Influenza Surveillance in Mainland of China Yuelong Shu

















- Slides: 47

Influenza Surveillance in Mainland of China Yuelong Shu Chinese National Influenza Center National Institute for Viral Disease Control and Prevention China CDC 2009 -8 -18, Beijing CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

l Management of Influenza Surveillance Network in Mainland China l Influenza Surveillance Data l Response to Pandemic H 1 N 1 2009 CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

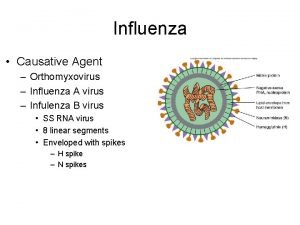
Influenza Surveillance in Mainland China l Ø Ø Ø Classification: Seasonal Influenza: Class C Notifiable Infectious Disease Pandemic A(H 1 N 1) 2009 : Class B Notifiable Infectious Disease H 5 N 1: Class B Notifiable Infectious Disease l Ø Ø Sentinel hospital-based surveillance: ILI surveillance Virological surveillance Influenza or ILI outbreak surveillance: Outbreak report Virological surveillance CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Surveillance Objectives l To monitor influenza activity in mainland China l To monitor the antigenic and genetic characterization of influenza virus: for recommendation of representative strain and vaccine strain l To monitor reassortant or mutation of the virus: more severe or/and transmissible? l Drug resistance surveillance l To monitor the novel influenza virus CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION 4

History l Influenza research initiated in 1952 in mainland of China l Influenza department of Institute of Virology was established in 1954 l Chinese National Influenza Center was established in 1957 l Rejoined WHO Global influenza surveillance network (GISN) in 1981 CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION 5

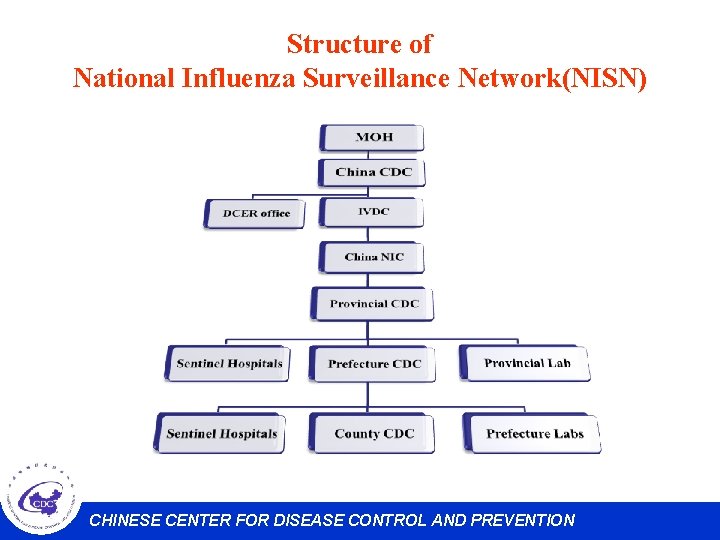
Structure of National Influenza Surveillance Network(NISN) CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

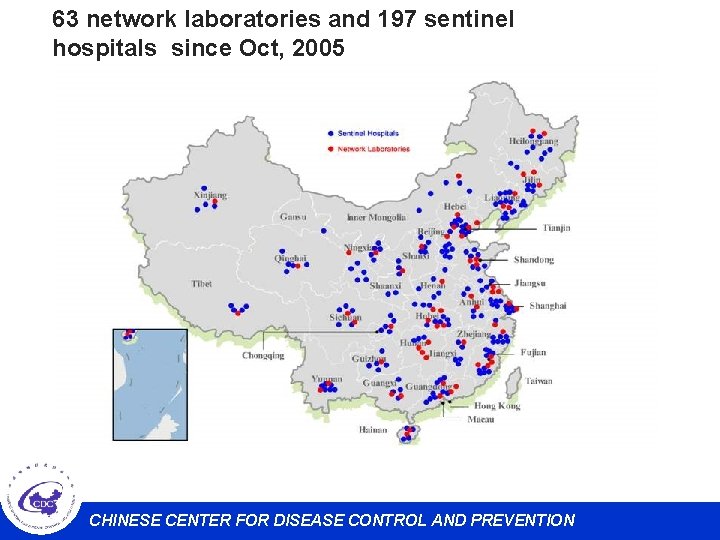
63 network laboratories and 197 sentinel hospitals since Oct, 2005 CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

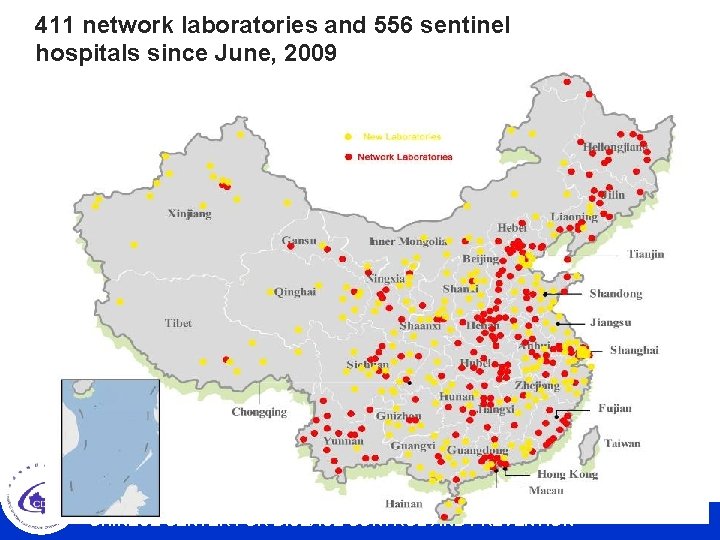
411 network laboratories and 556 sentinel hospitals since June, 2009 CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

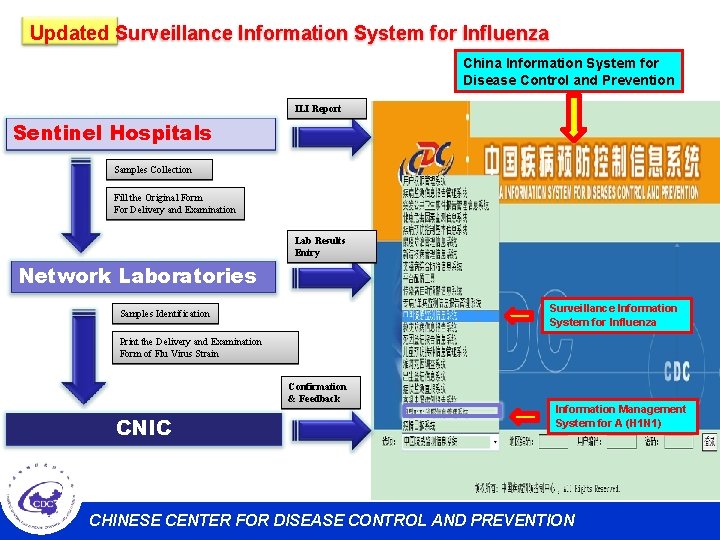
Updated Surveillance Information System for Influenza China Information System for Disease Control and Prevention ILI Report Sentinel Hospitals Samples Collection Fill the Original Form For Delivery and Examination Lab Results Entry Network Laboratories Surveillance Information System for Influenza Samples Identification Print the Delivery and Examination Form of Flu Virus Strain Confirmation & Feedback CNIC Information Management System for A (H 1 N 1) CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

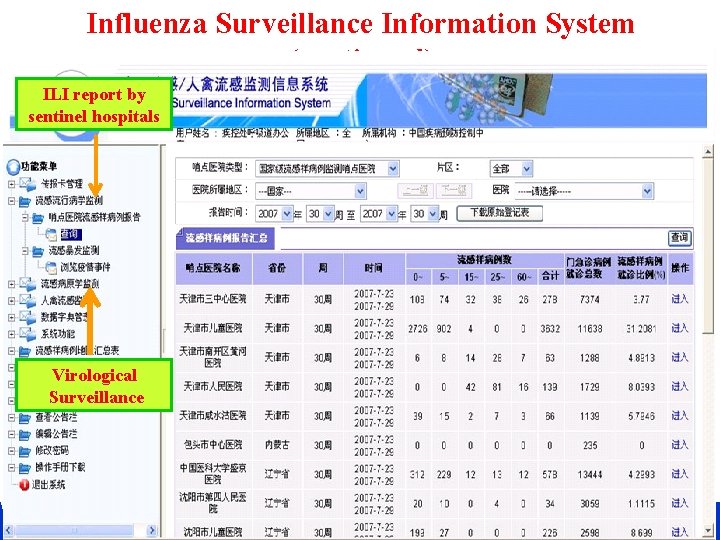
Influenza Surveillance Information System (continued) ILI report by sentinel hospitals Virological Surveillance CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Influenza Surveillance Information System (3) ILI by age groups No. of consultations

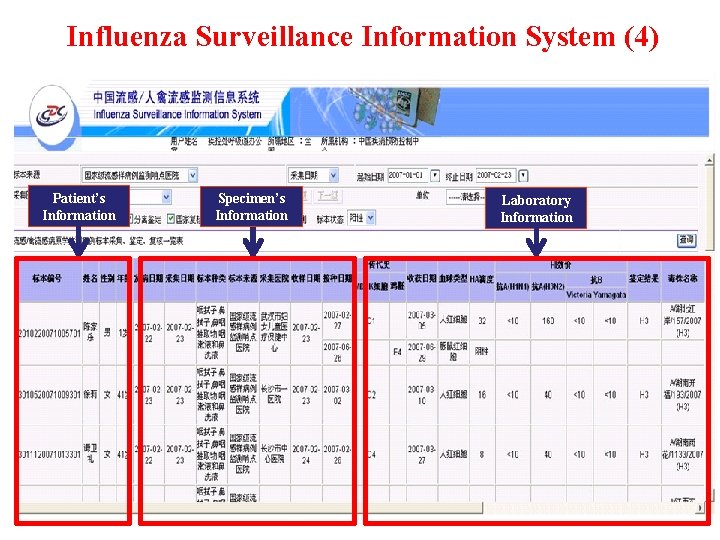
Influenza Surveillance Information System (4) Patient’s Information Specimen’s Information Laboratory Information

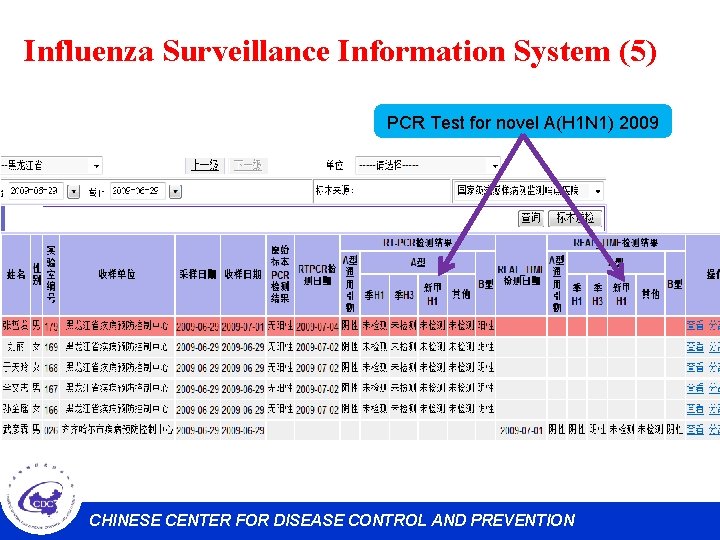
Influenza Surveillance Information System (5) PCR Test for novel A(H 1 N 1) 2009 CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Accelerating national and international cooperation and communication and continuing participation in the GISN CNIC Chinese Website http: //www. cnic. org. cn/ English Weekly Report CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Virus Information Database for NISN Virus Database • Add • Search Sequence Database • Add • Search • Download Multialignment & BLAST • Developing CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION 15

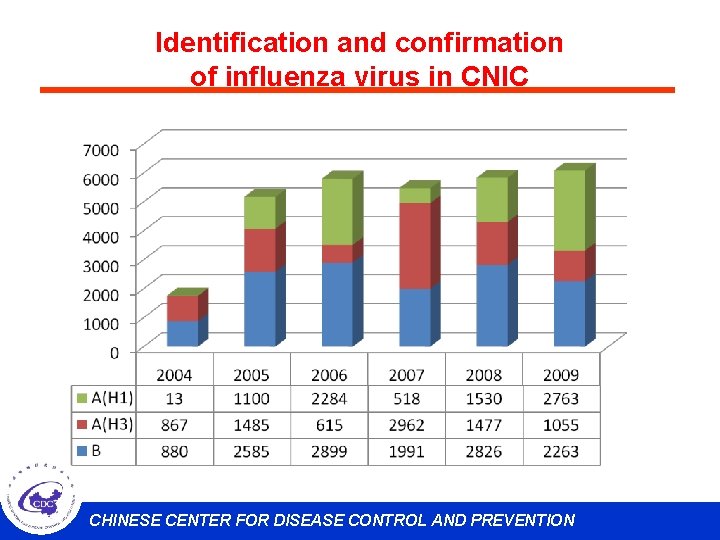
Identification and confirmation of influenza virus in CNIC CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

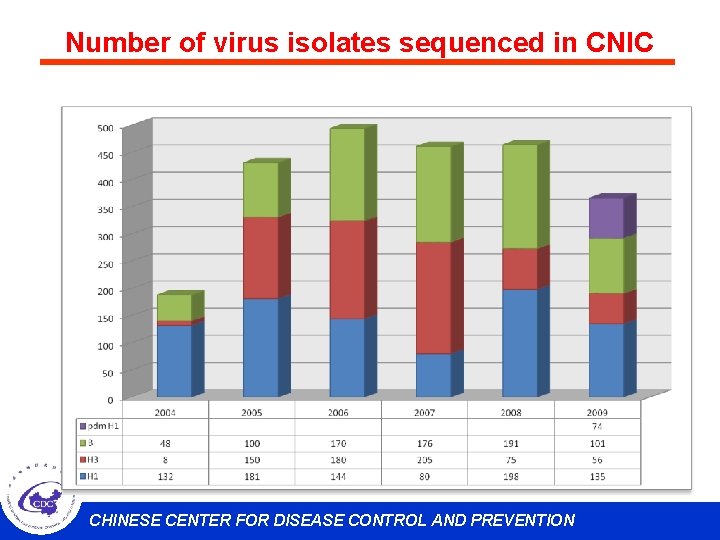
Number of virus isolates sequenced in CNIC CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Trainings to the NISN l Every year CNIC organizes various training courses for the NISN, such as epidemiology workshop, laboratory detection techniuqe training, Biosafety training, GLP training and laboratory hands on training, etc. l More than 2000 epidemiologists and laboratory technicians were trained in the last five years. 19 CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

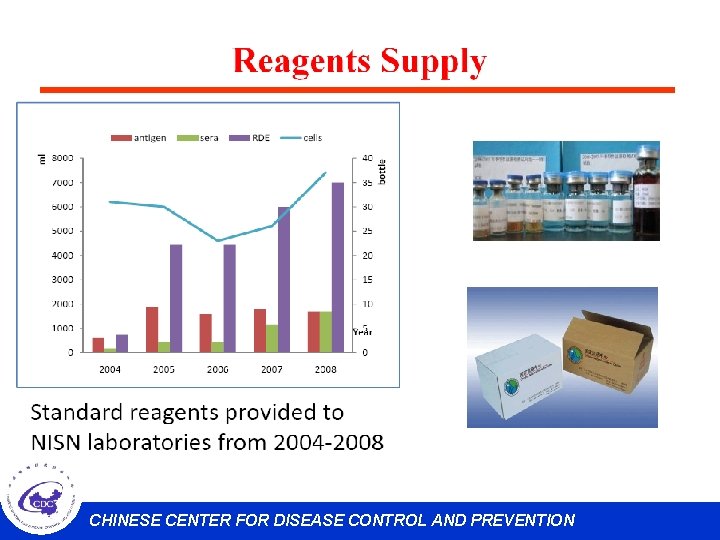
Supervision have been organized each year since 2000. CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

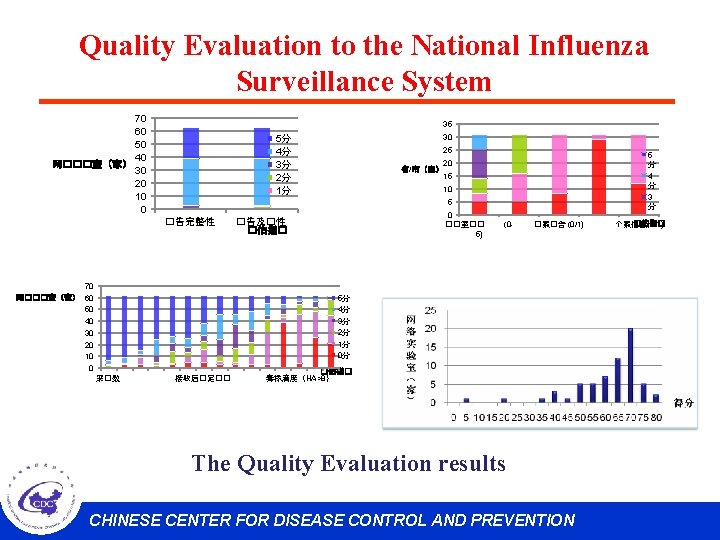
National Influenza Surveillance Quality Evaluation System l To strengthen the management of the NISN. l The evaluation system includes the following 4 parts: Ø Ø Virological Surveillance Epidemiological Surveillance Outbreak Surveillance Management of the Network Laboratory l The Quality Evaluation includes about 20 indicators. CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Quality Evaluation to the National Influenza Surveillance System 70 60 50 40 网���室(家) 30 20 10 0 35 30 5分 4分 3分 2分 1分 25 5 分 4 分 3 分 20 省/市(家) 15 10 5 �告完整性 70 网���室(家) 60 50 40 30 20 10 0 0 ��室�� 5) �告及�性 �估指� (0 - �案�告 (0/1) 5分 4分 3分 2分 1分 0分 采�数 接收后�定�� �估指� 毒株滴度(HA>8) The Quality Evaluation results CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION �估指� 个案信息(0/1)

International Cooperation & Communication CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

International Cooperation & Communication Tabletop Exercise Symposium CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Surveillance Data CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

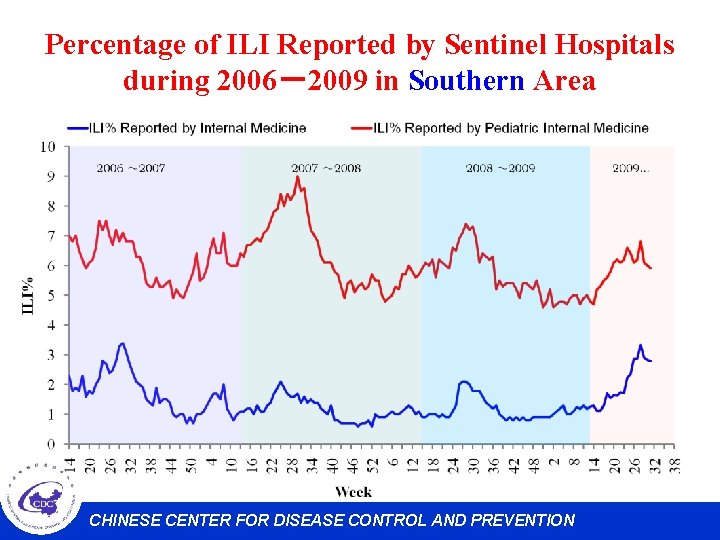
Percentage of ILI Reported by Sentinel Hospitals during 2006-2009 in Southern Area CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

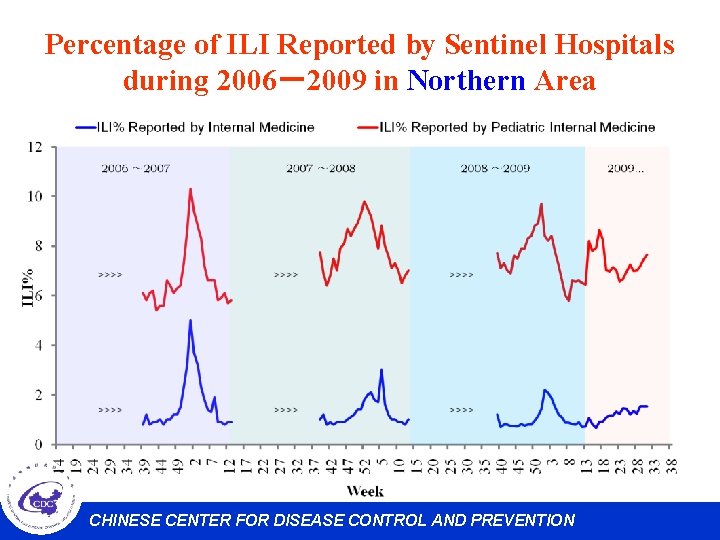
Percentage of ILI Reported by Sentinel Hospitals during 2006-2009 in Northern Area CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

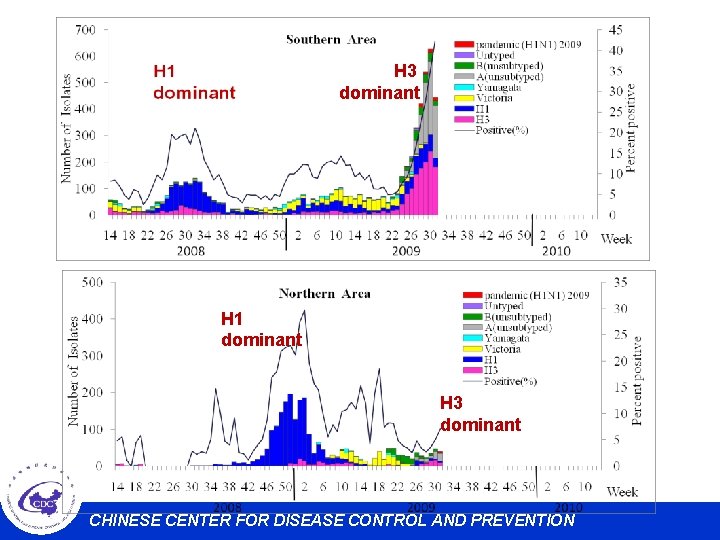
H 3 dominant H 1 dominant H 3 dominant CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

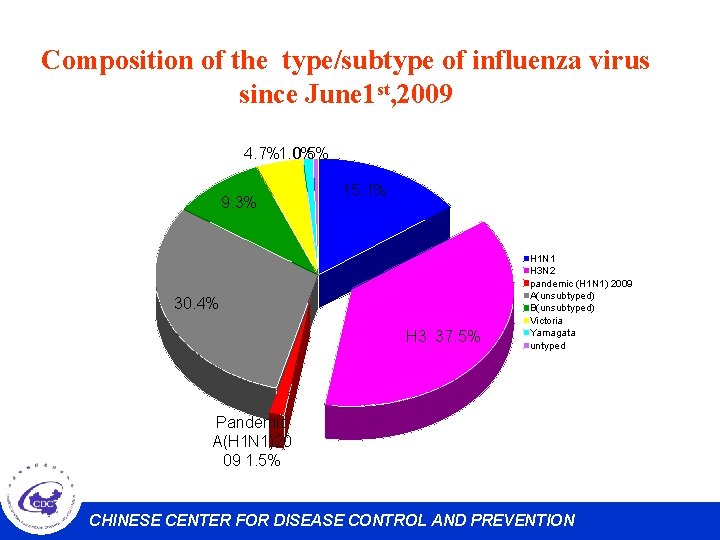
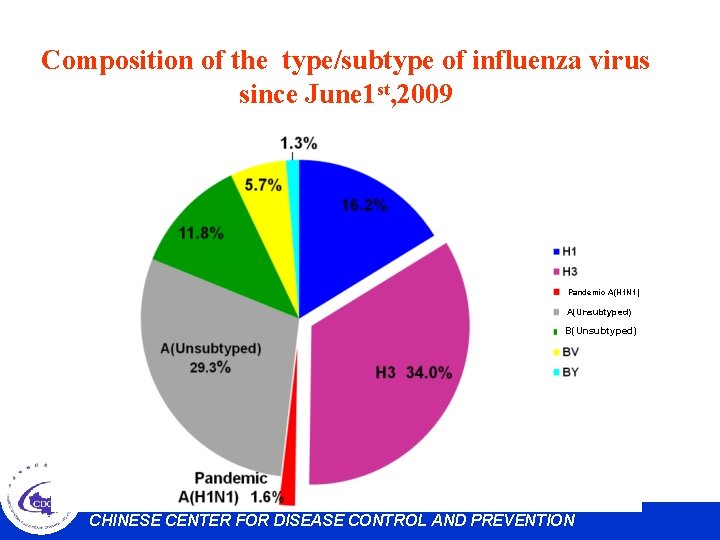
Composition of the type/subtype of influenza virus since June 1 st, 2009 4. 7%1. 0% 0. 5% 9. 3% 15. 1% 30. 4% H 3 37. 5% H 1 N 1 H 3 N 2 pandemic (H 1 N 1) 2009 A(unsubtyped) B(unsubtyped) Victoria Yamagata untyped Pandemic A(H 1 N 1)20 09 1. 5% CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

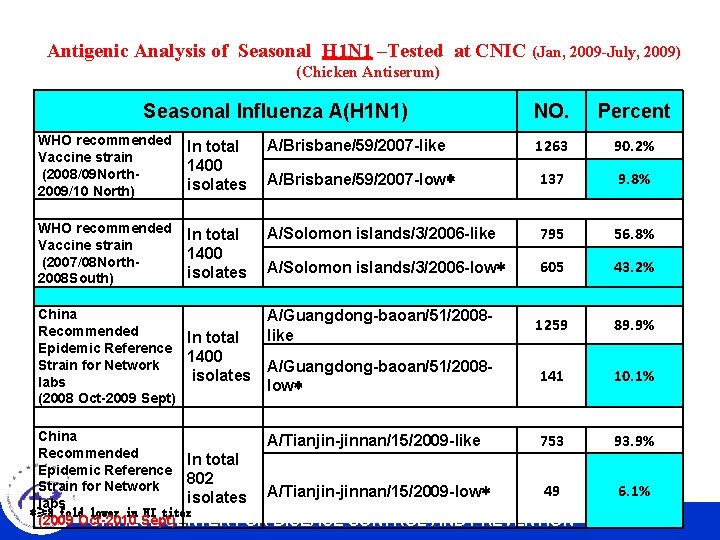
Antigenic Analysis of Seasonal H 1 N 1 –Tested at CNIC (Jan, 2009 -July, 2009) (Chicken Antiserum) Seasonal Influenza A(H 1 N 1) NO. Percent WHO recommended Vaccine strain (2008/09 North 2009/10 North) In total 1400 isolates A/Brisbane/59/2007 -like 1263 90. 2% A/Brisbane/59/2007 -low* 137 9. 8% WHO recommended Vaccine strain (2007/08 North 2008 South) In total 1400 isolates A/Solomon islands/3/2006 -like 795 56. 8% A/Solomon islands/3/2006 -low* 605 43. 2% A/Guangdong-baoan/51/2008 like 1259 89. 9% A/Guangdong-baoan/51/2008 low* 141 10. 1% China Recommended In total Epidemic Reference 1400 Strain for Network isolates labs (2008 Oct-2009 Sept) China 753 A/Tianjin-jinnan/15/2009 -like Recommended In total Epidemic Reference 802 Strain for Network 49 A/Tianjin-jinnan/15/2009 -low* isolates labs *≥ 8 fold lower in HI titer (2009 Oct-2010 Sept) CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION 93. 9% 6. 1%

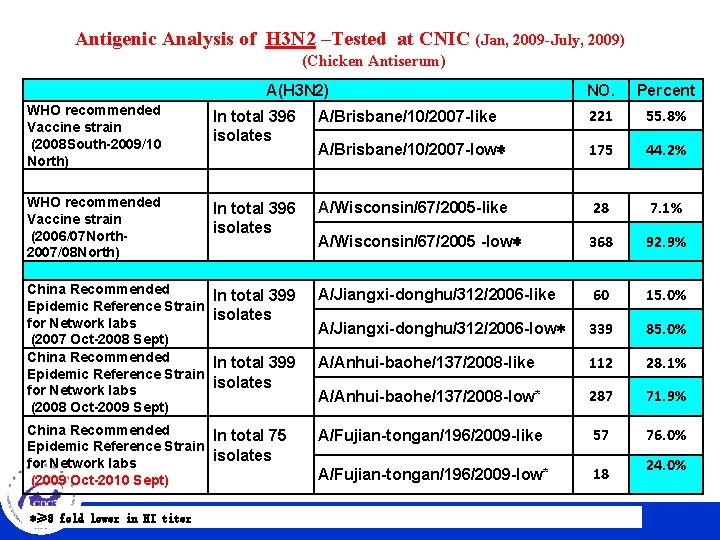
Antigenic Analysis of H 3 N 2 –Tested at CNIC (Jan, 2009 -July, 2009) (Chicken Antiserum) A(H 3 N 2) NO. Percent WHO recommended Vaccine strain (2008 South-2009/10 North) In total 396 isolates A/Brisbane/10/2007 -like 221 55. 8% A/Brisbane/10/2007 -low* 175 44. 2% WHO recommended Vaccine strain (2006/07 North 2007/08 North) In total 396 isolates A/Wisconsin/67/2005 -like 28 7. 1% A/Wisconsin/67/2005 -low* 368 92. 9% China Recommended Epidemic Reference Strain for Network labs (2007 Oct-2008 Sept) China Recommended Epidemic Reference Strain for Network labs (2008 Oct-2009 Sept) In total 399 isolates A/Jiangxi-donghu/312/2006 -like 60 15. 0% A/Jiangxi-donghu/312/2006 -low* 339 85. 0% In total 399 isolates A/Anhui-baohe/137/2008 -like 112 28. 1% A/Anhui-baohe/137/2008 -low* 287 71. 9% A/Fujian-tongan/196/2009 -like 57 76. 0% A/Fujian-tongan/196/2009 -low* 18 China Recommended In total 75 Epidemic Reference Strain isolates for Network labs (2009 Oct-2010 Sept) *≥ 8 fold CHINESE lower in HI titer CENTER FOR DISEASE CONTROL AND PREVENTION 24. 0%

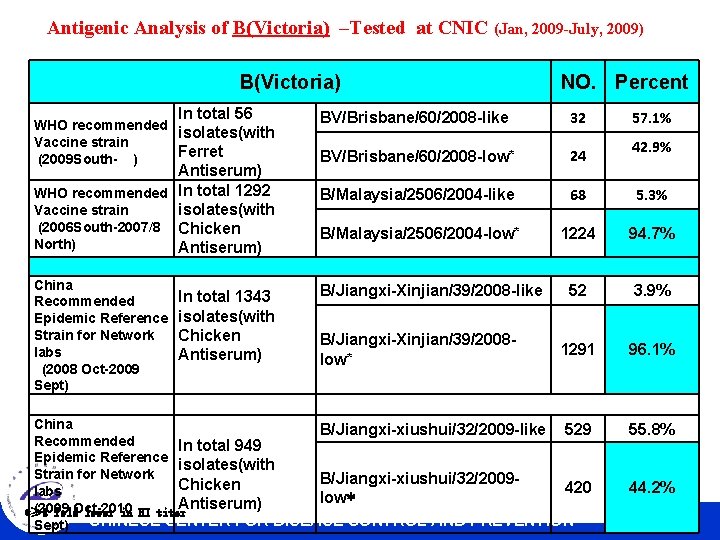
Antigenic Analysis of B(Victoria) –Tested at CNIC (Jan, 2009 -July, 2009) B(Victoria) In total 56 WHO recommended isolates(with Vaccine strain Ferret (2009 South- ) Antiserum) WHO recommended In total 1292 isolates(with Vaccine strain (2006 South-2007/8 Chicken North) Antiserum) China Recommended Epidemic Reference Strain for Network labs (2008 Oct-2009 Sept) In total 1343 isolates(with Chicken Antiserum) NO. Percent BV/Brisbane/60/2008 -like 32 BV/Brisbane/60/2008 -low* 24 B/Malaysia/2506/2004 -like 68 5. 3% B/Malaysia/2506/2004 -low* 1224 94. 7% 52 3. 9% 1291 96. 1% B/Jiangxi-Xinjian/39/2008 -like B/Jiangxi-Xinjian/39/2008 low* China B/Jiangxi-xiushui/32/2009 -like 529 Recommended In total 949 Epidemic Reference isolates(with Strain for Network B/Jiangxi-xiushui/32/2009 Chicken 420 labs low* Antiserum) (2009 *≥ 8 fold. Oct-2010 lower in HI titer Sept) CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION 57. 1% 42. 9% 55. 8% 44. 2%

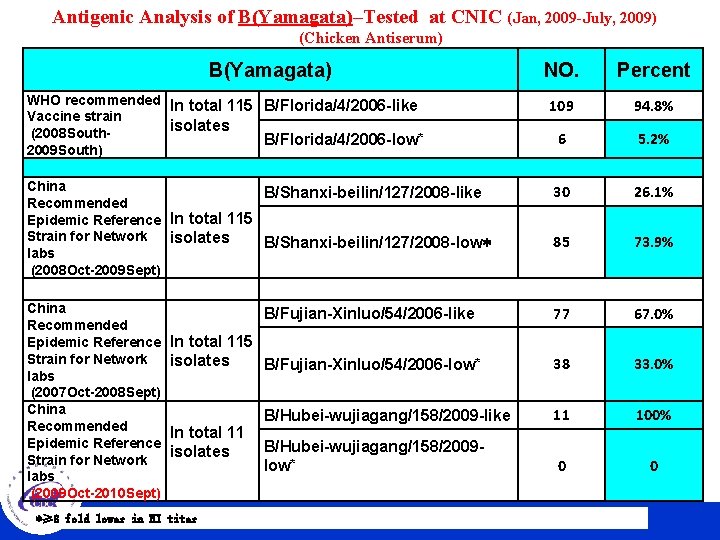
Antigenic Analysis of B(Yamagata)–Tested at CNIC (Jan, 2009 -July, 2009) (Chicken Antiserum) B(Yamagata) NO. Percent WHO recommended In total 115 B/Florida/4/2006 -like Vaccine strain isolates (2008 South. B/Florida/4/2006 -low* 2009 South) 109 94. 8% 6 5. 2% China B/Shanxi-beilin/127/2008 -like Recommended Epidemic Reference In total 115 Strain for Network isolates B/Shanxi-beilin/127/2008 -low* labs (2008 Oct-2009 Sept) 30 26. 1% 85 73. 9% China Recommended Epidemic Reference Strain for Network labs (2007 Oct-2008 Sept) China Recommended Epidemic Reference Strain for Network labs (2009 Oct-2010 Sept) 77 67. 0% 38 33. 0% B/Hubei-wujiagang/158/2009 -like 11 100% B/Hubei-wujiagang/158/2009 low* 0 0 B/Fujian-Xinluo/54/2006 -like In total 115 isolates B/Fujian-Xinluo/54/2006 -low* In total 11 isolates *≥ 8 fold. CHINESE lower in HI CENTER titer FOR DISEASE CONTROL AND PREVENTION

Drug resistance surveillance for seasonal influenza (bioinformatics) ( Collection date from Jan 2009 to July 2009) • 39. 0% (30/77) H 1 N 1 viruses and 100% (49/49) H 3 N 2 viruses isolated in mainland China from Jan 2009 to July 2009 are resistant to adamantine. • 61. 0% (47/77) H 1 N 1 viruses isolated in mainland China from Jan 2009 to July 2009 are resistant to Oseltamivir. No Oseltamivir resistance viruses were found in H 3 N 2(0/83) and B (0/49)viruses. CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Response to 2009 H 1 N 1 Pandemic CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Support to Chinese Influenza Surveillance Network l Special financial funds for Chinese Influenza Surveillance Network from Central finance l Timely development of diagnostic method l. Enhanced Surveillance: Ø Expansion of network laboratories Ø Expansion of sentinel hospitals l. A series of trainings and meetings On site or by video/teleconference CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

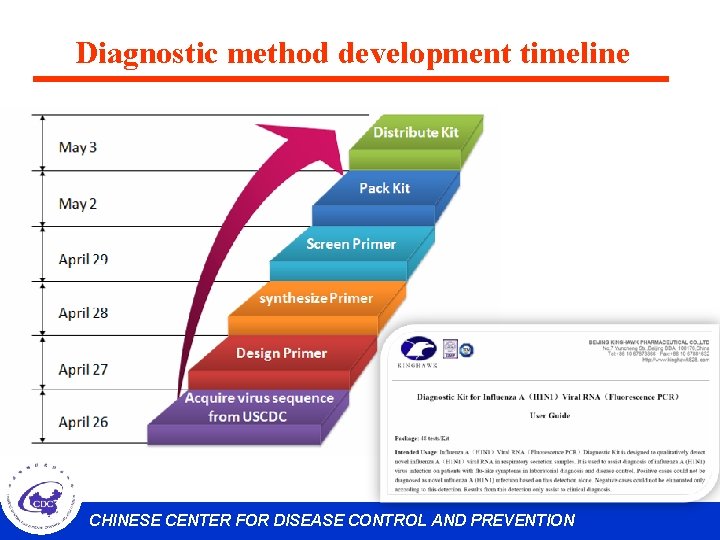
Diagnostic method development timeline CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

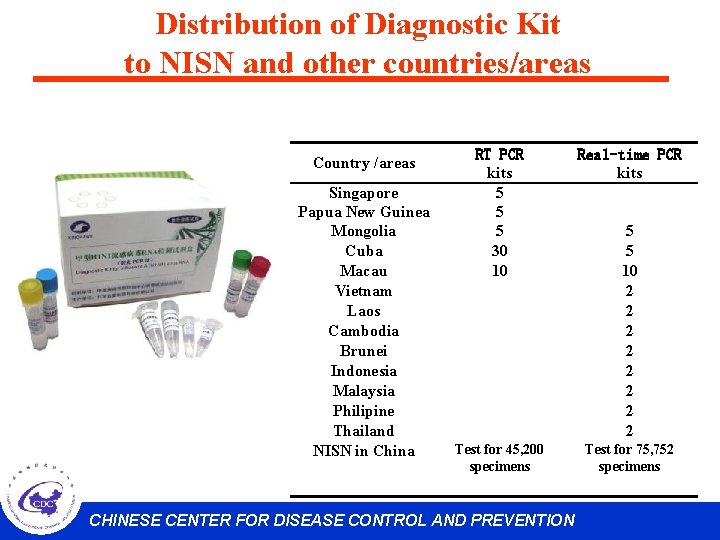
Distribution of Diagnostic Kit to NISN and other countries/areas Country /areas Singapore Papua New Guinea Mongolia Cuba Macau Vietnam Laos Cambodia Brunei Indonesia Malaysia Philipine Thailand NISN in China RT PCR kits 5 5 5 30 10 Real-time PCR kits Test for 45, 200 specimens Test for 75, 752 specimens CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION 5 5 10 2 2 2 2

Rapid information exchange CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

China-ASEAN workshop for Pandemic A(H 1 N 1) 2009 CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

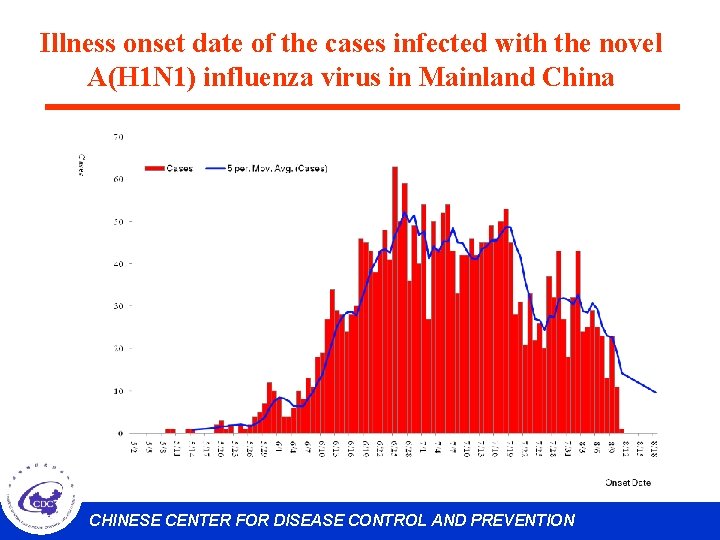
Illness onset date of the cases infected with the novel A(H 1 N 1) influenza virus in Mainland China CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

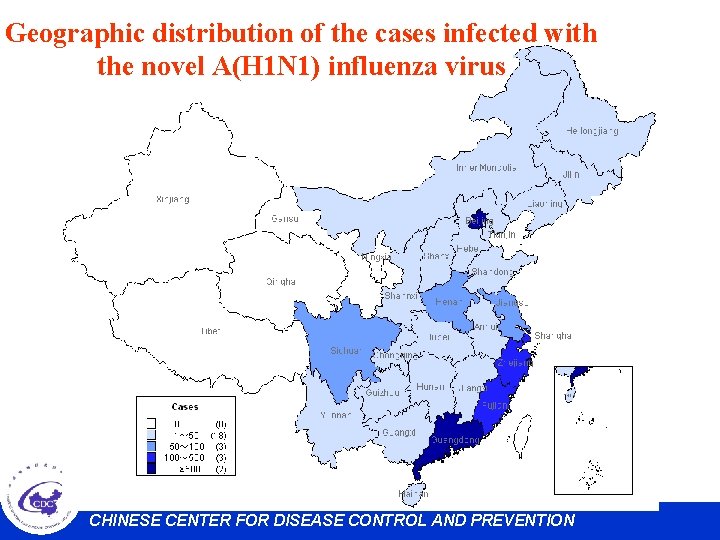
Geographic distribution of the cases infected with the novel A(H 1 N 1) influenza virus CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

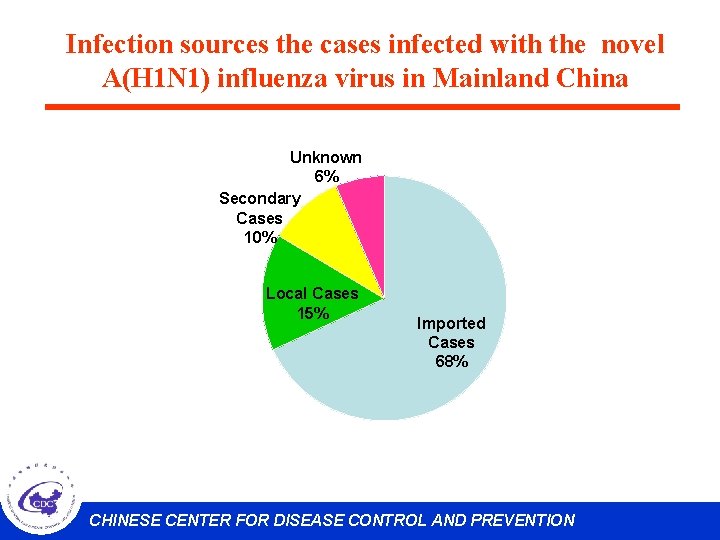
Infection sources the cases infected with the novel A(H 1 N 1) influenza virus in Mainland China Unknown 6% Secondary Cases 10% Local Cases 15% Imported Cases 68% CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Composition of the type/subtype of influenza virus since June 1 st, 2009 Pandemic A(H 1 N 1) A(Unsubtyped) B(Unsubtyped) CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

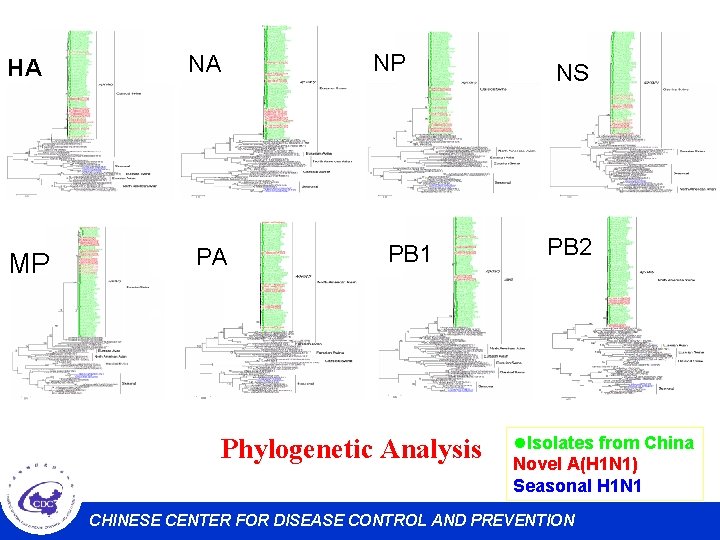
HA NA MP PA NP PB 1 Phylogenetic Analysis NS PB 2 l. Isolates from China Novel A(H 1 N 1) Seasonal H 1 N 1 CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

Drug Resistance Analysis by bioinformatics l All the A(H 1 N 1) viruses isolated in Mainland China are resistant to adamantine l A/Hunan/SWL 3/2009(H 1 N 1) has a histamine to tyrosine mutation at residue 274 of the NA (N 2 numbering; residue 275 by N 1 numbering), which confers a high level of resistance to Oseltamivir. The case was imported case from US, and took Oseltamivir for prophylactic purpose 1~6 days before the onset of illness. The sequence was reported to Genebank. l The other isolates are sensitive to Oseltamivir. CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION

CHINESE CENTER FOR DISEASE CONTROL AND PREVENTION
 Haksun li
Haksun li Pathology visions
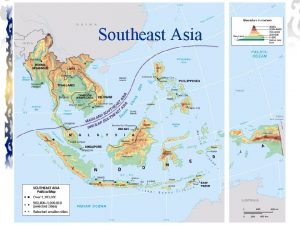
Pathology visions In 1500 mainland southeast asia was a relatively
In 1500 mainland southeast asia was a relatively Mainland se asia
Mainland se asia Mainland island metapopulation
Mainland island metapopulation Infulenza b
Infulenza b Stomach flu vs influenza
Stomach flu vs influenza Influenza virus replication
Influenza virus replication Influenza ww1
Influenza ww1 The great influenza rhetorical analysis
The great influenza rhetorical analysis Is influenza a airborne disease
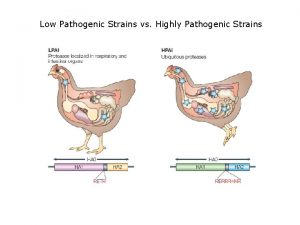
Is influenza a airborne disease Low pathogenic avian influenza
Low pathogenic avian influenza Influenza
Influenza Virus de la influenza
Virus de la influenza Piano di divisione delle staffe
Piano di divisione delle staffe Influenza
Influenza Influenza vaccine dosage chart 2019-2020
Influenza vaccine dosage chart 2019-2020 Noel shu
Noel shu 5400 shu
5400 shu Shu physiopathologie
Shu physiopathologie Bettencourt schueller
Bettencourt schueller Materi shu ekonomi kelas 10
Materi shu ekonomi kelas 10 Shu pao
Shu pao Informasi dasar shu
Informasi dasar shu Also all by shu ting
Also all by shu ting Apa arti shu
Apa arti shu Magisches quadrat 8x8 406
Magisches quadrat 8x8 406 Babi egyptian god
Babi egyptian god Cara menghitung shu
Cara menghitung shu Studiosity shu
Studiosity shu Analisa pearls
Analisa pearls Maytas login shu
Maytas login shu Punti shu antichi
Punti shu antichi Dú shū
Dú shū Also.all shu ting
Also.all shu ting Automated video surveillance
Automated video surveillance Sdr surveillance detection route
Sdr surveillance detection route Tipmp in english
Tipmp in english Reservoir surveillance definition
Reservoir surveillance definition Routine surveillance
Routine surveillance Ipmd surveillance guide
Ipmd surveillance guide 15012007 color
15012007 color Surveillance fixateur externe
Surveillance fixateur externe National institute for food and drug surveillance
National institute for food and drug surveillance Valise drainage thoracique
Valise drainage thoracique Cafe racer shooting surveillance video
Cafe racer shooting surveillance video Hai surveillance
Hai surveillance Developmental screening vs surveillance
Developmental screening vs surveillance