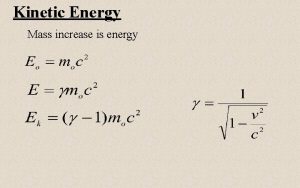Heat Kinetic Energy at the Nanoscale Heat vs



















- Slides: 19

Heat Kinetic Energy at the Nanoscale

Heat vs. Temperature n n Heat and temperature are related, but they are not the same Temperature is a measure of the average kinetic energy of particles (recall KE = ½mv 2); heat is energy that can be transferred

Heat n n Heat is the amount of energy that can be transferred from one object to another due to a temperature difference. Heat flows from warmer objects to cooler objects.

Heat vs. Temperature n n n Comparing a beaker of boiling water with one drop of boiling water, which is at a higher temperature? which contains more heat?

Temperature Units n Temperature is usually measured in o. F, or o. C n o. C = 5/9(o. F -32) n What is 75 o. F on the Celsius scale?

Temperature Units n The Kelvin scale is based on absolute zero When particles have lost all the kinetic energy they can lose, the object is at absolute zero, 0 K n K + 273 = o. C n

Heat Units n n Since heat is a form of energy, it is often reported in Joules, J Another common heat unit is the calorie 1 calorie = 1 cal = 4. 184 J

The calorie n n n One calorie is the amount of heat needed to raise the temperature of one gram of water by one degree Celsius How much heat to raise temperature of 2 g water by 1 o. C? How much heat to raise temperature of 1 g water by 2 o. C?

Specific Heat n n The amount of heat needed to raise the temperature of one gram of a substance by one degree Celsius is the specific heat, c. The specific heat of water is 1 cal/(g o. C)

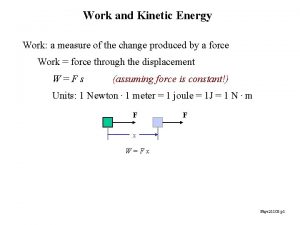
Heat n n n q = mcΔT q is the amount of heat that flows m is the mass of the substance c is the specific heat of the substance ΔT is the temperature change

example n n If 100 calories flows into 20 g of water (c = 1 cal/(g o. C)) initially at 20 o. C, what will its temperature become? Why does hot pizza burn the roof of your mouth but not your tongue?

Heat & Phase Changes n Ice bath activity n n Did the temperature of the ice bath change? Did the temperature of the surroundings change? Did heat flow? Was energy conserved?

Heat & Phase Changes n A flow of heat can cause a change in the. . . n n kinetic energy of particles (q = mcΔT) potential energy of the particles (q = m. L) where L is the latent heat

Heat & Phase Changes n Adding heat causes a temperature change UNTIL a phase change begins, then the heat causes the phase change. Ice -10°C heat Temperature change Ice 0°C heat Phase change Water 0°C

OBJECT (large objects are about the same size) MASS (grams) largest marble 59 large marble 21 large steel sphere 67 large aluminum sphere 23 ice cubes 21 ice-cold water 21 INDIVIDUAL RANK 1=largest effect, 6=smallest effect Initial temperature of water: _____ Final temperature of water: ______ GROUP RANK 1=largest effect, 6=smallest effect expt. temp change OBJECT: _______ temp change: ______

Heat, Temperature & Phase Change

Heat & Phase Changes n How much heat is needed to melt 250 g of ice at 0 o. C into water at 0 o. C? The latent heat of fusion for ice is 333 J/g. q = m. L q = (250 g)(333 J/g) = 83, 250 J

Heat Flow n n When heat is absorbed by a system, the process is called endothermic When heat is released from a system, the process is called exothermic

Phase Changes c mi er th o nd E Gas do Ex ic m er the oth h xot E En rm ic erm ic Endothermic Solid Exothermic Liquid
 What is nanoscale
What is nanoscale Nanoscale lithography
Nanoscale lithography Micro and nanoscale fluid mechanics
Micro and nanoscale fluid mechanics Spring potential energy
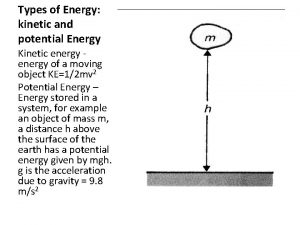
Spring potential energy Kinetic energy and gravity
Kinetic energy and gravity Gravitational potential energy vs kinetic energy
Gravitational potential energy vs kinetic energy Thermal energy and mass
Thermal energy and mass Kinetic energy vs potential energy
Kinetic energy vs potential energy Example of kinetic energy
Example of kinetic energy Potential energy
Potential energy Potential energy
Potential energy Formula of potential energy
Formula of potential energy Thermal energy vs heat energy
Thermal energy vs heat energy Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Temperature is a measure of the average
Temperature is a measure of the average Acceleration energy
Acceleration energy Ads=vdv example
Ads=vdv example Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Normalizable wave function
Normalizable wave function