INTRINSIC SEMICONDUCTOR q A pure semiconductor q Its

- Slides: 24

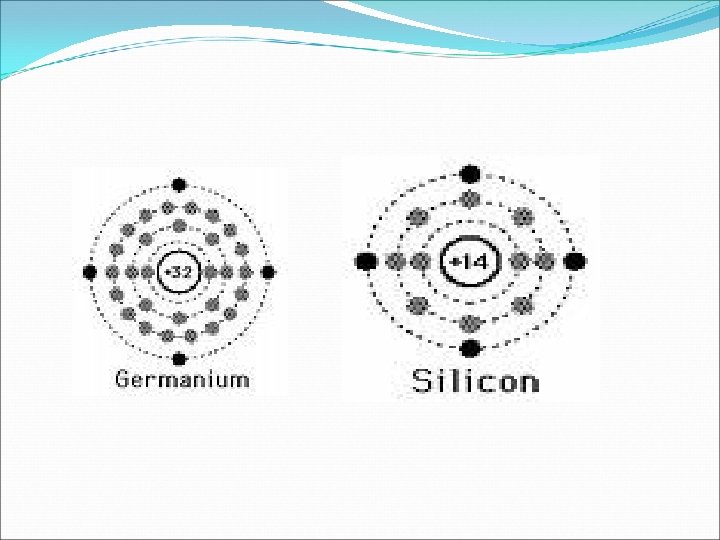
INTRINSIC SEMICONDUCTOR q. A pure semiconductor. q. Its conductivity is low. q. It has thermally generated current carries. q. Examples of pure or intrinsic semiconductor used frequently are germanium and silicon.



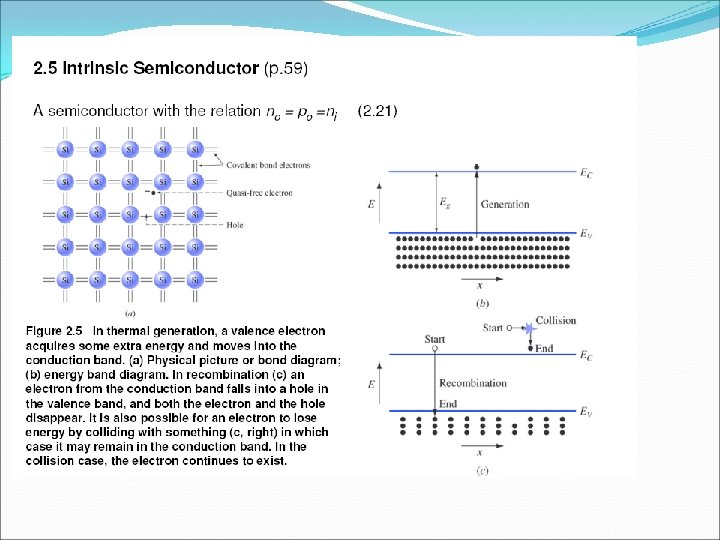
q At 0 K, all the covalent bonds is complete. Therefore, no free electron is available in the crystal for the conduction of current. Hence, silicon crystal behaves as an insulator at 0 K. q At room temperature, a covalent bond breaks, an electron becomes free. The electron which leave the bonds is called free electron and the vacancy created in the covalent bond due to the release of electron is called a hole. q If the potential difference is applied across an intrinsic semiconductor, electrons will moves towards the positive terminal, while the holes will drift toward the negative terminal.


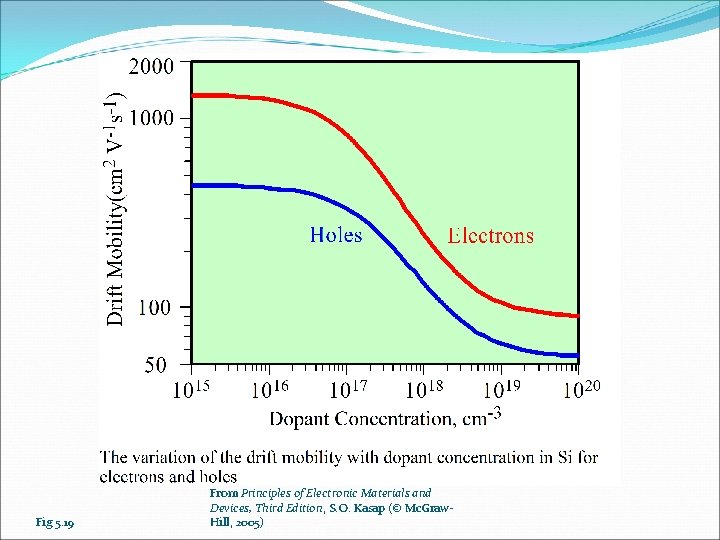
Fig 5. 19 From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw. Hill, 2005)


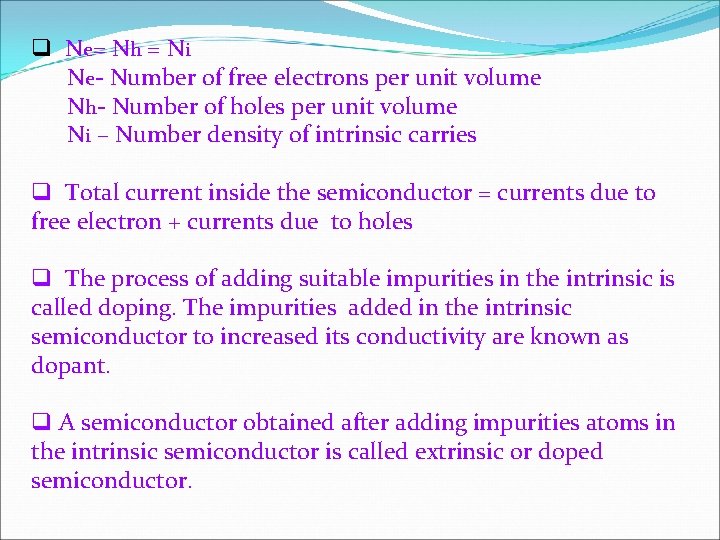
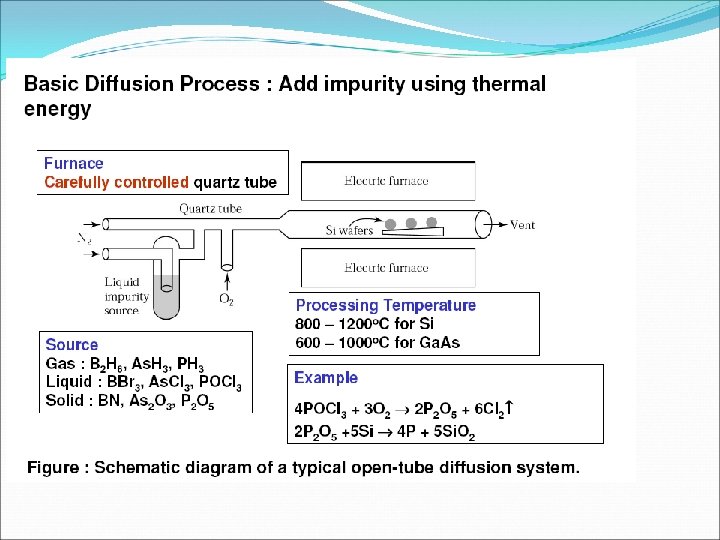
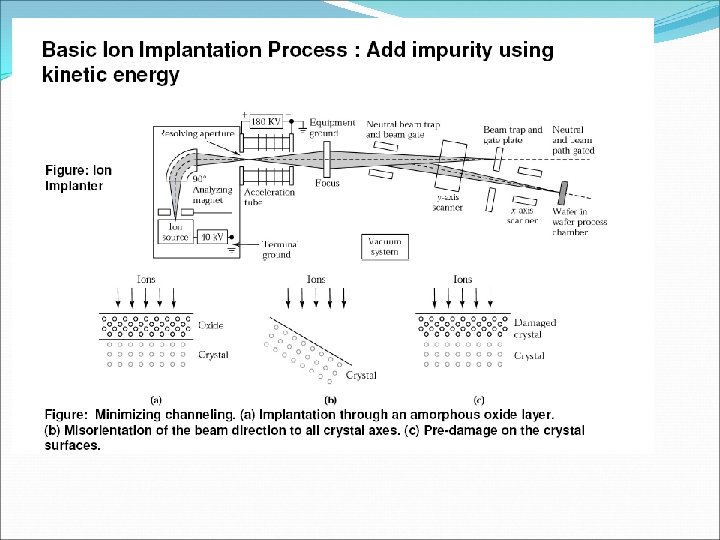
q N e= N h = N i Ne- Number of free electrons per unit volume Nh- Number of holes per unit volume Ni – Number density of intrinsic carries q Total current inside the semiconductor = currents due to free electron + currents due to holes q The process of adding suitable impurities in the intrinsic is called doping. The impurities added in the intrinsic semiconductor to increased its conductivity are known as dopant. q A semiconductor obtained after adding impurities atoms in the intrinsic semiconductor is called extrinsic or doped semiconductor.

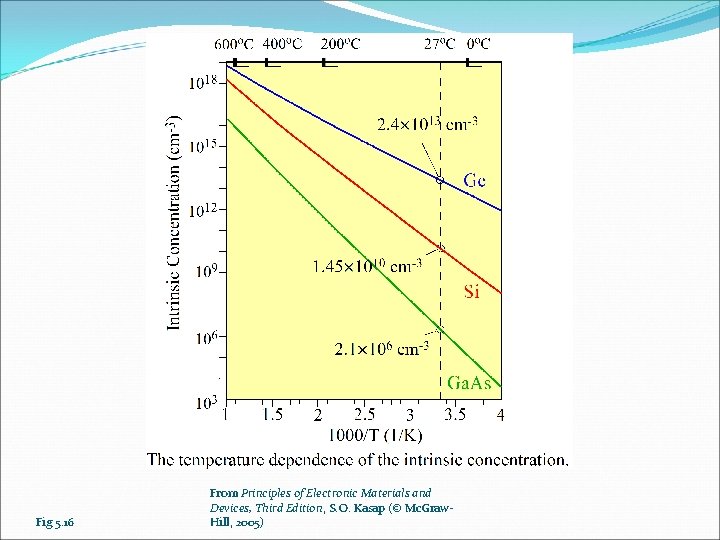
Fig 5. 16 From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw. Hill, 2005)

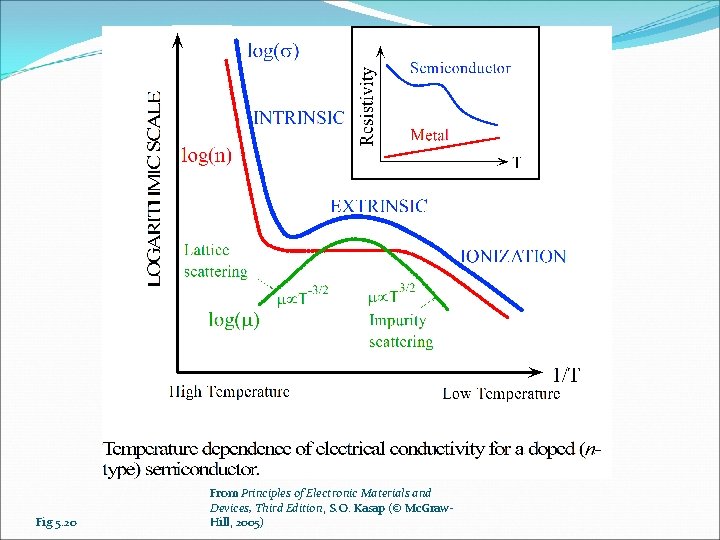
Fig 5. 20 From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw. Hill, 2005)

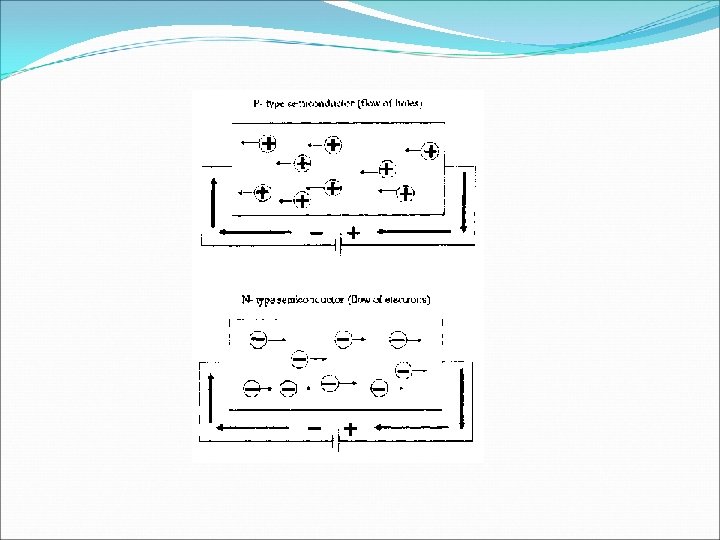
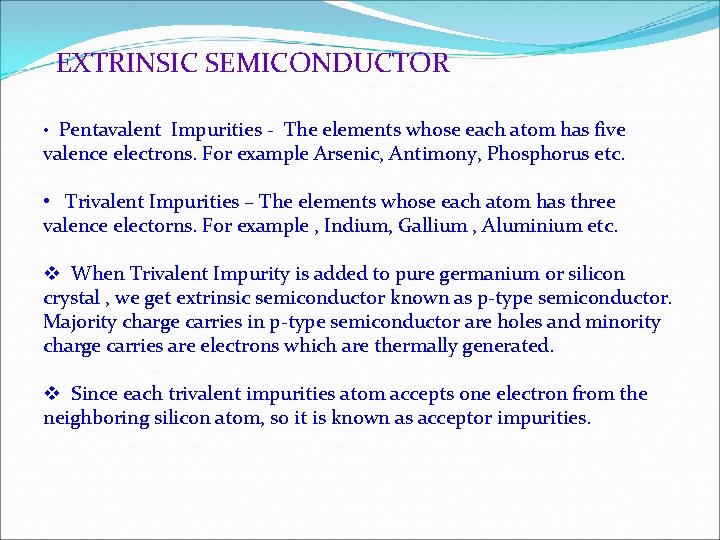
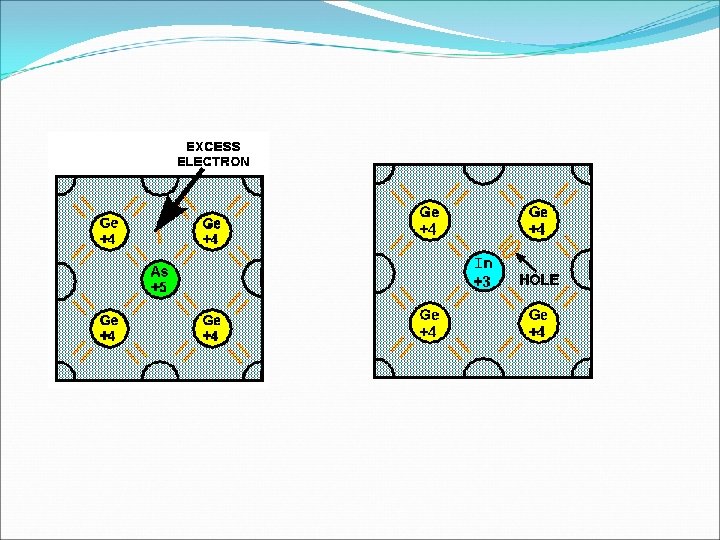
EXTRINSIC SEMICONDUCTOR • Pentavalent Impurities - The elements whose each atom has five valence electrons. For example Arsenic, Antimony, Phosphorus etc. • Trivalent Impurities – The elements whose each atom has three valence electorns. For example , Indium, Gallium , Aluminium etc. v When Trivalent Impurity is added to pure germanium or silicon crystal , we get extrinsic semiconductor known as p-type semiconductor. Majority charge carries in p-type semiconductor are holes and minority charge carries are electrons which are thermally generated. v Since each trivalent impurities atom accepts one electron from the neighboring silicon atom, so it is known as acceptor impurities.


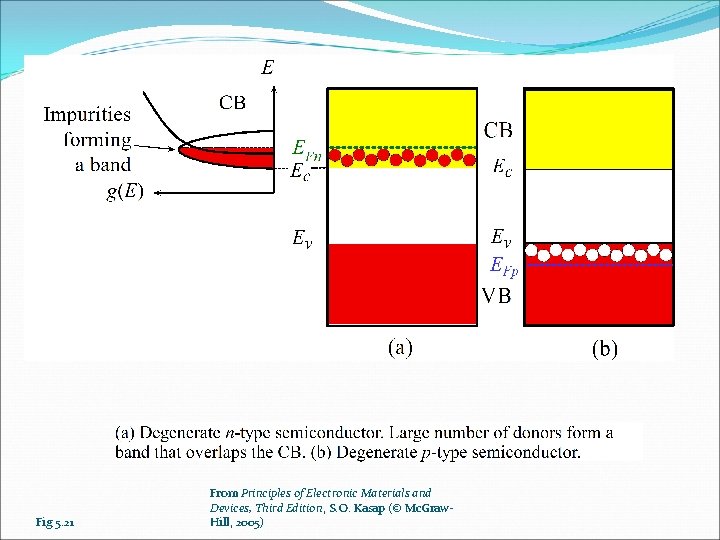
Fig 5. 21 From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw. Hill, 2005)

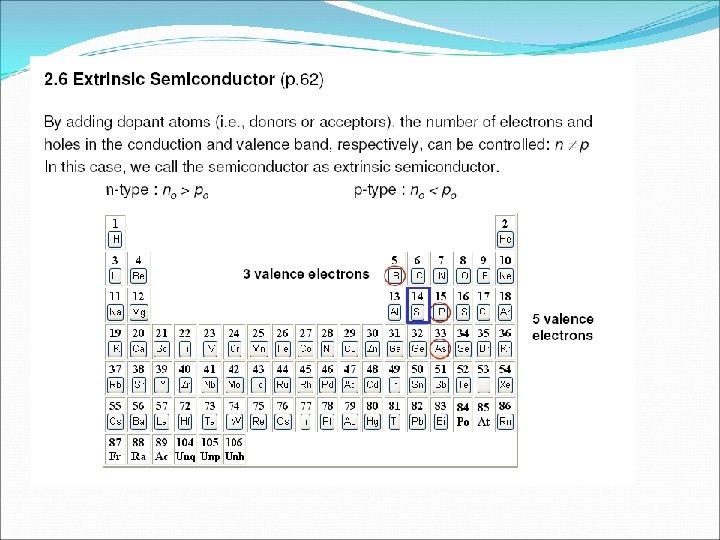
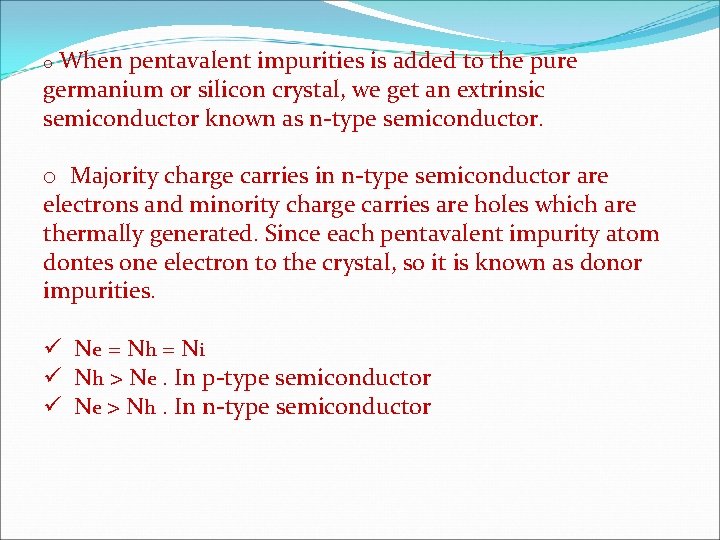
o When pentavalent impurities is added to the pure germanium or silicon crystal, we get an extrinsic semiconductor known as n-type semiconductor. o Majority charge carries in n-type semiconductor are electrons and minority charge carries are holes which are thermally generated. Since each pentavalent impurity atom dontes one electron to the crystal, so it is known as donor impurities. ü Ne = N h = Ni ü Nh > Ne. In p-type semiconductor ü Ne > Nh. In n-type semiconductor






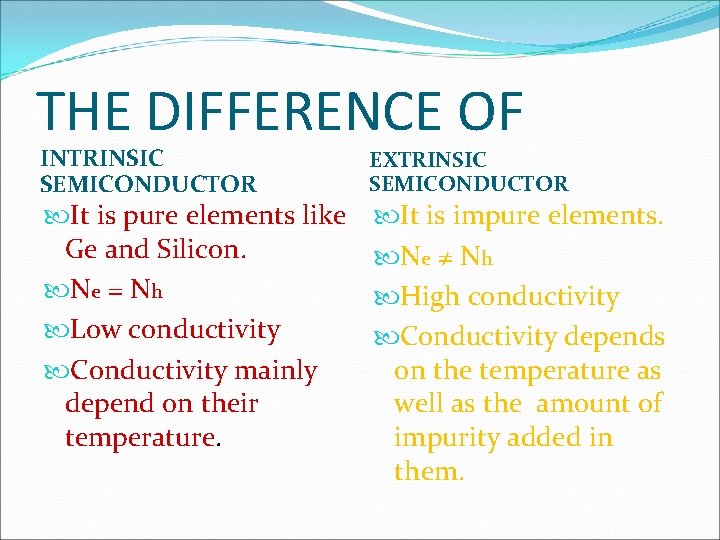
THE DIFFERENCE OF INTRINSIC SEMICONDUCTOR It is pure elements like Ge and Silicon. Ne = Nh Low conductivity Conductivity mainly depend on their temperature. EXTRINSIC SEMICONDUCTOR It is impure elements. Ne ≠ Nh High conductivity Conductivity depends on the temperature as well as the amount of impurity added in them.

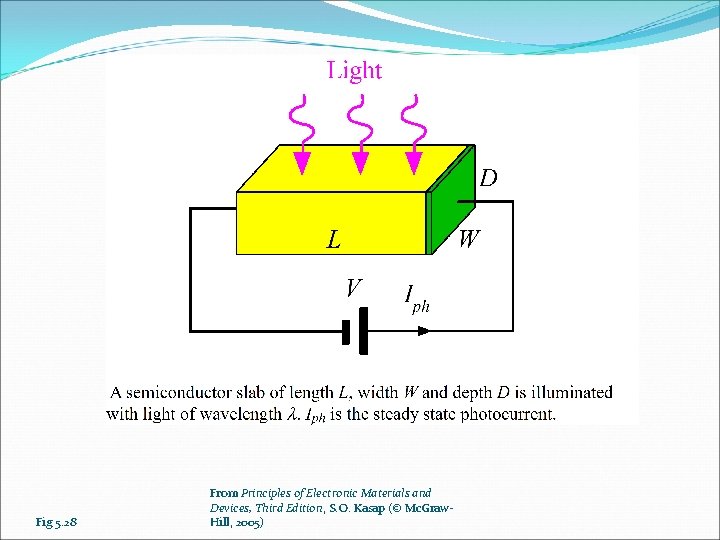
Fig 5. 28 From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw. Hill, 2005)

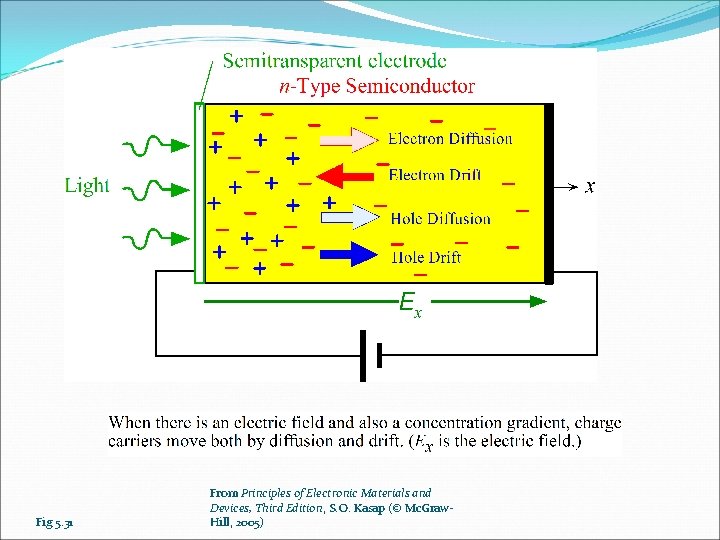
Fig 5. 31 From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw. Hill, 2005)

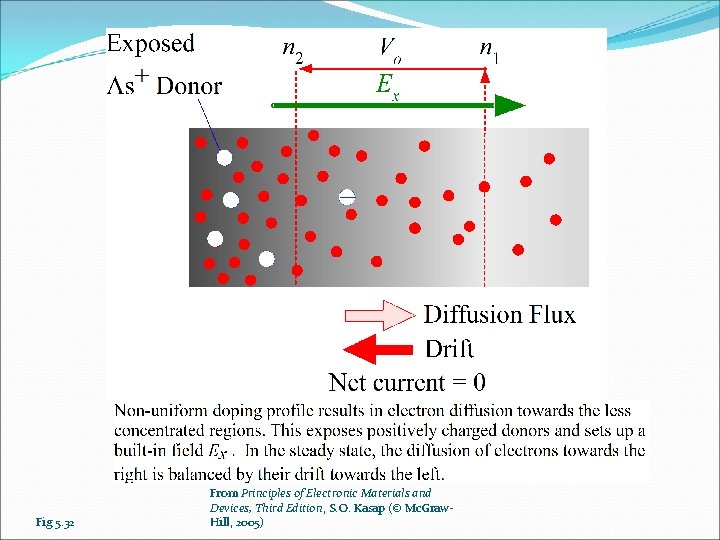
Fig 5. 32 From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw. Hill, 2005)

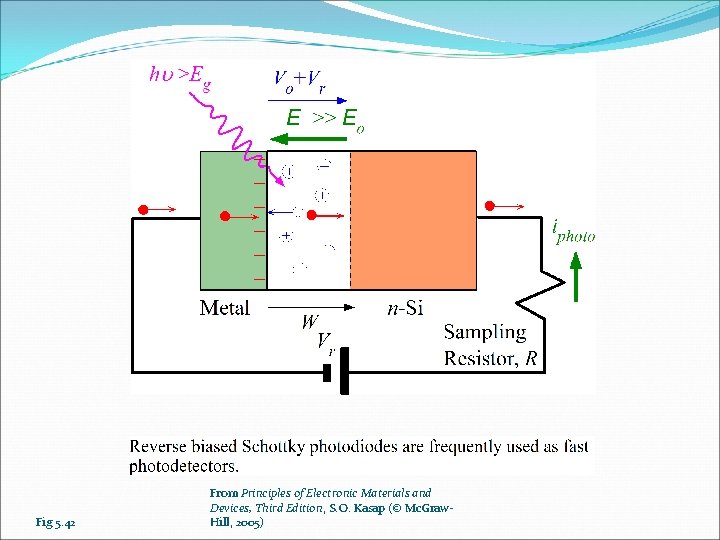
Fig 5. 42 From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw. Hill, 2005)