Glycoproteins Sialylation and the Ashwell Receptor The glycan






- Slides: 27

Glycoproteins, Sialylation and the Ashwell Receptor The glycan branches of glycoproteins often have sialic acid (N-acetylneuraminic acid) added to their termini The process of adding sialic acid to these glycoproteins is called “SIALYLATION” and this process is critical for the function and clearance of glycoproteins from the body Glycoproteins without sialic acid residues (Asialoglycoproteins) can be rapidly cleared by the hepatic asialoglycoprotein receptor, also known as the Ashwell receptor

THE ASHWELL RECEPTOR • 1965 Gilbert Ashwell was on sabbatical at Columbia • He was having dinner with Anatol Morell from AECOM who mentioned that he was having difficulty determining the half life of the glycoprotein that he was working on, ceruloplasmin • Since Ashwell was working in carbohydrates, he suggested that they label a terminal galactose with a long-lasting tritium isotope to determine the half life of ceruloplasmin • So they injected this material into a rabbit which rapidly disappeared from the circulation within 5 -10 minutes and was recovered completely intact in the liver • In 1974 they isolated and characterized this hepatic receptor as the first mammalian lectin, a receptor which recognizes particular glycoproteins, the asialoglycoproteins (glycoproteins with glycan chains lacking sialic acid) • Being modest gentlemen they felt the appropriate name for this receptor should be the “Ashwell-Morell receptor’

THE ASHWELL RECEPTOR Ashwell Receptor is one of many lectins that bind asialoglycoproteins (recent data suggests that this receptor may bind sialylated glycans as well) Asialoglycoprotein receptors (ASGPR’s) are involved in finding and endocytosing various glycoproteins Ashwell Receptor is on the hepatocyte cell surface where it can remove and degrade potentially harmful circulating glycoproteins Yet the story of the Ashwell Receptor has remained mysterious since endogenous ligands have never been discovered Hopefully our discussion today will begin to shed light on the role of the Ashwell Receptor in regulating sepsis Dr. Ashwell commented that he was dumbfounded by the results of this paper since he had been working on this protein for over 30 years trying to determine its real biologic function (the knockout mice seemed to function normally) With the discovery of a ligand for the Ashwell Receptor, we have the first real evidence for the receptor’s physiologic function which may explain why it has been conserved throughout vertebrate evolution

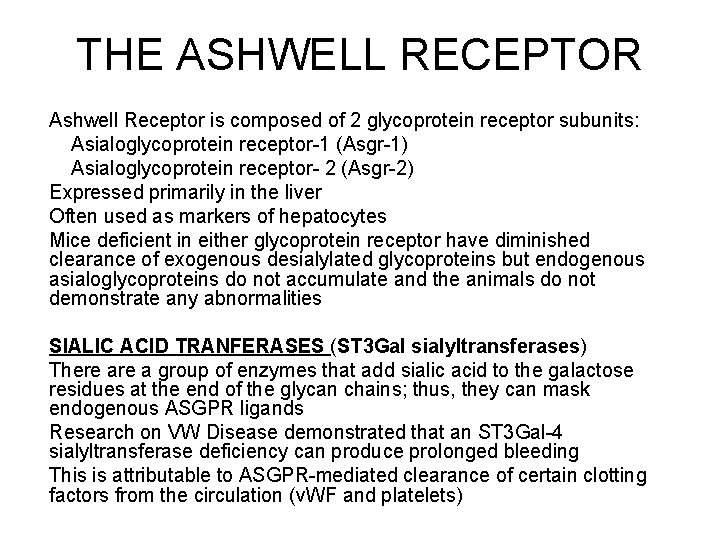
THE ASHWELL RECEPTOR Ashwell Receptor is composed of 2 glycoprotein receptor subunits: Asialoglycoprotein receptor-1 (Asgr-1) Asialoglycoprotein receptor- 2 (Asgr-2) Expressed primarily in the liver Often used as markers of hepatocytes Mice deficient in either glycoprotein receptor have diminished clearance of exogenous desialylated glycoproteins but endogenous asialoglycoproteins do not accumulate and the animals do not demonstrate any abnormalities SIALIC ACID TRANFERASES (ST 3 Gal sialyltransferases) There a group of enzymes that add sialic acid to the galactose residues at the end of the glycan chains; thus, they can mask endogenous ASGPR ligands Research on VW Disease demonstrated that an ST 3 Gal-4 sialyltransferase deficiency can produce prolonged bleeding This is attributable to ASGPR-mediated clearance of certain clotting factors from the circulation (v. WF and platelets)

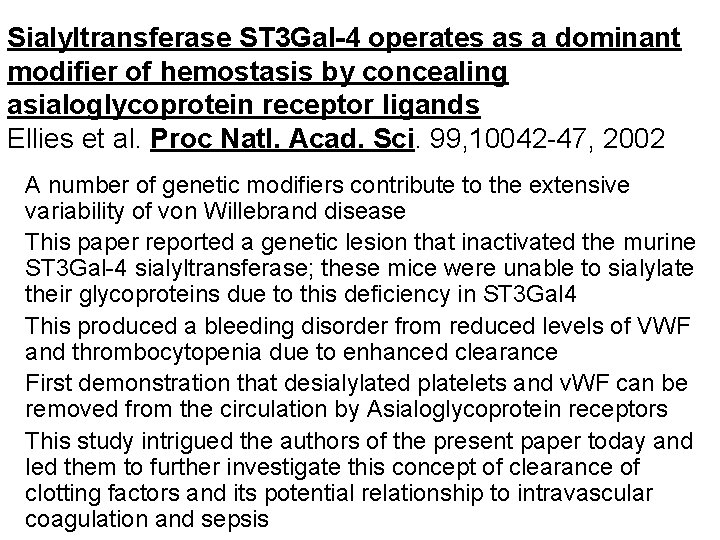
Sialyltransferase ST 3 Gal-4 operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands Ellies et al. Proc Natl. Acad. Sci. 99, 10042 -47, 2002 A number of genetic modifiers contribute to the extensive variability of von Willebrand disease This paper reported a genetic lesion that inactivated the murine ST 3 Gal-4 sialyltransferase; these mice were unable to sialylate their glycoproteins due to this deficiency in ST 3 Gal 4 This produced a bleeding disorder from reduced levels of VWF and thrombocytopenia due to enhanced clearance First demonstration that desialylated platelets and v. WF can be removed from the circulation by Asialoglycoprotein receptors This study intrigued the authors of the present paper today and led them to further investigate this concept of clearance of clotting factors and its potential relationship to intravascular coagulation and sepsis


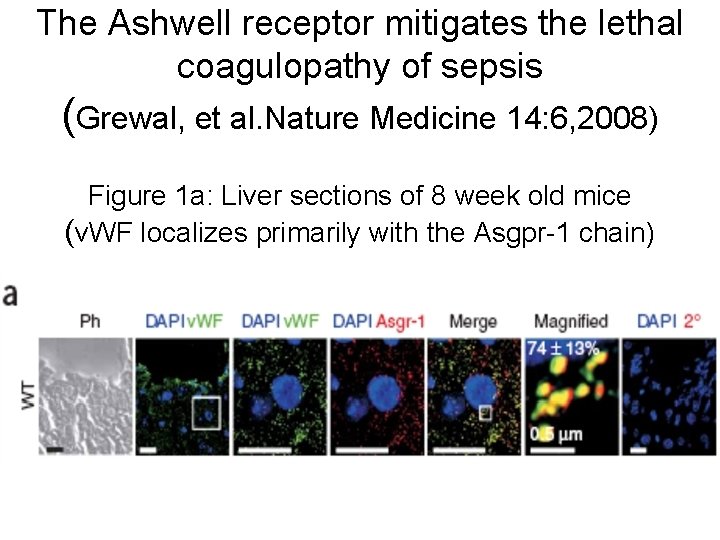
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Figure 1 a: Liver sections of 8 week old mice (v. WF localizes primarily with the Asgpr-1 chain)

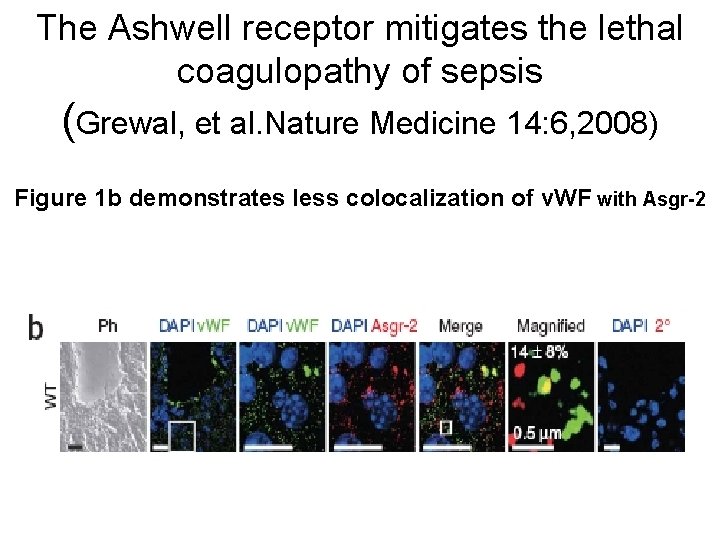
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Figure 1 b demonstrates less colocalization of v. WF with Asgr-2

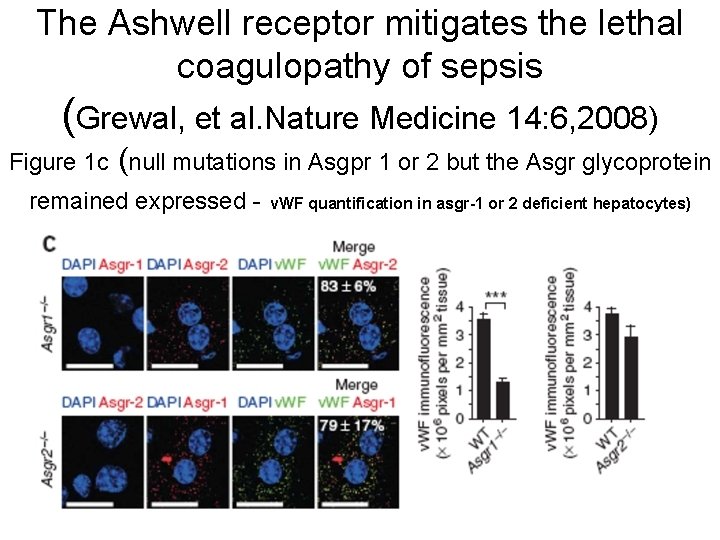
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Figure 1 c (null mutations in Asgpr 1 or 2 but the Asgr glycoprotein remained expressed - v. WF quantification in asgr-1 or 2 deficient hepatocytes)

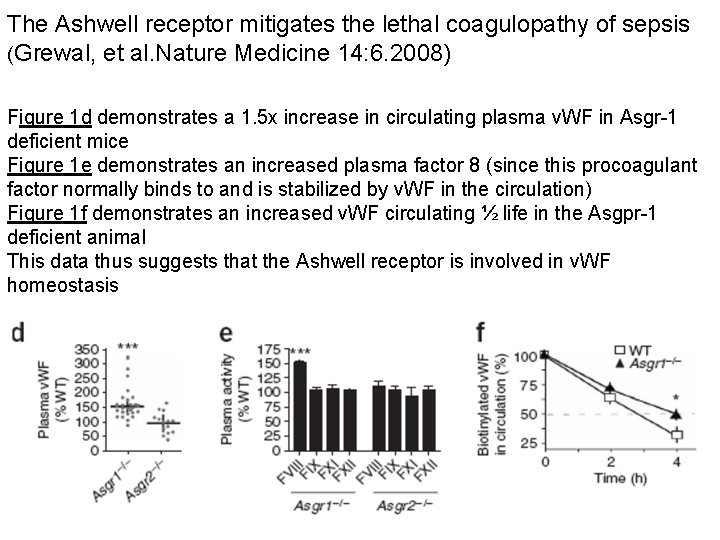
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6. 2008) Figure 1 d demonstrates a 1. 5 x increase in circulating plasma v. WF in Asgr-1 deficient mice Figure 1 e demonstrates an increased plasma factor 8 (since this procoagulant factor normally binds to and is stabilized by v. WF in the circulation) Figure 1 f demonstrates an increased v. WF circulating ½ life in the Asgpr-1 deficient animal This data thus suggests that the Ashwell receptor is involved in v. WF homeostasis

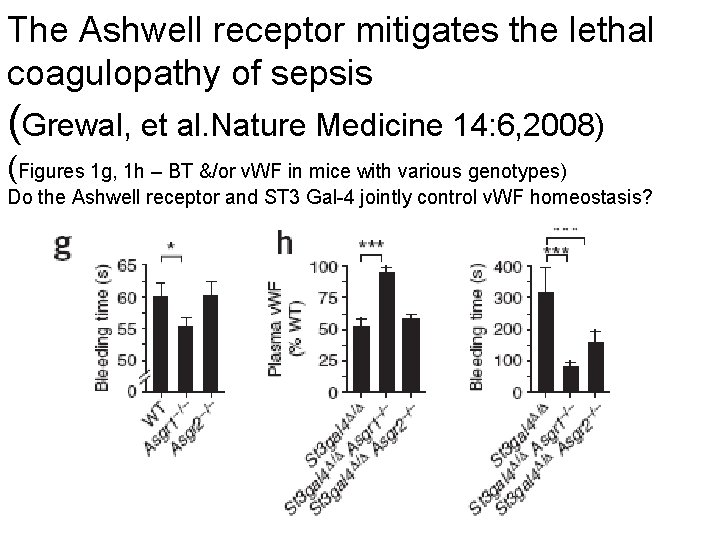
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) (Figures 1 g, 1 h – BT &/or v. WF in mice with various genotypes) Do the Ashwell receptor and ST 3 Gal-4 jointly control v. WF homeostasis?

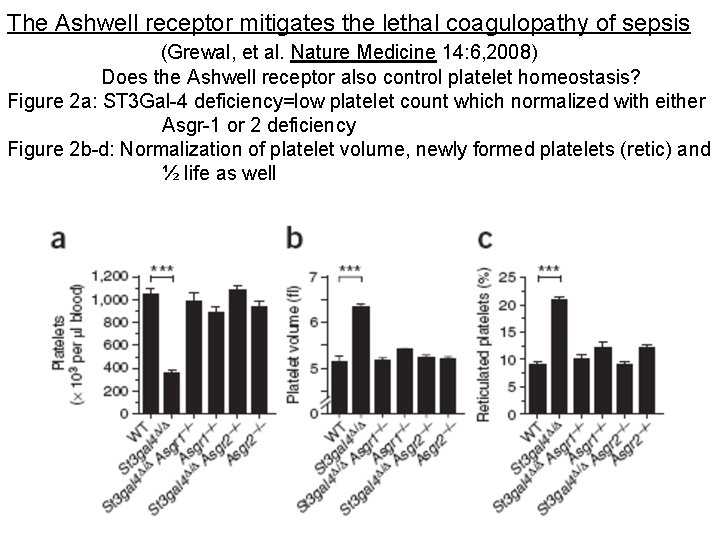
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Does the Ashwell receptor also control platelet homeostasis? Figure 2 a: ST 3 Gal-4 deficiency=low platelet count which normalized with either Asgr-1 or 2 deficiency Figure 2 b-d: Normalization of platelet volume, newly formed platelets (retic) and ½ life as well

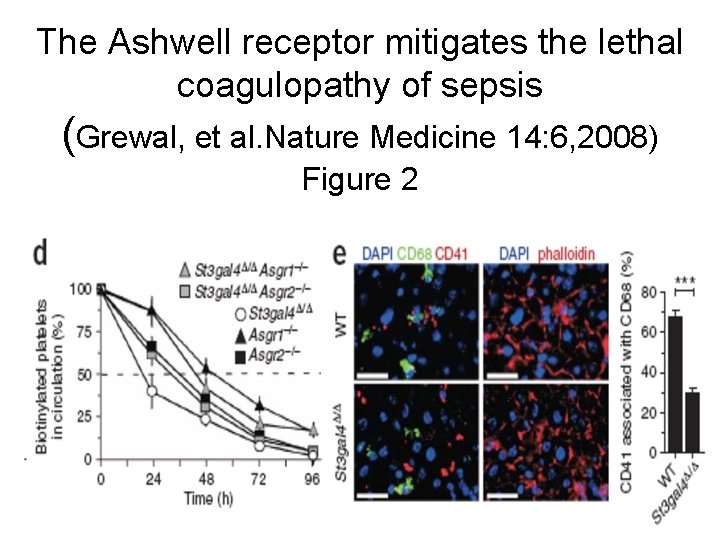
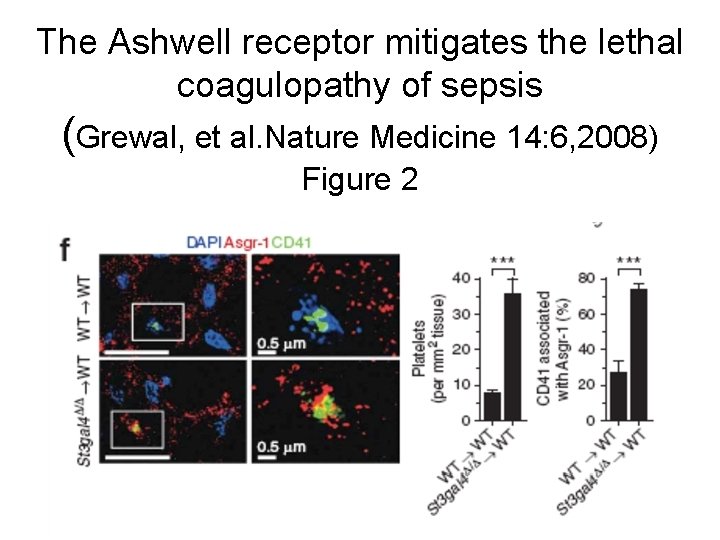
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Figure 2

The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Figure 2

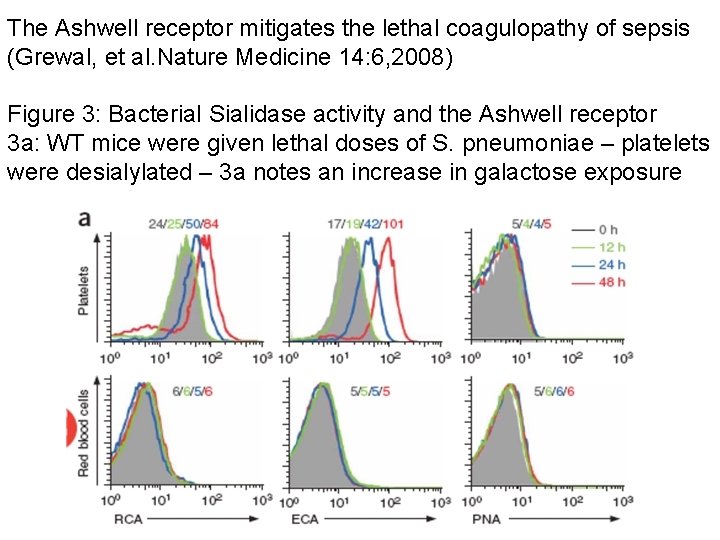
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Figure 3: Bacterial Sialidase activity and the Ashwell receptor 3 a: WT mice were given lethal doses of S. pneumoniae – platelets were desialylated – 3 a notes an increase in galactose exposure

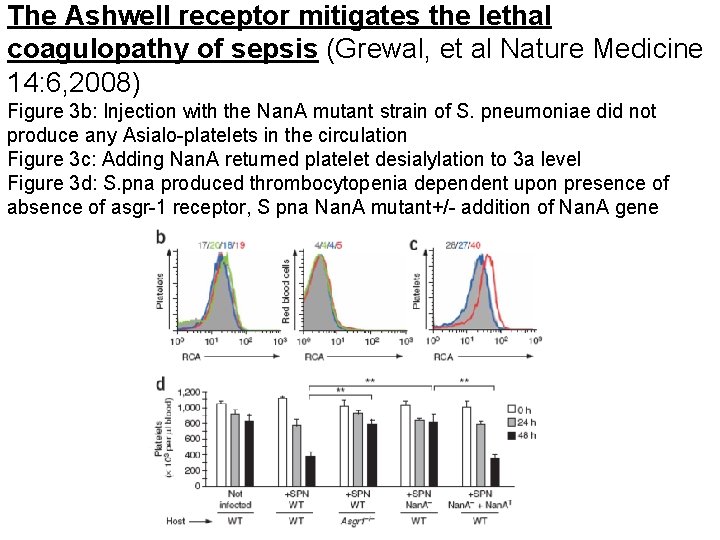
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al Nature Medicine 14: 6, 2008) Figure 3 b: Injection with the Nan. A mutant strain of S. pneumoniae did not produce any Asialo-platelets in the circulation Figure 3 c: Adding Nan. A returned platelet desialylation to 3 a level Figure 3 d: S. pna produced thrombocytopenia dependent upon presence of absence of asgr-1 receptor, S pna Nan. A mutant+/- addition of Nan. A gene

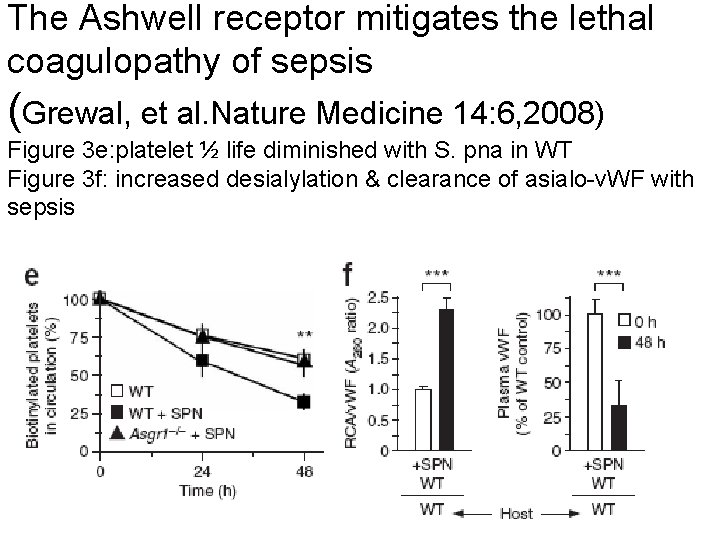
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Figure 3 e: platelet ½ life diminished with S. pna in WT Figure 3 f: increased desialylation & clearance of asialo-v. WF with sepsis

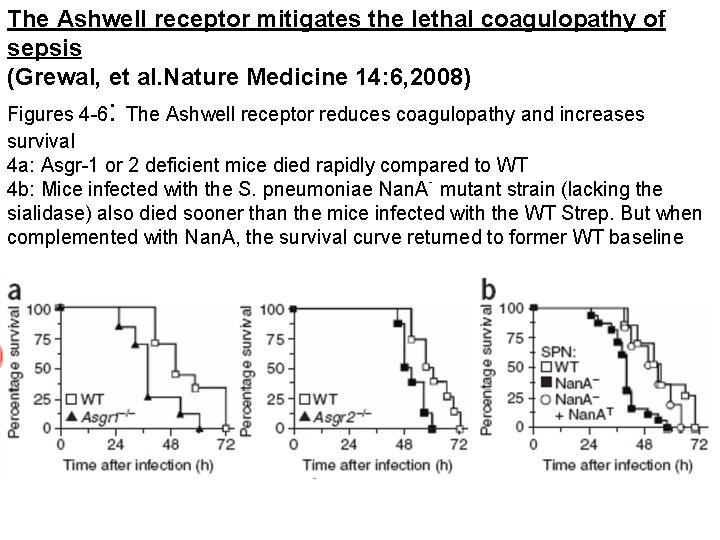
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) : Figures 4 -6 The Ashwell receptor reduces coagulopathy and increases survival 4 a: Asgr-1 or 2 deficient mice died rapidly compared to WT 4 b: Mice infected with the S. pneumoniae Nan. A- mutant strain (lacking the sialidase) also died sooner than the mice infected with the WT Strep. But when complemented with Nan. A, the survival curve returned to former WT baseline

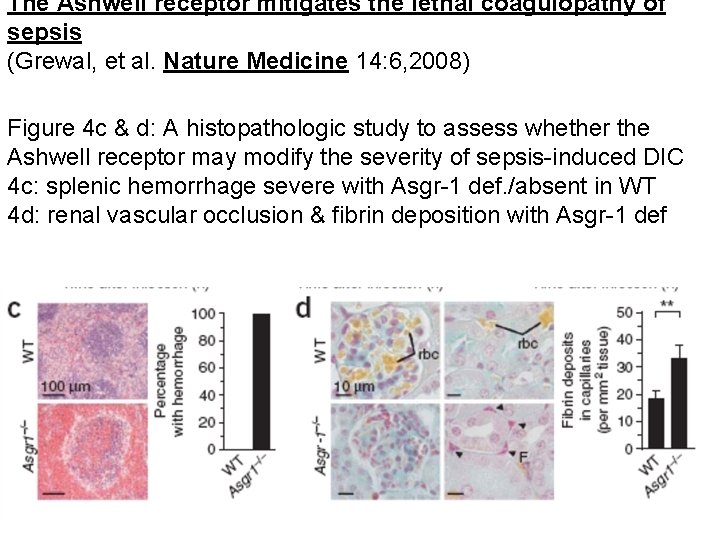
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Figure 4 c & d: A histopathologic study to assess whether the Ashwell receptor may modify the severity of sepsis-induced DIC 4 c: splenic hemorrhage severe with Asgr-1 def. /absent in WT 4 d: renal vascular occlusion & fibrin deposition with Asgr-1 def

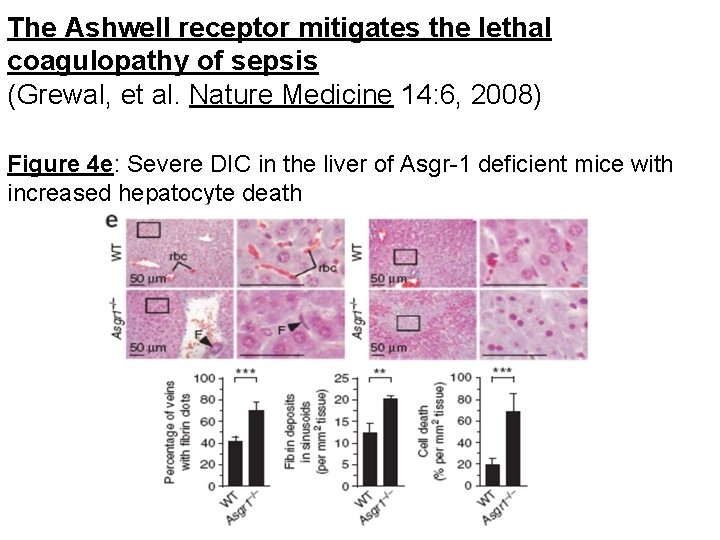
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Figure 4 e: Severe DIC in the liver of Asgr-1 deficient mice with increased hepatocyte death

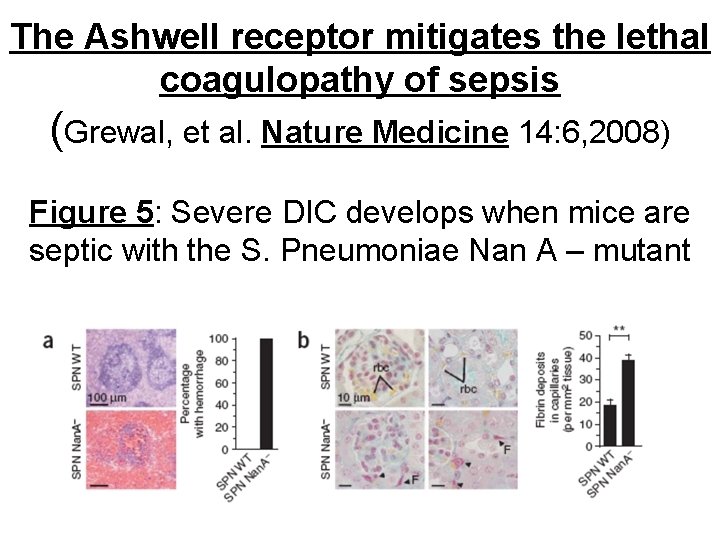
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Figure 5: Severe DIC develops when mice are septic with the S. Pneumoniae Nan A – mutant

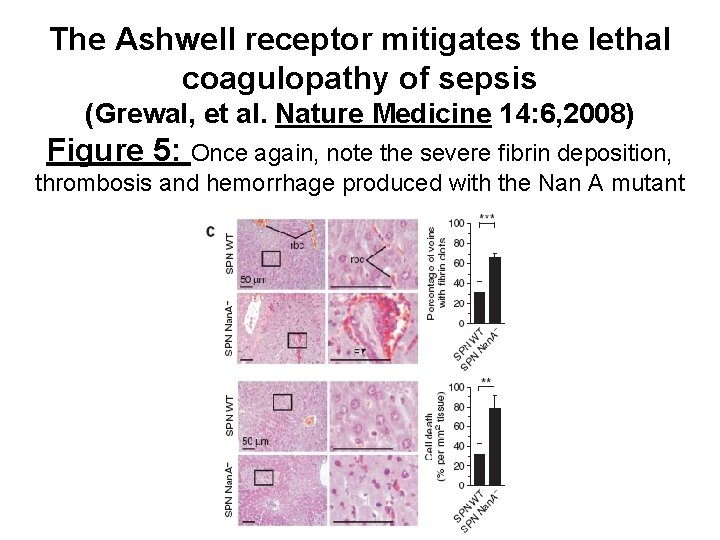
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6, 2008) Figure 5: Once again, note the severe fibrin deposition, thrombosis and hemorrhage produced with the Nan A mutant

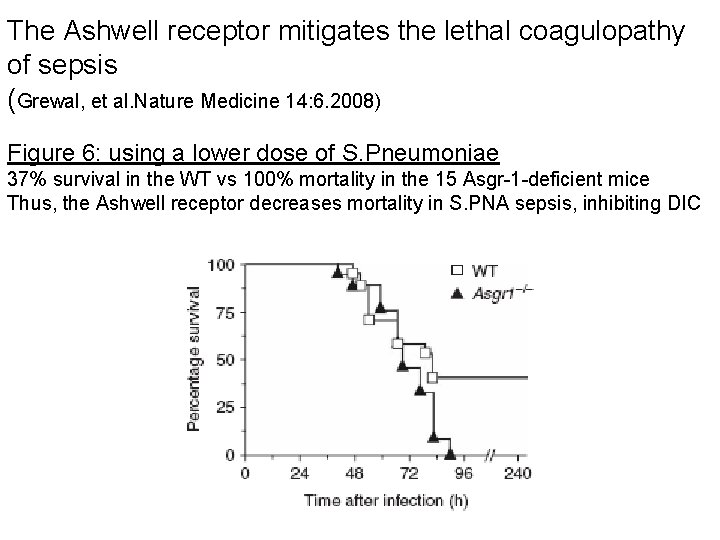
The Ashwell receptor mitigates the lethal coagulopathy of sepsis (Grewal, et al. Nature Medicine 14: 6. 2008) Figure 6: using a lower dose of S. Pneumoniae 37% survival in the WT vs 100% mortality in the 15 Asgr-1 -deficient mice Thus, the Ashwell receptor decreases mortality in S. PNA sepsis, inhibiting DIC

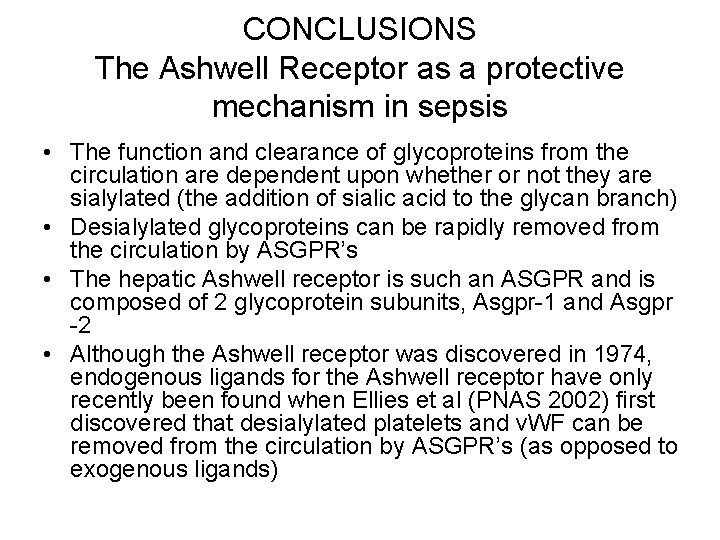
CONCLUSIONS The Ashwell Receptor as a protective mechanism in sepsis • The function and clearance of glycoproteins from the circulation are dependent upon whether or not they are sialylated (the addition of sialic acid to the glycan branch) • Desialylated glycoproteins can be rapidly removed from the circulation by ASGPR’s • The hepatic Ashwell receptor is such an ASGPR and is composed of 2 glycoprotein subunits, Asgpr-1 and Asgpr -2 • Although the Ashwell receptor was discovered in 1974, endogenous ligands for the Ashwell receptor have only recently been found when Ellies et al (PNAS 2002) first discovered that desialylated platelets and v. WF can be removed from the circulation by ASGPR’s (as opposed to exogenous ligands)

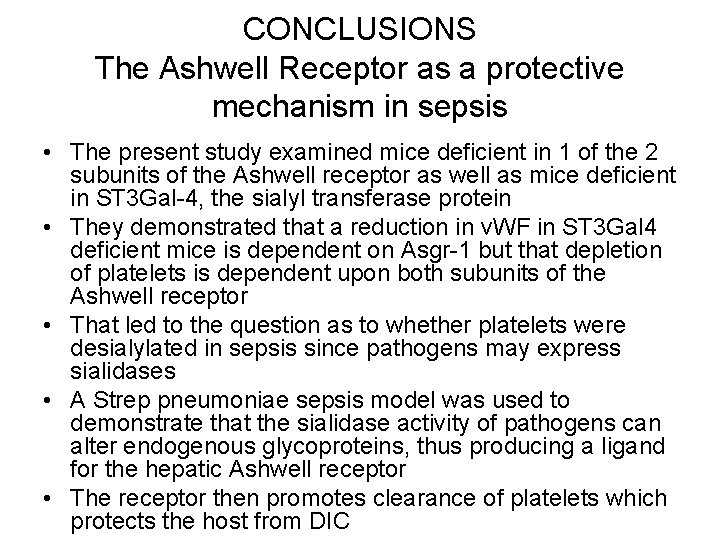
CONCLUSIONS The Ashwell Receptor as a protective mechanism in sepsis • The present study examined mice deficient in 1 of the 2 subunits of the Ashwell receptor as well as mice deficient in ST 3 Gal-4, the sialyl transferase protein • They demonstrated that a reduction in v. WF in ST 3 Gal 4 deficient mice is dependent on Asgr-1 but that depletion of platelets is dependent upon both subunits of the Ashwell receptor • That led to the question as to whether platelets were desialylated in sepsis since pathogens may express sialidases • A Strep pneumoniae sepsis model was used to demonstrate that the sialidase activity of pathogens can alter endogenous glycoproteins, thus producing a ligand for the hepatic Ashwell receptor • The receptor then promotes clearance of platelets which protects the host from DIC

CONCLUSIONS The Ashwell Receptor as a protective mechanism in sepsis • Thus, thrombocytopenia in sepsis may be due to hepatic uptake of platelets by the Ashwell receptor , once desialylated • The hepatocyte may play a role in the procoagulant response to sepsis via the Ashwell receptor • Sepsis by a mutant Strep pneumoniae strain that lacked sialidase could not produce asialylated platelets and did not produce thrombocytopenia • Asgr-1 deficient mice also did not develop thrombocytopenia • Thrombocytopenia produced by the Ashwell receptor clearance of asgp-covered platelets protected against DIC and those animals survived longer

CONCLUSIONS The Ashwell Receptor as a protective mechanism in sepsis • • • This study demonstrates the presence of endogenous ligands for the Ashwell receptor which may play a role in mitigating the severe coagulopathy of sepsis This study puts forth a new concept in the pathophysiology of sepsis – the development of thrombocytopenia as a way to limit the lethal coagulation cascade complicating sepsis What are the important questions that need to be addressed in determining the relevance of these experiments re: sepsis, DIC and the role of asialylated glycoproteins in disease? Needless to say, there are many. There a number of pathways involved in the coagulation cascade. Since DIC is a complicated disease, these other pathways also need to be explored in terms of their involvement in promoting the prothrombotic state in sepsis Sepsis is a very complicated pathophysiologic state and there also many pathogens that do not express sialidases. So does the Ashwell receptor play a role in sepsis from these other pathogens? The Ashwell receptor is present on the hepatocytes of mammalian species and is thought to have originated from a single ancestral gene. It may have a number of important pathophysiologic roles and this study may just be touching the tip of the iceberg
 Rapifluor glycan performance test standards
Rapifluor glycan performance test standards Hunter syndrome
Hunter syndrome Classification of glycoproteins
Classification of glycoproteins Elements of connective tissue
Elements of connective tissue Nature medicine
Nature medicine Synthesis of insulin
Synthesis of insulin Insulin and insulin receptor
Insulin and insulin receptor Que es endocitosis mediada por receptor
Que es endocitosis mediada por receptor Olfactory receptors
Olfactory receptors Classification of somatic senses
Classification of somatic senses Emisor receptor mensaje código canal
Emisor receptor mensaje código canal Diametricamente
Diametricamente Dr abdel
Dr abdel Reflejo flexor y extensor cruzado
Reflejo flexor y extensor cruzado Angulation for maxillary teeth
Angulation for maxillary teeth What is an image receptor
What is an image receptor The image receptor in xeroradiography is
The image receptor in xeroradiography is Receptor-mediated endocytosis
Receptor-mediated endocytosis Emissor e receptor
Emissor e receptor Sujeto lírico en un poema
Sujeto lírico en un poema Raprazole
Raprazole Insulin binding to receptor
Insulin binding to receptor Emisor canal receptor
Emisor canal receptor Imagen de emisor y receptor
Imagen de emisor y receptor Linguistica acustica
Linguistica acustica Agente y receptor de una fuerza ejemplos
Agente y receptor de una fuerza ejemplos Nicotinic vs muscarinic receptors
Nicotinic vs muscarinic receptors Receptor protein
Receptor protein