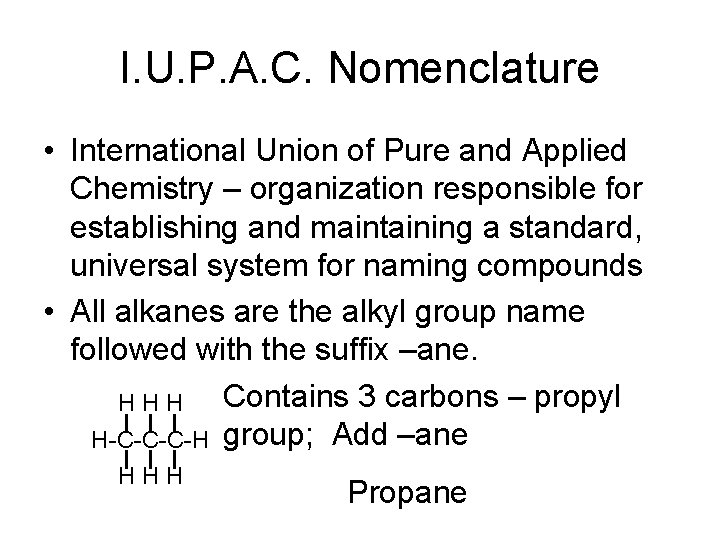
I. U. P. A. C. Nomenclature • International Union of Pure and Applied Chemistry – organization responsible for establishing and maintaining a standard, universal system for naming compounds • All alkanes are the alkyl group name followed with the suffix –ane. Contains 3 carbons – propyl HHH H-C-C-C-H group; Add –ane HHH Propane

Nomenclature of Alkanes 1. Find the parent chain. A. Find the longest continuous chain of carbon atoms present in the molecule, and use the name of that chain as the parent name. B. If two different chains of equal length are present, choose the one with the larger number of branches.

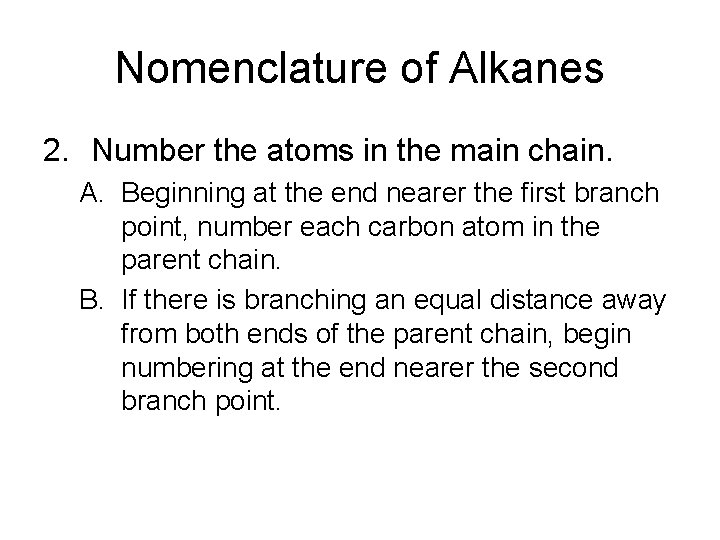
Nomenclature of Alkanes 2. Number the atoms in the main chain. A. Beginning at the end nearer the first branch point, number each carbon atom in the parent chain. B. If there is branching an equal distance away from both ends of the parent chain, begin numbering at the end nearer the second branch point.

Nomenclature of Alkanes 3. Identify and number the substituents. A. Assign a number to each substituent according to its point of attachment to the main chain. B. If there are two substituents on the same carbon, assign them both the same number. There must be as many numbers in the name as there are substituents.

Nomenclature of Alkanes 4. Write the name as a single word, using hyphens to separate the different prefixes and using commas to separate numbers. A. If two or more different substitutents are present, cite them in alphabetical order. B. If two or more identical substituents are present, use one of the prefixes di-, tri-, tetra -, etc but do not use these for alphabetizing purposes.

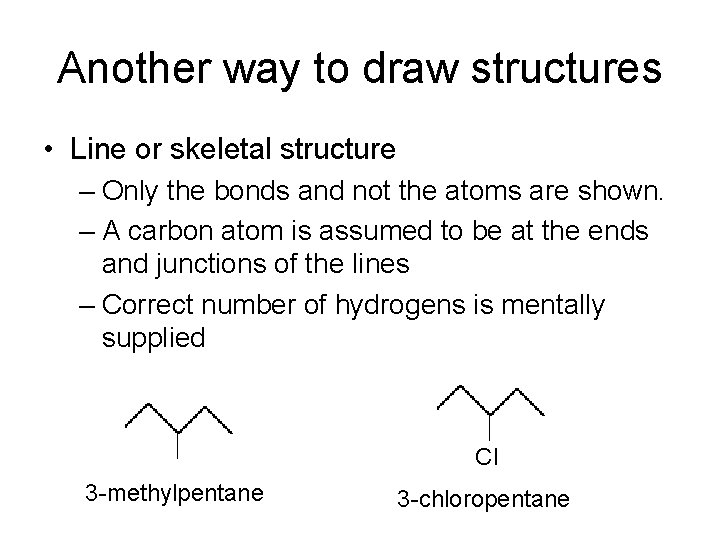
Another way to draw structures • Line or skeletal structure – Only the bonds and not the atoms are shown. – A carbon atom is assumed to be at the ends and junctions of the lines – Correct number of hydrogens is mentally supplied Cl 3 -methylpentane 3 -chloropentane
 Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Organic chemistry introduction
Organic chemistry introduction Ethane
Ethane Saponifiable lipids example
Saponifiable lipids example Octane lewis structure
Octane lewis structure Acetoacetic ester synthesis mechanism
Acetoacetic ester synthesis mechanism Compairson test
Compairson test Alkanes solubility
Alkanes solubility Isomers of pentane
Isomers of pentane Alkane molecular formula
Alkane molecular formula Uses of alkanes
Uses of alkanes Alkanes list
Alkanes list General formula for alkyl group
General formula for alkyl group