Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver




- Slides: 20

Hepatocellular Carcinoma in Patients with Non-alcoholic Fatty Liver Disease IM R 3 전유경 / Pf. 이동현

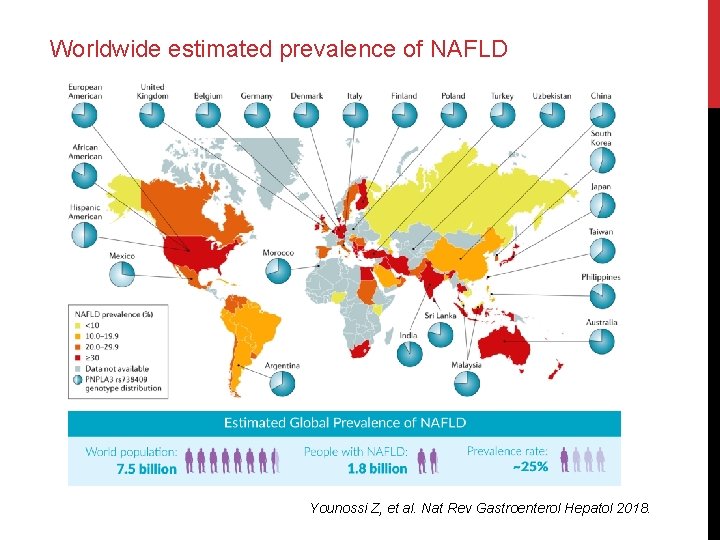
Worldwide estimated prevalence of NAFLD Younossi Z, et al. Nat Rev Gastroenterol Hepatol 2018.

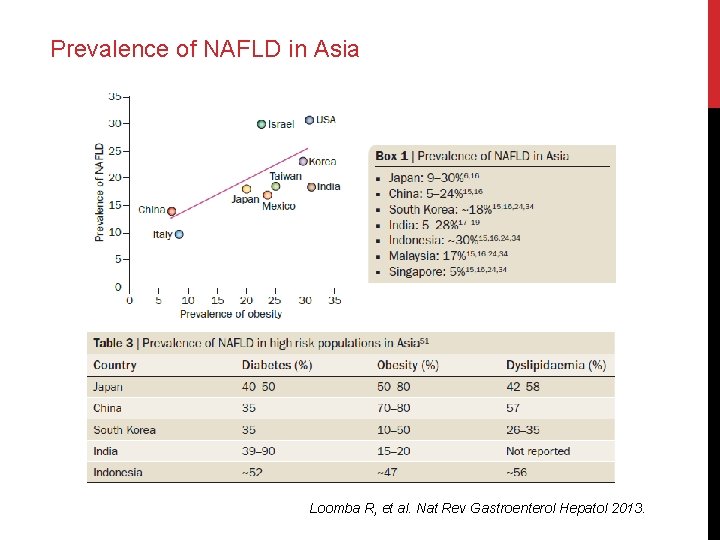
Prevalence of NAFLD in Asia Loomba R, et al. Nat Rev Gastroenterol Hepatol 2013.

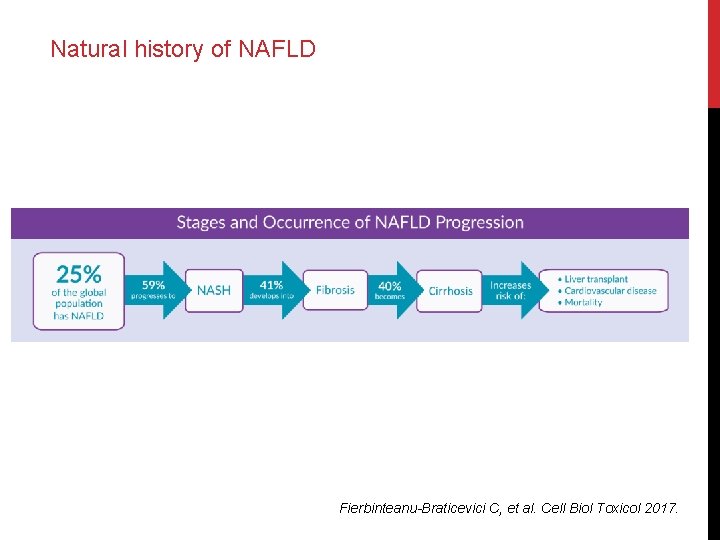
Natural history of NAFLD Fierbinteanu-Braticevici C, et al. Cell Biol Toxicol 2017.

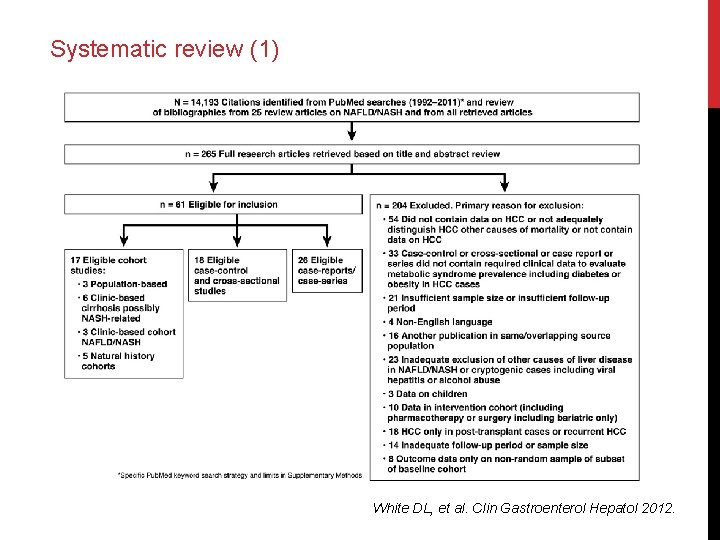
Systematic review (1) White DL, et al. Clin Gastroenterol Hepatol 2012.

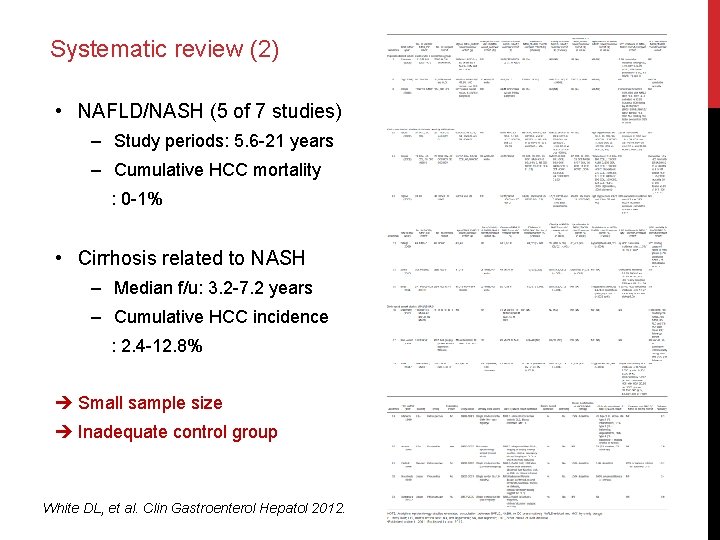
Systematic review (2) • NAFLD/NASH (5 of 7 studies) ‒ Study periods: 5. 6 -21 years ‒ Cumulative HCC mortality : 0 -1% • Cirrhosis related to NASH ‒ Median f/u: 3. 2 -7. 2 years ‒ Cumulative HCC incidence : 2. 4 -12. 8% Small sample size Inadequate control group White DL, et al. Clin Gastroenterol Hepatol 2012.


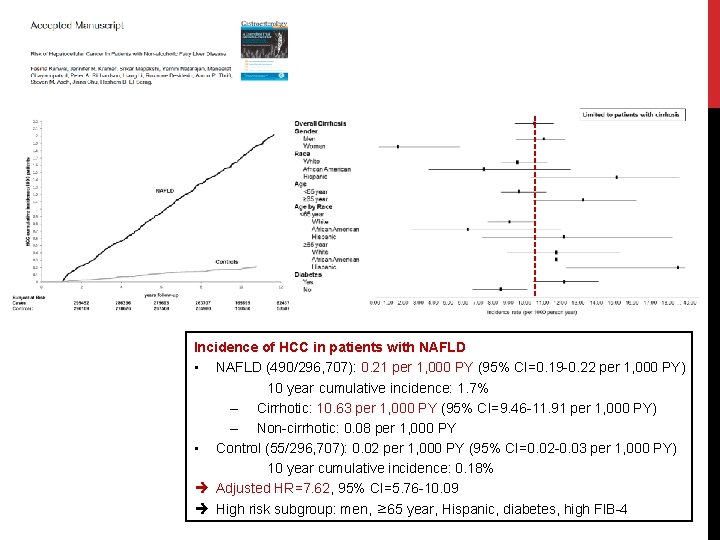
Incidence of HCC in patients with NAFLD • NAFLD (490/296, 707): 0. 21 per 1, 000 PY (95% CI=0. 19 -0. 22 per 1, 000 PY) 10 year cumulative incidence: 1. 7% – Cirrhotic: 10. 63 per 1, 000 PY (95% CI=9. 46 -11. 91 per 1, 000 PY) – Non-cirrhotic: 0. 08 per 1, 000 PY • Control (55/296, 707): 0. 02 per 1, 000 PY (95% CI=0. 02 -0. 03 per 1, 000 PY) 10 year cumulative incidence: 0. 18% Adjusted HR=7. 62, 95% CI=5. 76 -10. 09 High risk subgroup: men, ≥ 65 year, Hispanic, diabetes, high FIB-4

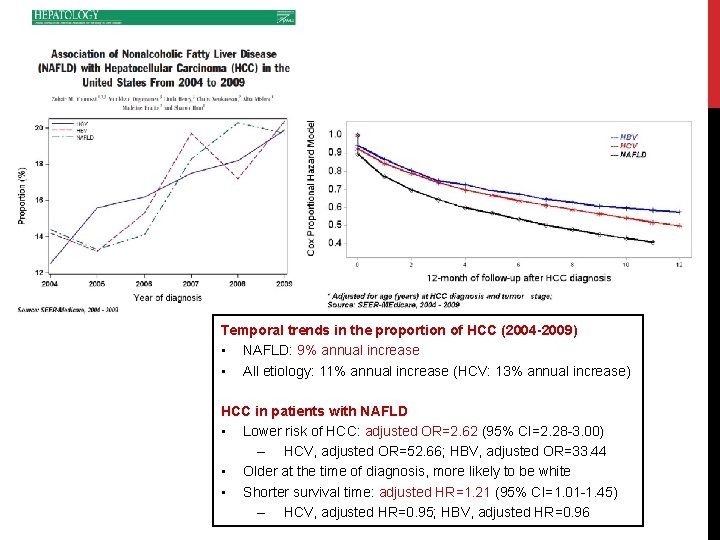
Temporal trends in the proportion of HCC (2004 -2009) • NAFLD: 9% annual increase • All etiology: 11% annual increase (HCV: 13% annual increase) HCC in patients with NAFLD • Lower risk of HCC: adjusted OR=2. 62 (95% CI=2. 28 -3. 00) – HCV, adjusted OR=52. 66; HBV, adjusted OR=33. 44 • Older at the time of diagnosis, more likely to be white • Shorter survival time: adjusted HR=1. 21 (95% CI=1. 01 -1. 45) – HCV, adjusted HR=0. 95; HBV, adjusted HR=0. 96

Limitation • Retrospective population-based study ‒ Lack of clinical information Ø Unable to conduct adjusting for clinical variables Ø Misclassification bias Sensitive analyses ‒ Imperfect definition of NAFLD Chart validation (PPV=89%, NPV=98%), Sensitive analyses Too low risk for evaluation ! Kanwal F, et al. Gastroenterology 2018. Younossi ZM, et al. Hepatology 2015.

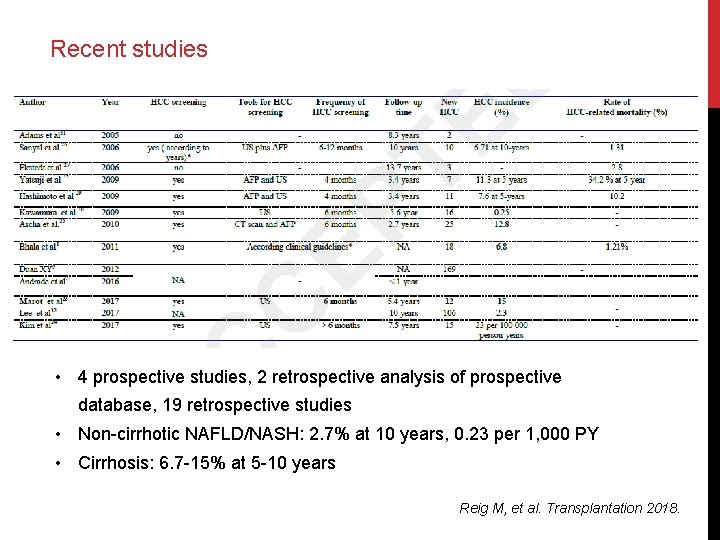
Recent studies • 4 prospective studies, 2 retrospective analysis of prospective database, 19 retrospective studies • Non-cirrhotic NAFLD/NASH: 2. 7% at 10 years, 0. 23 per 1, 000 PY • Cirrhosis: 6. 7 -15% at 5 -10 years Reig M, et al. Transplantation 2018.

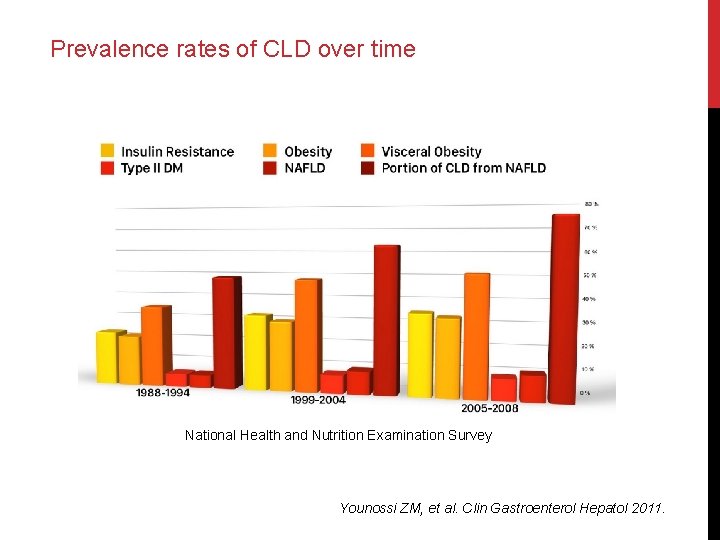
Prevalence rates of CLD over time National Health and Nutrition Examination Survey Younossi ZM, et al. Clin Gastroenterol Hepatol 2011.

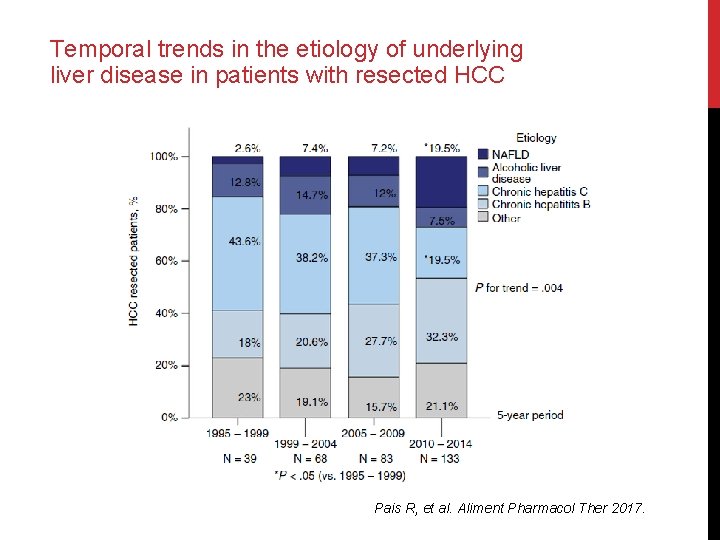
Temporal trends in the etiology of underlying liver disease in patients with resected HCC Pais R, et al. Aliment Pharmacol Ther 2017.

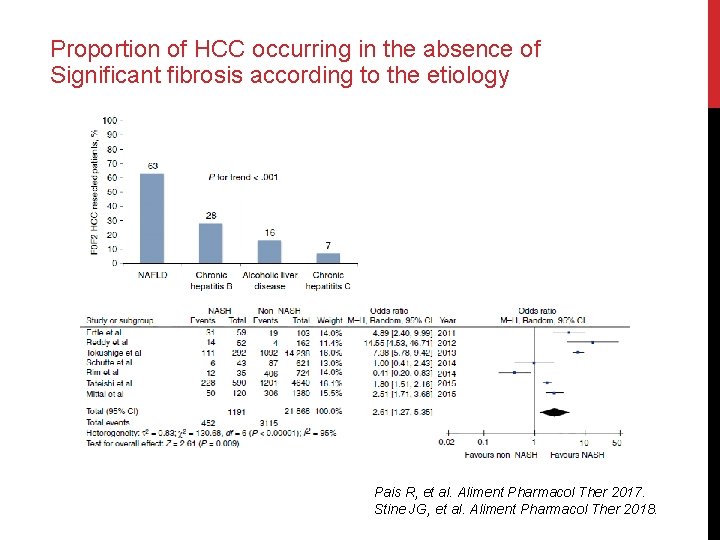
Proportion of HCC occurring in the absence of Significant fibrosis according to the etiology Pais R, et al. Aliment Pharmacol Ther 2017. Stine JG, et al. Aliment Pharmacol Ther 2018.

Surveillance

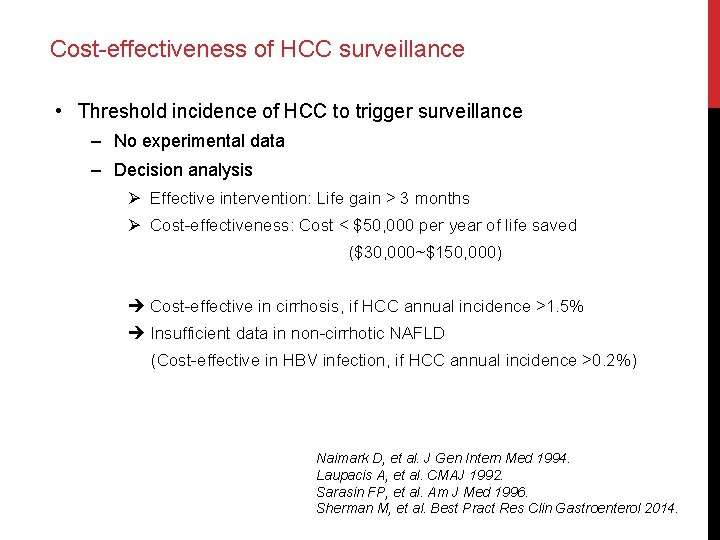
Cost-effectiveness of HCC surveillance • Threshold incidence of HCC to trigger surveillance ‒ No experimental data ‒ Decision analysis Ø Effective intervention: Life gain > 3 months Ø Cost-effectiveness: Cost < $50, 000 per year of life saved ($30, 000~$150, 000) Cost-effective in cirrhosis, if HCC annual incidence >1. 5% Insufficient data in non-cirrhotic NAFLD (Cost-effective in HBV infection, if HCC annual incidence >0. 2%) Naimark D, et al. J Gen Intern Med 1994. Laupacis A, et al. CMAJ 1992. Sarasin FP, et al. Am J Med 1996. Sherman M, et al. Best Pract Res Clin Gastroenterol 2014.

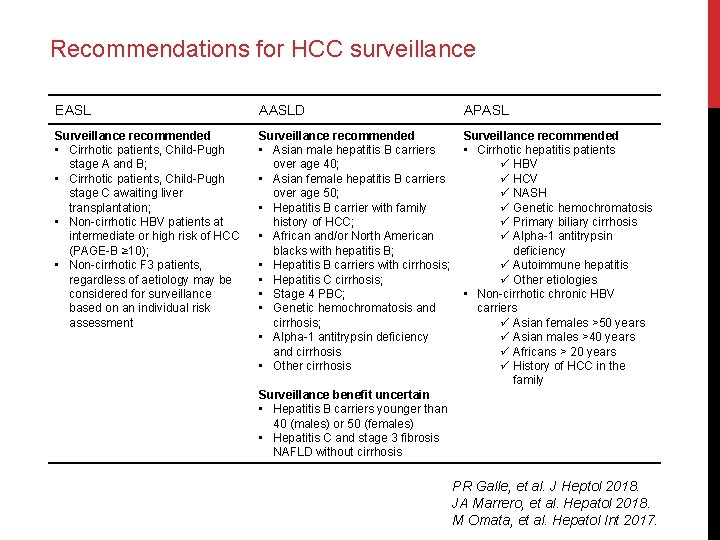
Recommendations for HCC surveillance EASL AASLD APASL Surveillance recommended • Cirrhotic patients, Child-Pugh stage A and B; • Cirrhotic patients, Child-Pugh stage C awaiting liver transplantation; • Non-cirrhotic HBV patients at intermediate or high risk of HCC (PAGE-B ≥ 10); • Non-cirrhotic F 3 patients, regardless of aetiology may be considered for surveillance based on an individual risk assessment Surveillance recommended • Asian male hepatitis B carriers over age 40; • Asian female hepatitis B carriers over age 50; • Hepatitis B carrier with family history of HCC; • African and/or North American blacks with hepatitis B; • Hepatitis B carriers with cirrhosis; • Hepatitis C cirrhosis; • Stage 4 PBC; • Genetic hemochromatosis and cirrhosis; • Alpha-1 antitrypsin deficiency and cirrhosis • Other cirrhosis Surveillance recommended • Cirrhotic hepatitis patients ü HBV ü HCV ü NASH ü Genetic hemochromatosis ü Primary biliary cirrhosis ü Alpha-1 antitrypsin deficiency ü Autoimmune hepatitis ü Other etiologies • Non-cirrhotic chronic HBV carriers ü Asian females >50 years ü Asian males >40 years ü Africans > 20 years ü History of HCC in the family Surveillance benefit uncertain • Hepatitis B carriers younger than 40 (males) or 50 (females) • Hepatitis C and stage 3 fibrosis NAFLD without cirrhosis PR Galle, et al. J Heptol 2018. JA Marrero, et al. Hepatol 2018. M Omata, et al. Hepatol Int 2017.

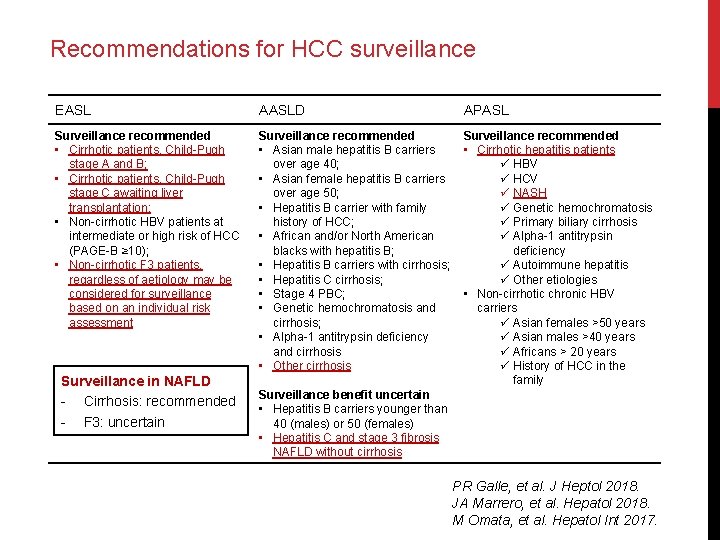
Recommendations for HCC surveillance EASL AASLD APASL Surveillance recommended • Cirrhotic patients, Child-Pugh stage A and B; • Cirrhotic patients, Child-Pugh stage C awaiting liver transplantation; • Non-cirrhotic HBV patients at intermediate or high risk of HCC (PAGE-B ≥ 10); • Non-cirrhotic F 3 patients, regardless of aetiology may be considered for surveillance based on an individual risk assessment Surveillance recommended • Asian male hepatitis B carriers over age 40; • Asian female hepatitis B carriers over age 50; • Hepatitis B carrier with family history of HCC; • African and/or North American blacks with hepatitis B; • Hepatitis B carriers with cirrhosis; • Hepatitis C cirrhosis; • Stage 4 PBC; • Genetic hemochromatosis and cirrhosis; • Alpha-1 antitrypsin deficiency and cirrhosis • Other cirrhosis Surveillance recommended • Cirrhotic hepatitis patients ü HBV ü HCV ü NASH ü Genetic hemochromatosis ü Primary biliary cirrhosis ü Alpha-1 antitrypsin deficiency ü Autoimmune hepatitis ü Other etiologies • Non-cirrhotic chronic HBV carriers ü Asian females >50 years ü Asian males >40 years ü Africans > 20 years ü History of HCC in the family Surveillance in NAFLD - Cirrhosis: recommended - F 3: uncertain Surveillance benefit uncertain • Hepatitis B carriers younger than 40 (males) or 50 (females) • Hepatitis C and stage 3 fibrosis NAFLD without cirrhosis PR Galle, et al. J Heptol 2018. JA Marrero, et al. Hepatol 2018. M Omata, et al. Hepatol Int 2017.

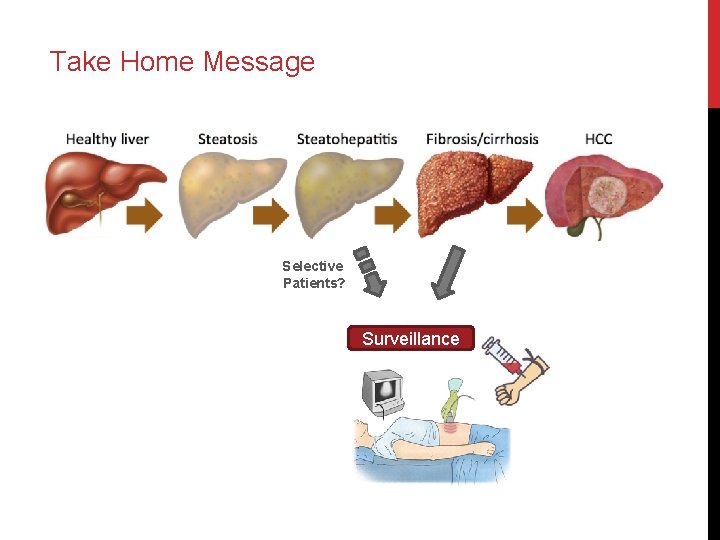
Take Home Message Selective Patients? Surveillance

 Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease Hepatocellular jaundice
Hepatocellular jaundice Acute fatty liver of pregnancy
Acute fatty liver of pregnancy Liversoc
Liversoc Non-alcoholic fatty liver disease (nafld)
Non-alcoholic fatty liver disease (nafld) Acute fatty liver of pregnancy
Acute fatty liver of pregnancy Chapter 58 care of patients with liver problems
Chapter 58 care of patients with liver problems Stdis
Stdis Prurito clitoride
Prurito clitoride Carcinoma in situ
Carcinoma in situ Carcinoma
Carcinoma Tumor carcinoide
Tumor carcinoide Cervix carcinoma
Cervix carcinoma Arteria axilar
Arteria axilar Lobular breast
Lobular breast Thymus gland location
Thymus gland location Carcinoma squamoso polmone
Carcinoma squamoso polmone Carcinoma vs adenoma
Carcinoma vs adenoma Squamous cell carcinoma
Squamous cell carcinoma Haggit kriterleri
Haggit kriterleri Invasive ductal carcinoma with medullary features
Invasive ductal carcinoma with medullary features