GSF Groupe Sarcome Franais GETO Groupe dEtudes Tumeurs



- Slides: 13

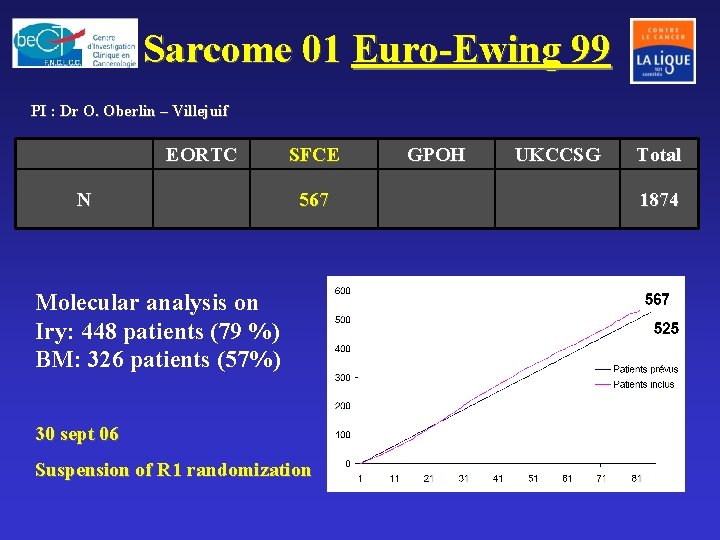
GSF: Groupe Sarcome Français GETO: Groupe d’Etudes Tumeurs Osseuses Lille Rouen Caen N = 36 institutions Rennes Reims Paris- RP Villejuif Nancy Strasbourg Angers Tours Sarcoma Database: 3300 pts Frozen tissue bank: 1300 sarcomas Nantes Dijon Besançon Poitiers Clermont-Ferrand Lyon Bordeaux Toulouse Nice Montpellier Marseille

Sarcome 01 Euro-Ewing 99 PI : Dr O. Oberlin – Villejuif EORTC N SFCE 567 Molecular analysis on Iry: 448 patients (79 %) BM: 326 patients (57%) 30 sept 06 Suspension of R 1 randomization GPOH UKCCSG Total 1874

Sarcome 02 PALSAR II PI : Dr N B BUI – Bordeaux Main end-point: overall survival Statistical hypothesis: 3 -yr OS: 40% vs 15% (N® per arm: 50) October 06: N = 207 patients N® = 65/100 patients

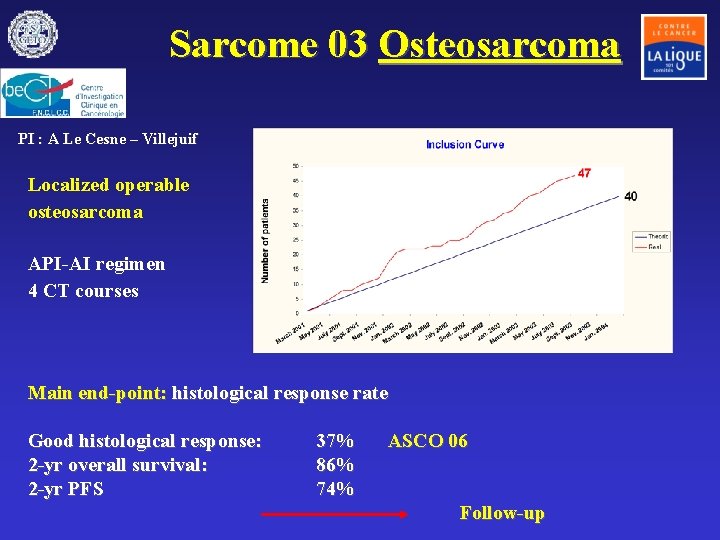
Sarcome 03 Osteosarcoma PI : A Le Cesne – Villejuif Localized operable osteosarcoma API-AI regimen 4 CT courses Main end-point: histological response rate Good histological response: 2 -yr overall survival: 2 -yr PFS 37% 86% 74% ASCO 06 Follow-up

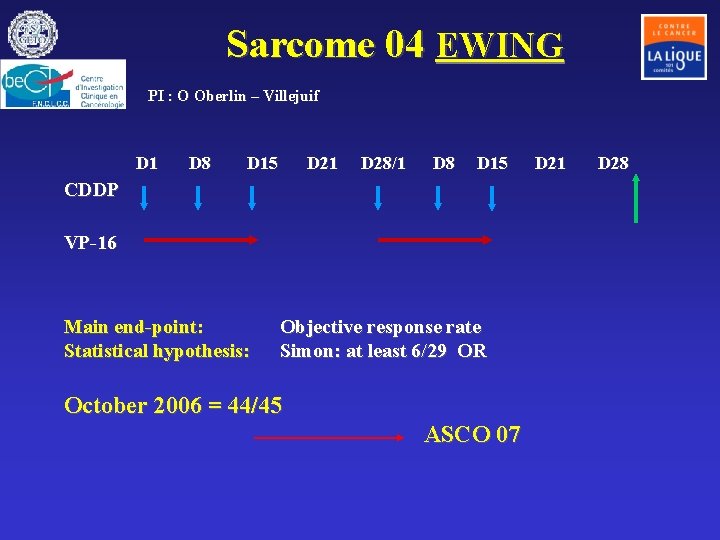
Sarcome 04 EWING PI : O Oberlin – Villejuif D 1 D 8 D 15 D 21 D 28/1 D 8 D 15 CDDP VP-16 Main end-point: Statistical hypothesis: Objective response rate Simon: at least 6/29 OR October 2006 = 44/45 ASCO 07 D 21 D 28

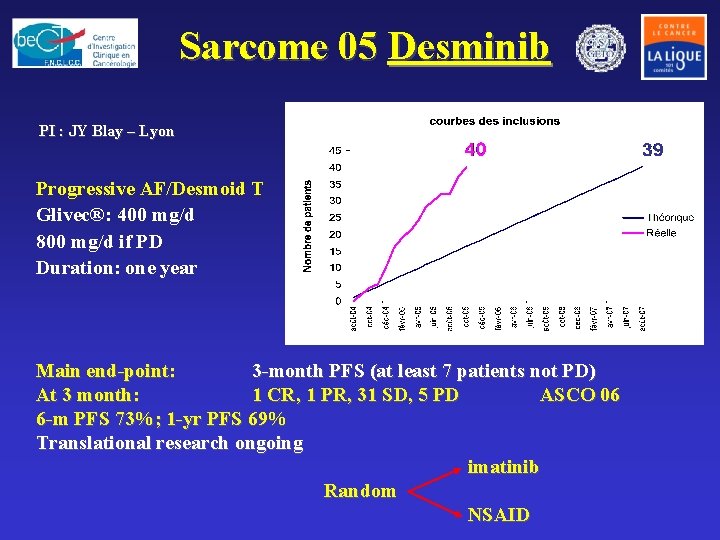
Sarcome 05 Desminib PI : JY Blay – Lyon Progressive AF/Desmoid T Glivec®: 400 mg/d 800 mg/d if PD Duration: one year Main end-point: 3 -month PFS (at least 7 patients not PD) At 3 month: 1 CR, 1 PR, 31 SD, 5 PD ASCO 06 6 -m PFS 73%; 1 -yr PFS 69% Translational research ongoing imatinib Random NSAID

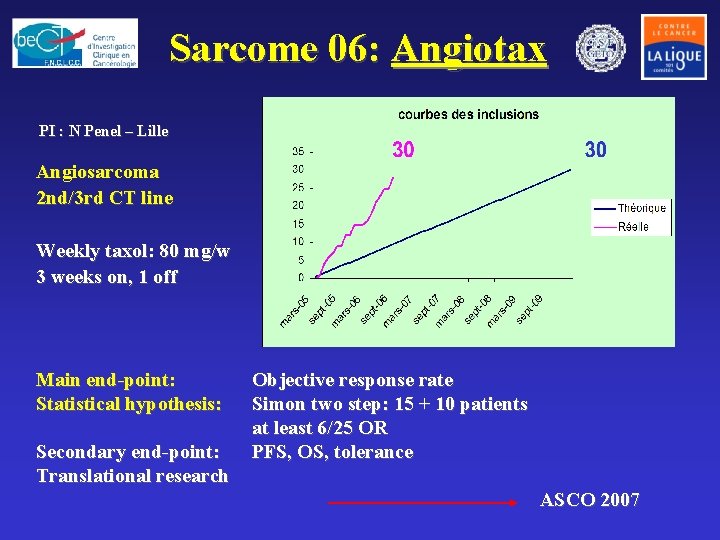
Sarcome 06: Angiotax PI : N Penel – Lille Angiosarcoma 2 nd/3 rd CT line Weekly taxol: 80 mg/w 3 weeks on, 1 off Main end-point: Statistical hypothesis: Secondary end-point: Translational research Objective response rate Simon two step: 15 + 10 patients at least 6/25 OR PFS, OS, tolerance ASCO 2007

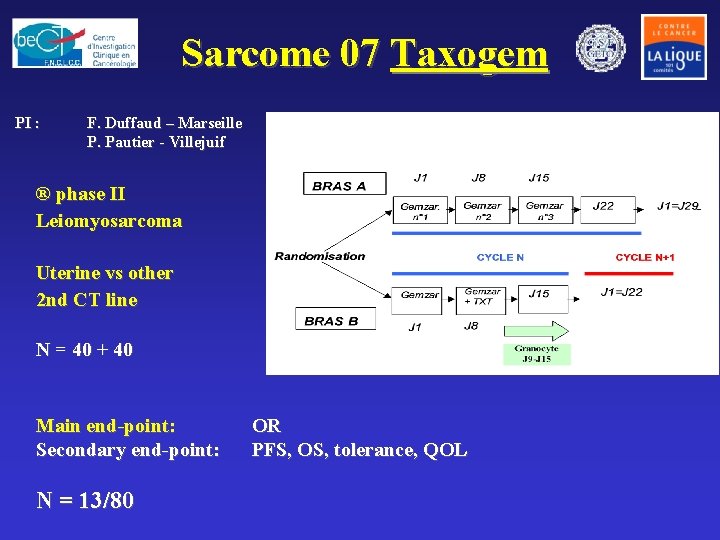
Sarcome 07 Taxogem PI : F. Duffaud – Marseille P. Pautier - Villejuif ® phase II Leiomyosarcoma Uterine vs other 2 nd CT line N = 40 + 40 Main end-point: Secondary end-point: N = 13/80 OR PFS, OS, tolerance, QOL

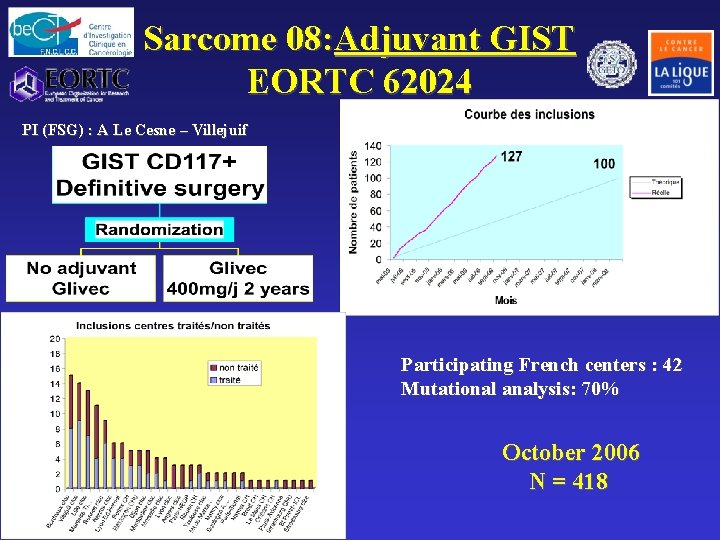
Sarcome 08: Adjuvant GIST EORTC 62024 PI (FSG) : A Le Cesne – Villejuif Participating French centers : 42 Mutational analysis: 70% October 2006 N = 418

Sarcome 09 OS 2006 PI : F Duffaud – Curie / L Brugieres - Villejuif Intergroup SFCE/GSF/GETO Adult/children OS – phase III Zoledronate N = 470 3 -yr EFS > 13% in exp arm Translational research

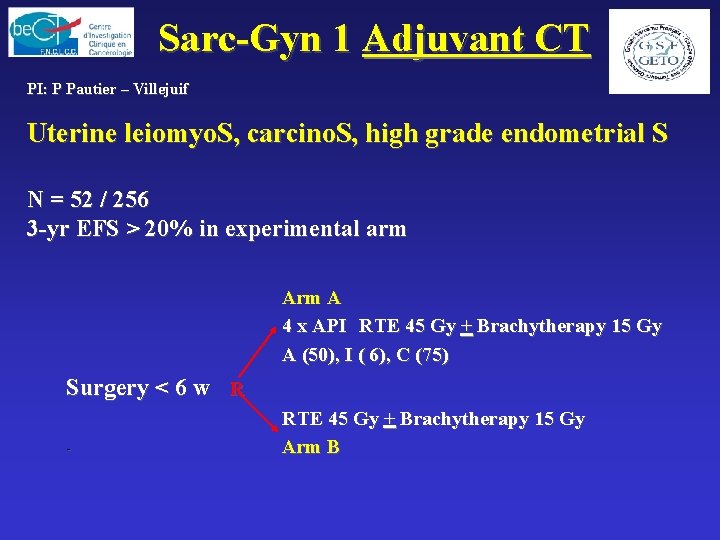
Sarc-Gyn 1 Adjuvant CT PI: P Pautier – Villejuif Uterine leiomyo. S, carcino. S, high grade endometrial S N = 52 / 256 3 -yr EFS > 20% in experimental arm A 4 x API RTE 45 Gy + Brachytherapy 15 Gy A (50), I ( 6), C (75) Surgery < 6 w R - RTE 45 Gy + Brachytherapy 15 Gy Arm B

BFR 14 trial - Advanced GIST (June 2002) PI : J. Y Blay – Lyon A. Le Cesne - Villejuif Imatinib Stop Non PD R Imatinib N patients included in October 2005: 271 1) N randomized (interrupted) pts at 1 year: 58 (ASCO 04, 05) Stop imatinib arm: 32 imatinib arm: 26 2) N randomized (ongoing) pts at 3 years: 32 (ASCO 07)

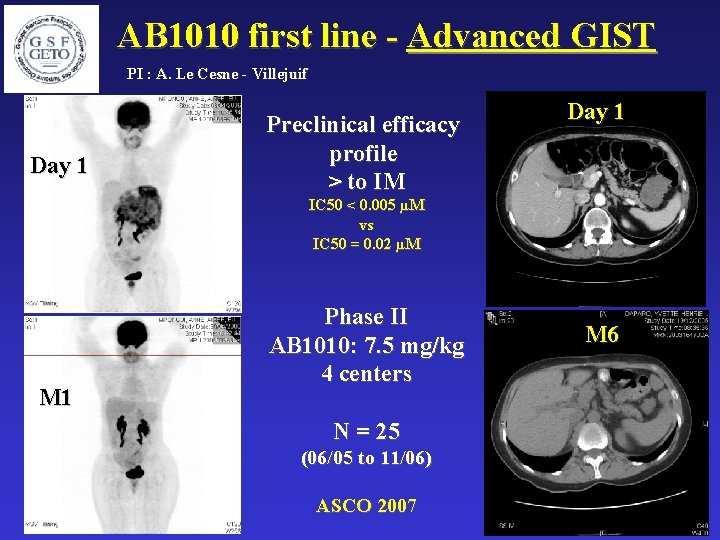
AB 1010 first line - Advanced GIST PI : A. Le Cesne - Villejuif Day 1 Preclinical efficacy profile > to IM Day 1 IC 50 < 0. 005 µM vs IC 50 = 0. 02 µM M 1 Phase II AB 1010: 7. 5 mg/kg 4 centers N = 25 (06/05 to 11/06) ASCO 2007 M 6
























