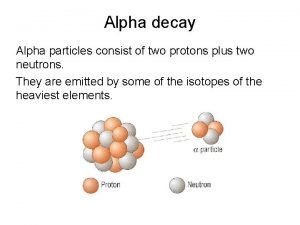
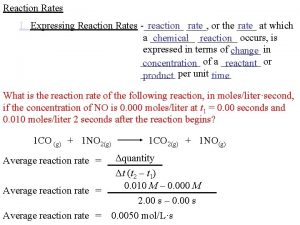
III Nuclear Reaction Rates Nuclear reactions generate energy
- Slides: 24

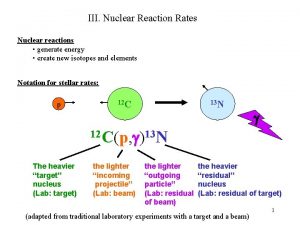
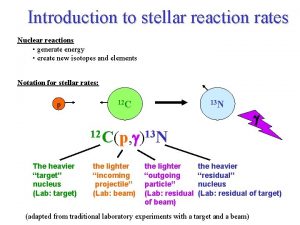
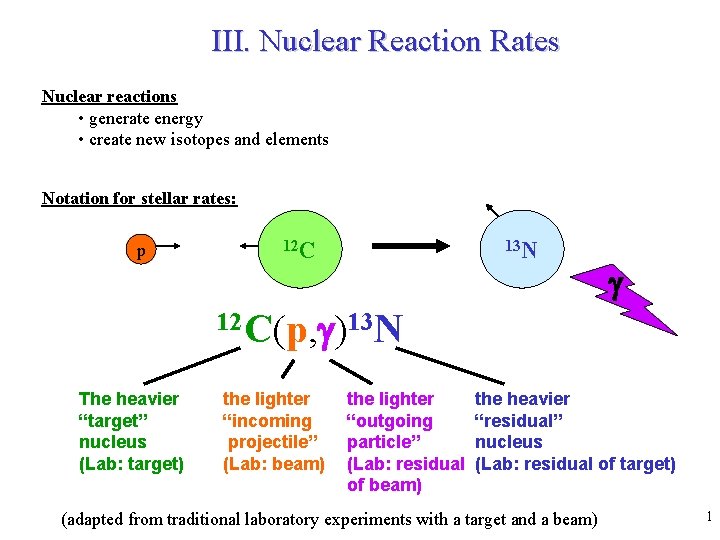
III. Nuclear Reaction Rates Nuclear reactions • generate energy • create new isotopes and elements Notation for stellar rates: p 12 C 13 N g 12 C(p, g)13 N The heavier “target” nucleus (Lab: target) the lighter “incoming projectile” (Lab: beam) the lighter “outgoing particle” (Lab: residual of beam) the heavier “residual” nucleus (Lab: residual of target) (adapted from traditional laboratory experiments with a target and a beam) 1

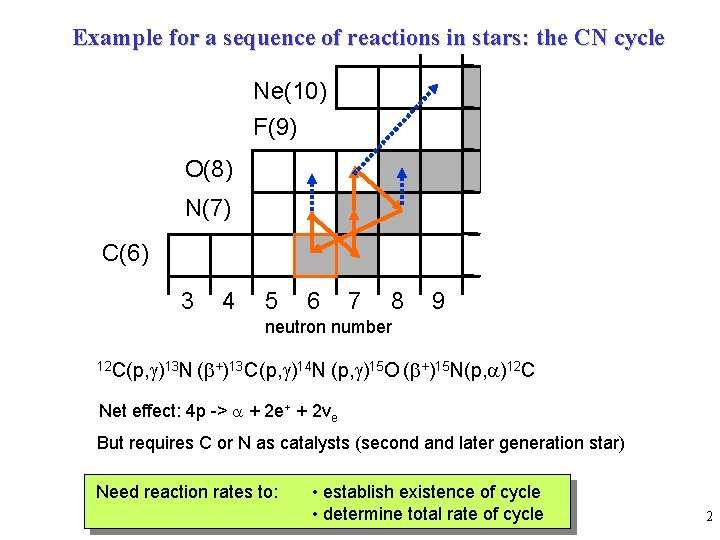
Example for a sequence of reactions in stars: the CN cycle Ne(10) F(9) O(8) N(7) C(6) 3 4 5 6 7 8 9 neutron number 12 C(p, g)13 N (b+)13 C(p, g)14 N (p, g)15 O (b+)15 N(p, a)12 C Net effect: 4 p -> a + 2 e+ + 2 ve But requires C or N as catalysts (second and later generation star) Need reaction rates to: • establish existence of cycle • determine total rate of cycle 2

Sun pp reaction chain: 3

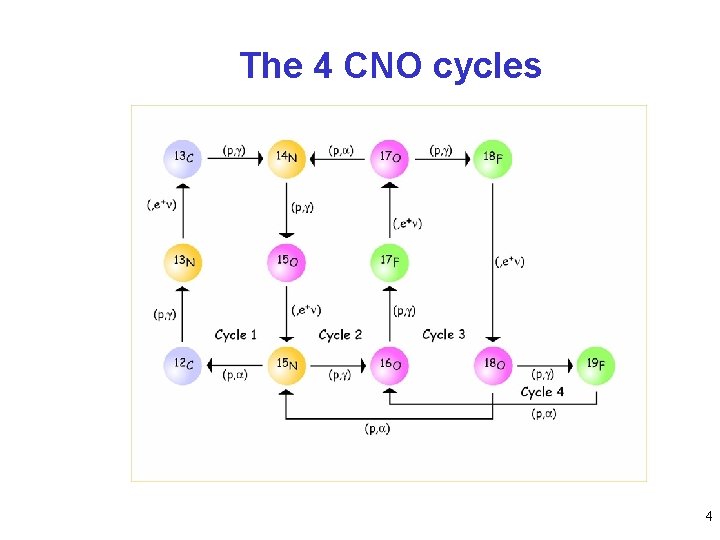
The 4 CNO cycles 4

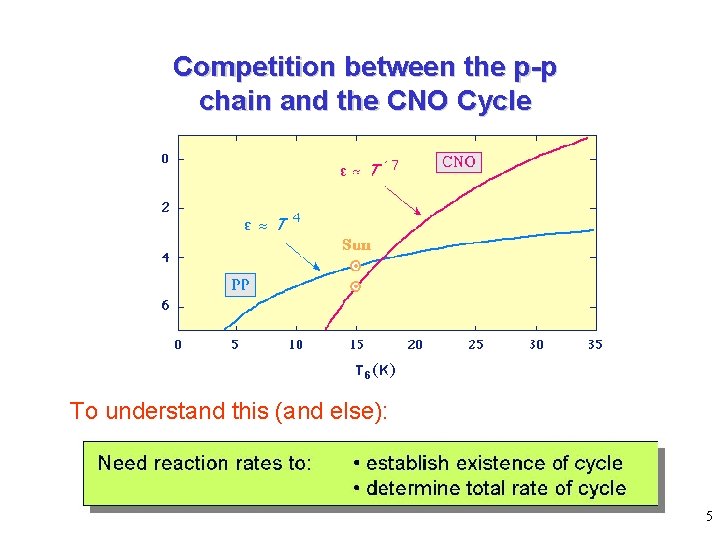
Competition between the p-p chain and the CNO Cycle To understand this (and else): 5

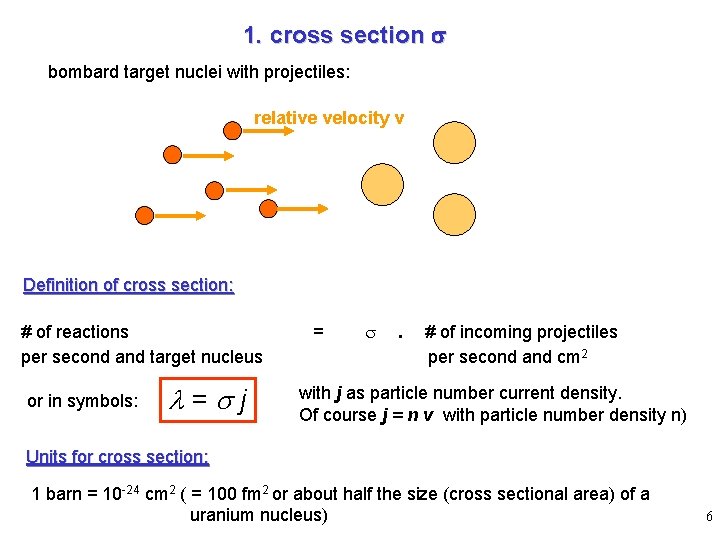
1. cross section s bombard target nuclei with projectiles: relative velocity v Definition of cross section: # of reactions per second and target nucleus or in symbols: l=sj = . # of incoming projectiles per second and cm 2 with j as particle number current density. Of course j = n v with particle number density n) Units for cross section: 1 barn = 10 -24 cm 2 ( = 100 fm 2 or about half the size (cross sectional area) of a uranium nucleus) 6

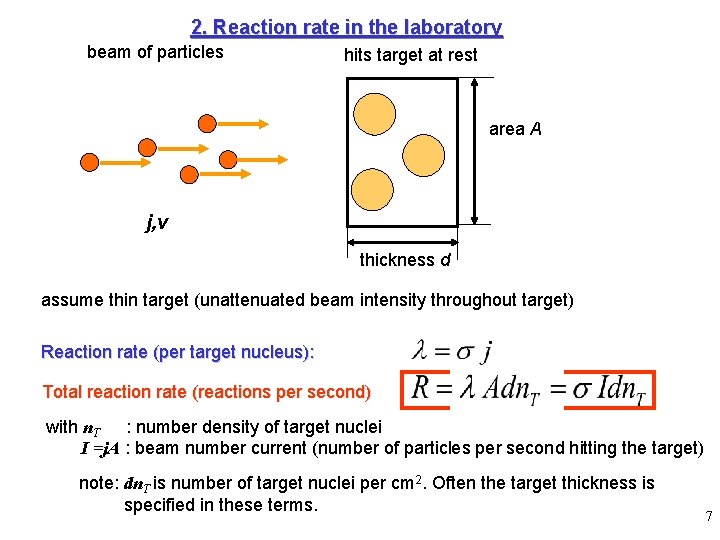
2. Reaction rate in the laboratory beam of particles hits target at rest area A j, v thickness d assume thin target (unattenuated beam intensity throughout target) Reaction rate (per target nucleus): Total reaction rate (reactions per second) with n. T : number density of target nuclei I =j. A : beam number current (number of particles per second hitting the target) note: dn. T is number of target nuclei per cm 2. Often the target thickness is specified in these terms. 7

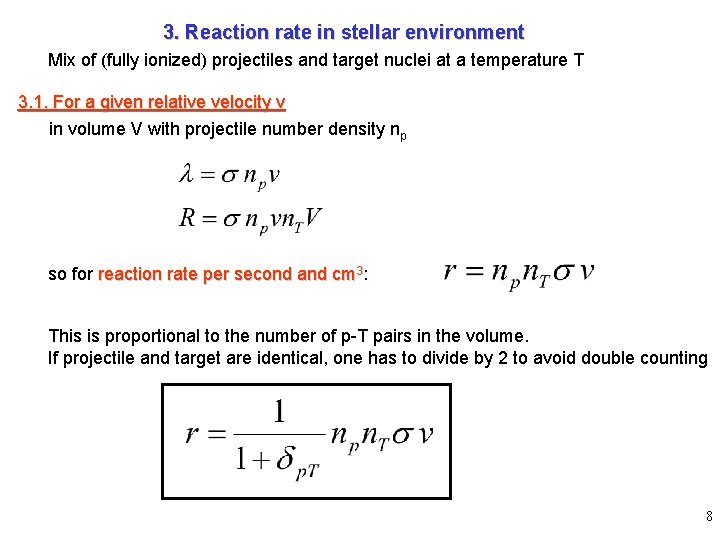
3. Reaction rate in stellar environment Mix of (fully ionized) projectiles and target nuclei at a temperature T 3. 1. For a given relative velocity v in volume V with projectile number density np so for reaction rate per second and cm 3: This is proportional to the number of p-T pairs in the volume. If projectile and target are identical, one has to divide by 2 to avoid double counting 8

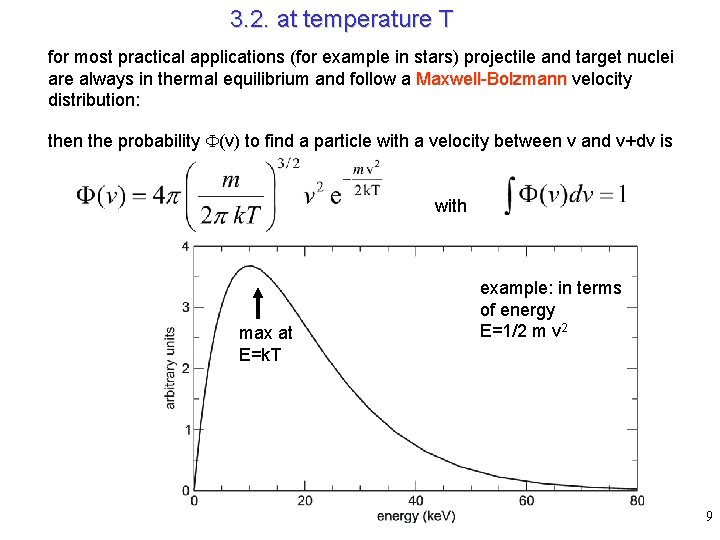
3. 2. at temperature T for most practical applications (for example in stars) projectile and target nuclei are always in thermal equilibrium and follow a Maxwell-Bolzmann velocity distribution: then the probability F(v) to find a particle with a velocity between v and v+dv is with max at E=k. T example: in terms of energy E=1/2 m v 2 9

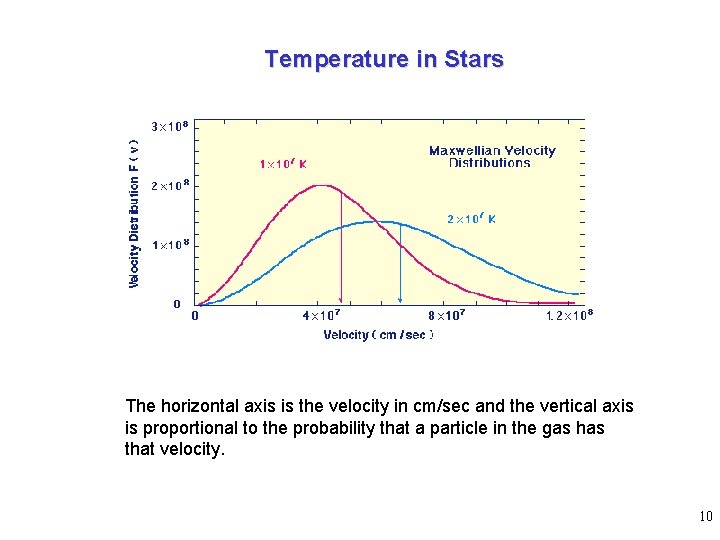
Temperature in Stars The horizontal axis is the velocity in cm/sec and the vertical axis is proportional to the probability that a particle in the gas has that velocity. 10

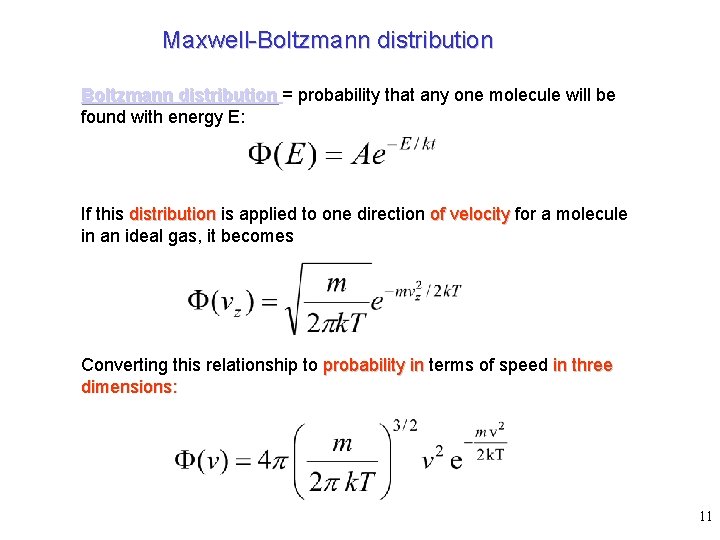
Maxwell-Boltzmann distribution = probability that any one molecule will be found with energy E: If this distribution is applied to one direction of velocity for a molecule in an ideal gas, it becomes Converting this relationship to probability in terms of speed in three dimensions: 11

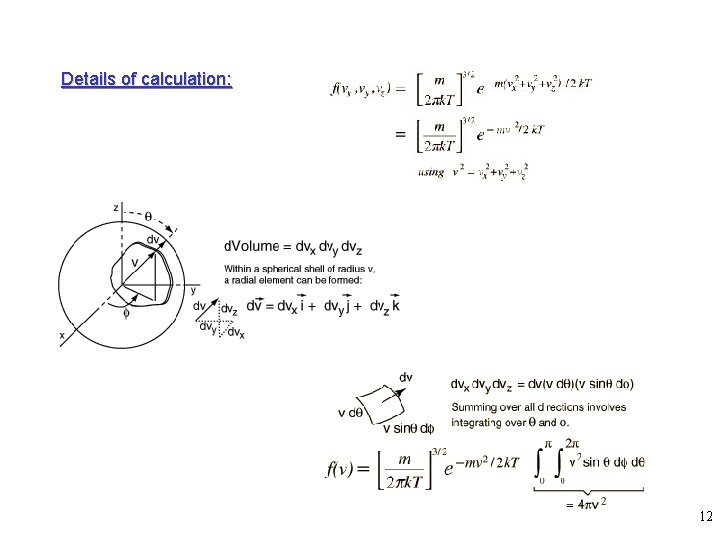
Details of calculation: 12

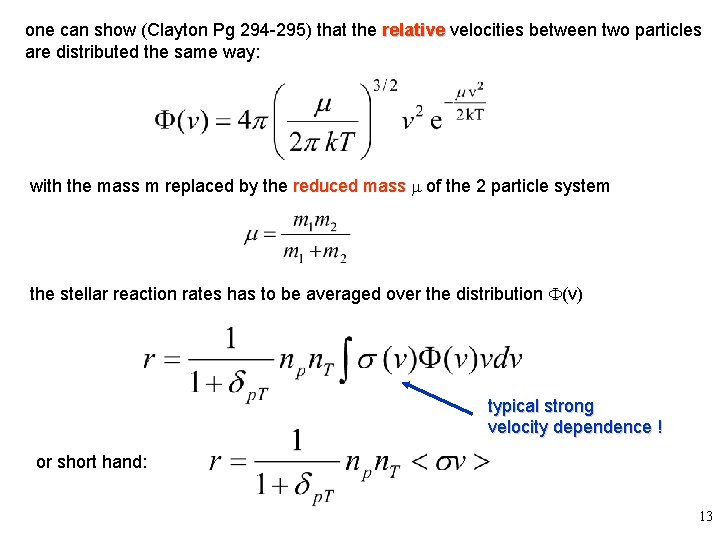
one can show (Clayton Pg 294 -295) that the relative velocities between two particles are distributed the same way: with the mass m replaced by the reduced mass m of the 2 particle system the stellar reaction rates has to be averaged over the distribution F(v) typical strong velocity dependence ! or short hand: 13

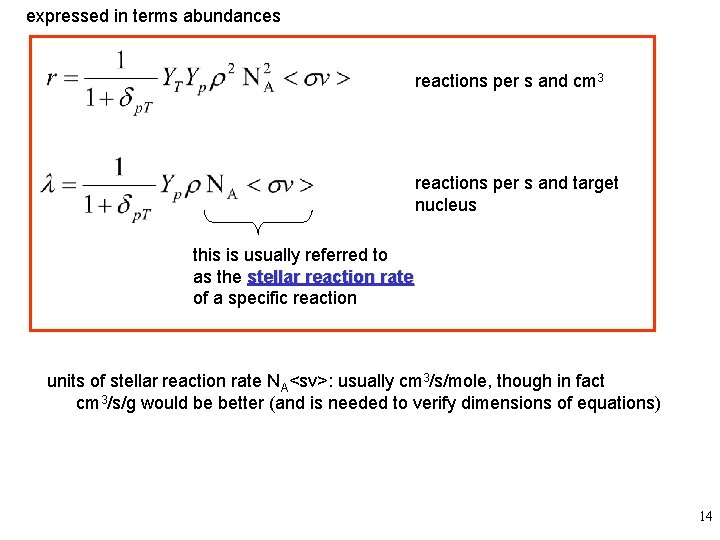
expressed in terms abundances reactions per s and cm 3 reactions per s and target nucleus this is usually referred to as the stellar reaction rate of a specific reaction units of stellar reaction rate NA<sv>: usually cm 3/s/mole, though in fact cm 3/s/g would be better (and is needed to verify dimensions of equations) 14

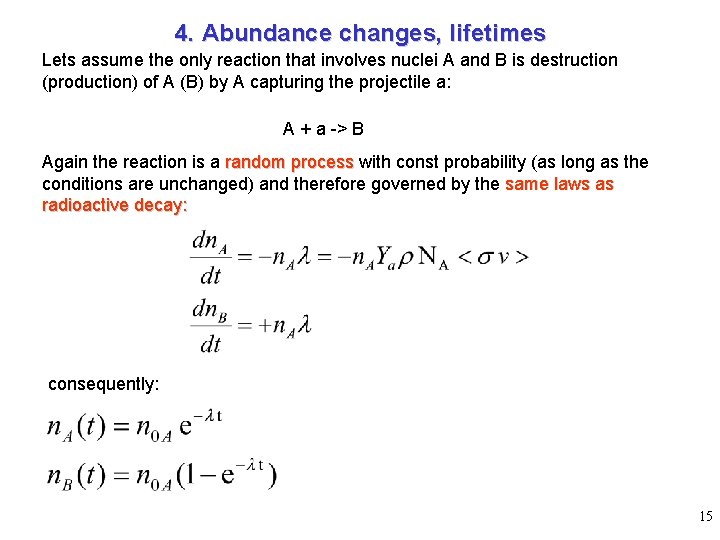
4. Abundance changes, lifetimes Lets assume the only reaction that involves nuclei A and B is destruction (production) of A (B) by A capturing the projectile a: A + a -> B Again the reaction is a random process with const probability (as long as the conditions are unchanged) and therefore governed by the same laws as radioactive decay: consequently: 15

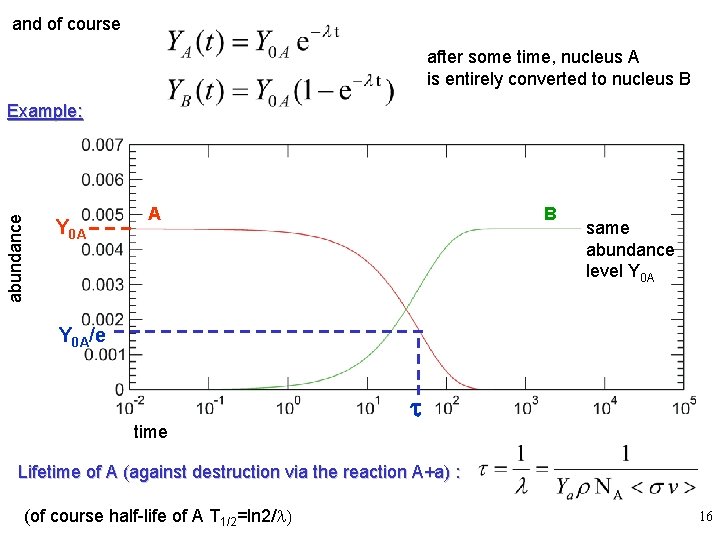
and of course after some time, nucleus A is entirely converted to nucleus B abundance Example: Y 0 A A B same abundance level Y 0 A/e time t Lifetime of A (against destruction via the reaction A+a) : (of course half-life of A T 1/2=ln 2/l) 16

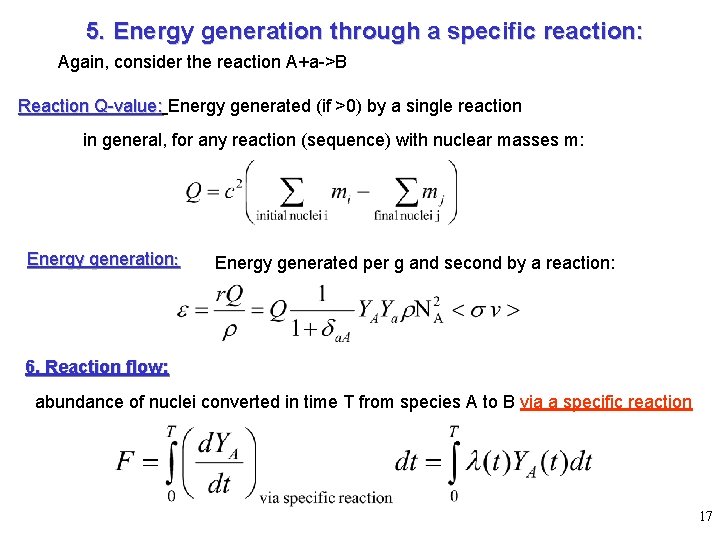
5. Energy generation through a specific reaction: Again, consider the reaction A+a->B Reaction Q-value: Energy generated (if >0) by a single reaction in general, for any reaction (sequence) with nuclear masses m: Energy generation: Energy generated per g and second by a reaction: 6. Reaction flow: abundance of nuclei converted in time T from species A to B via a specific reaction 17

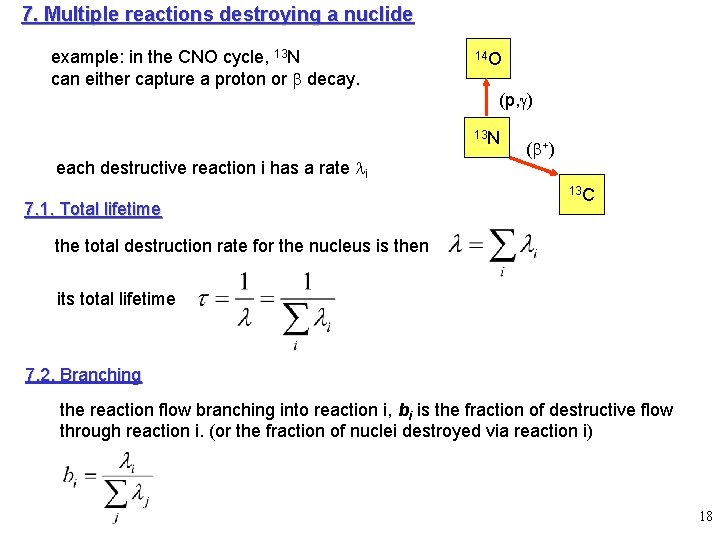
7. Multiple reactions destroying a nuclide example: in the CNO cycle, 13 N can either capture a proton or b decay. 14 O (p, g) 13 N each destructive reaction i has a rate li 7. 1. Total lifetime (b+) 13 C the total destruction rate for the nucleus is then its total lifetime 7. 2. Branching the reaction flow branching into reaction i, bi is the fraction of destructive flow through reaction i. (or the fraction of nuclei destroyed via reaction i) 18

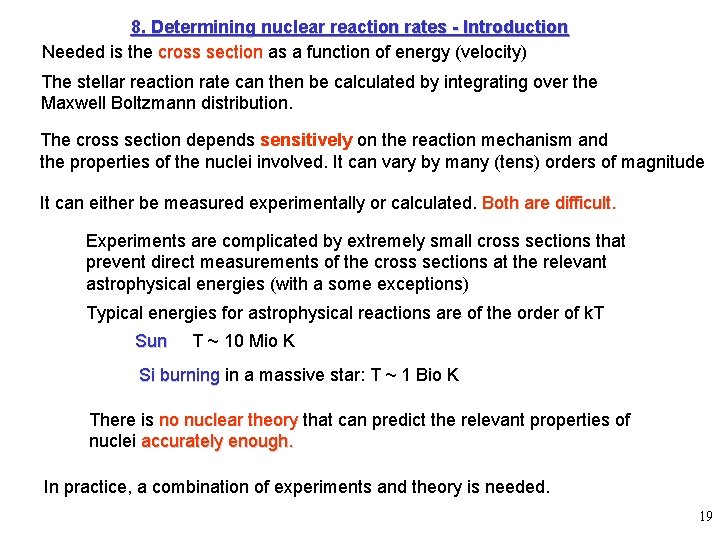
8. Determining nuclear reaction rates - Introduction Needed is the cross section as a function of energy (velocity) The stellar reaction rate can then be calculated by integrating over the Maxwell Boltzmann distribution. The cross section depends sensitively on the reaction mechanism and the properties of the nuclei involved. It can vary by many (tens) orders of magnitude It can either be measured experimentally or calculated. Both are difficult. Experiments are complicated by extremely small cross sections that prevent direct measurements of the cross sections at the relevant astrophysical energies (with a some exceptions) Typical energies for astrophysical reactions are of the order of k. T Sun T ~ 10 Mio K Si burning in a massive star: T ~ 1 Bio K There is no nuclear theory that can predict the relevant properties of nuclei accurately enough. In practice, a combination of experiments and theory is needed. 19

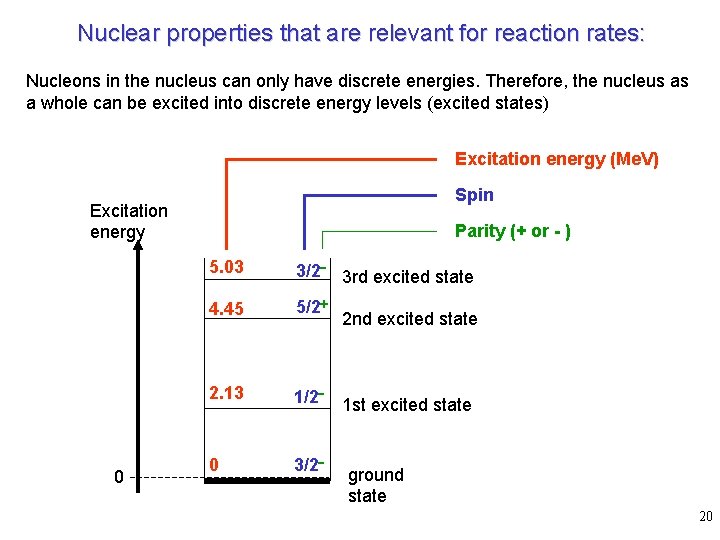
Nuclear properties that are relevant for reaction rates: Nucleons in the nucleus can only have discrete energies. Therefore, the nucleus as a whole can be excited into discrete energy levels (excited states) Excitation energy (Me. V) Spin Excitation energy 0 Parity (+ or - ) 5. 03 3/2 - 3 rd excited state 4. 45 5/2+ 2. 13 1/2 - 1 st excited state 0 3/2 - 2 nd excited state ground state 20

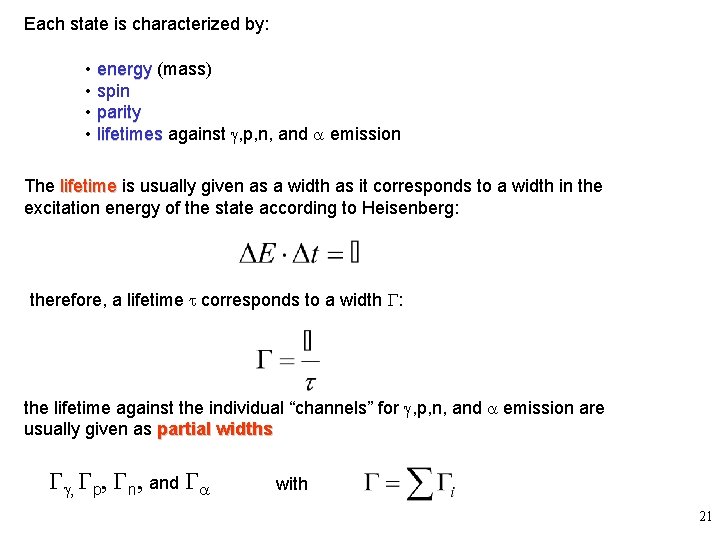
Each state is characterized by: • energy (mass) • spin • parity • lifetimes against g, p, n, and a emission The lifetime is usually given as a width as it corresponds to a width in the excitation energy of the state according to Heisenberg: therefore, a lifetime t corresponds to a width G: the lifetime against the individual “channels” for g, p, n, and a emission are usually given as partial widths Gg, Gp, Gn, and Ga with 21

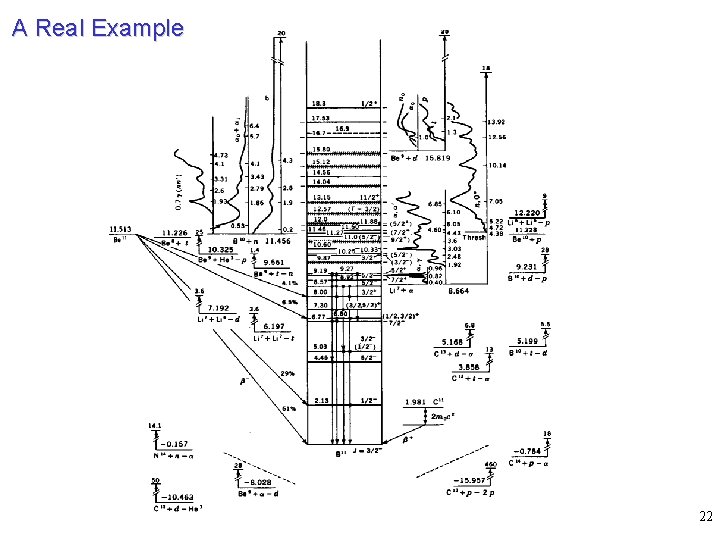
A Real Example 22

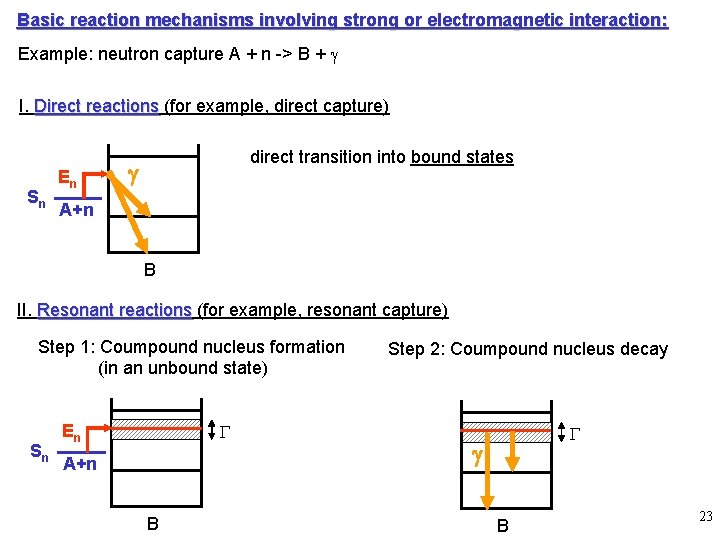
Basic reaction mechanisms involving strong or electromagnetic interaction: Example: neutron capture A + n -> B + g I. Direct reactions (for example, direct capture) Sn En direct transition into bound states g A+n B II. Resonant reactions (for example, resonant capture) Step 1: Coumpound nucleus formation (in an unbound state) Sn G En A+n B Step 2: Coumpound nucleus decay G g B 23

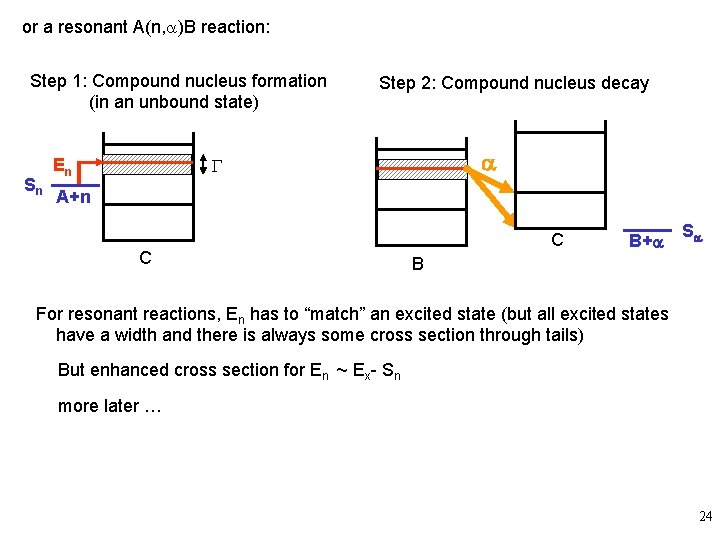
or a resonant A(n, a)B reaction: Step 1: Compound nucleus formation (in an unbound state) Sn Step 2: Compound nucleus decay a G En A+n C C B+a Sa B For resonant reactions, En has to “match” an excited state (but all excited states have a width and there is always some cross section through tails) But enhanced cross section for En ~ Ex- Sn more later … 24
 Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Bomb power
Bomb power What is a unit ratio
What is a unit ratio Equivalent ratios definition
Equivalent ratios definition Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers An example of redox reaction
An example of redox reaction Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Hamlet act iii scene iii
Hamlet act iii scene iii Nuclear decays and reactions section 2
Nuclear decays and reactions section 2 U 235 fission products
U 235 fission products Natural transmutation
Natural transmutation Balancing nuclear reactions
Balancing nuclear reactions Gamma decay nuclear equation
Gamma decay nuclear equation Flerov laboratory of nuclear reactions
Flerov laboratory of nuclear reactions Nuclear decay reactions examples
Nuclear decay reactions examples Two types of nuclear reactions
Two types of nuclear reactions Nuclear bombardment reactions
Nuclear bombardment reactions Nuclear reactions are at
Nuclear reactions are at Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium