HTA Methods Guidelines for Medical Devices How can















- Slides: 19

HTA Methods Guidelines for Medical Devices: How can We Address the Gaps? The International Federation of Medical and Biological Engineering Perspective Presented by: Julie Polisena, Ph. D

Disclosure • IFMBE and the University of Warwick provided financial support for this study. • Authors declare no COI.

IFMBE The International Federation for Medical and Biological Engineering (IFMBE) is primarily a federation of national and transnational societies. These professional organizations represent interests in medical and biological engineering. The IFMBE is also a Non-Governmental Organization (NGO) for the United Nations and the World Health Organization (WHO).

Introduction Drug Therapies Medical Devices

Introduction

Study Objectives • Review and identify gaps in HTA MD guidelines • Propose recommendations • Comparison between guidelines and recommendations • Reach consensus among clinical and biomedical engineers

Methods • Focus groups o Narrative summary of HTA MD guidelines o Discussions with IFMBE-HTA Division • Differences between drugs and MDs • Impact on HTA methods • Recommendations for MD assessment

Methods • Modified Delphi survey o. Survey design o. Online o. Delphi process o. Agreement level on 30 recommendations o 5 -point Likert scale

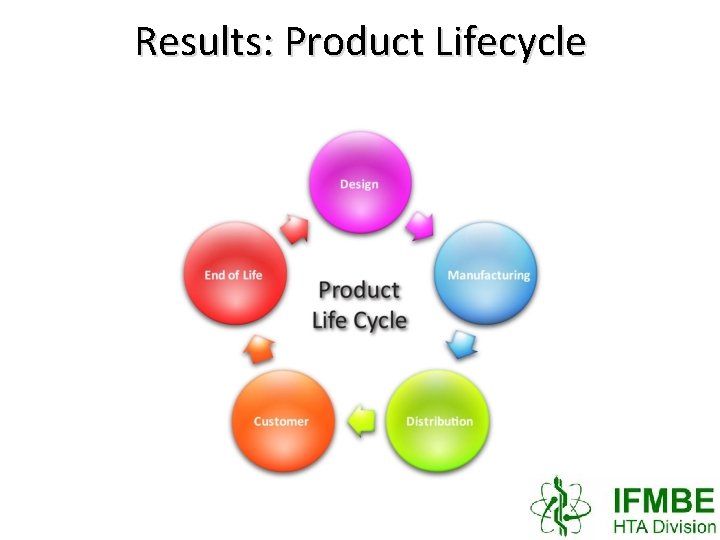
Results • Focus Group o Product lifecycle o Clinical evaluation o Issues in use o Costs and economic evaluation

Results: Product Lifecycle

Results: Clinical Evaluation

Results: Issues in Use

Results: Costs and Economic Evaluation

Results • Delphi survey Characteristics of Panelists (N=32): 37. 6% response rate 17 countries > 25% were clinical engineers or managers > 65% graduate studies > 70% involved in HTA

Results • Delphi survey o Consensus on recommendations o Consensus with strong agreement reached in 90% recommendations o Consensus with agreement reached in 10% recommendations

Discussion • Comments: o Feasibility of accurate assessment throughout product lifecycle o Interoperability of medical device with other devices or within hospital systems o Availability of data or evidence o Adoption of medical device in organization o New methods may be required o Risk assessment

Limitations • Uncertain about interpretation of recommendations and descriptions in survey • Not all perspectives were represented

Directions for Future Research

Reference • Polisena J, Castaldo R, Ciani O, Federici C, Borsci S, Ritrovato M, Clark D, and Pecchia L. “HTA Methods Guidelines for Medical Devices: How can We Address the Gaps? ” The International Federation of Medical and Biological Engineering Perspective. 2018. (International Journal of Technology Assessment in Health Care, 34: 3 (2018), 276– 289))