Gas Chromatography Gas chromatography q one of most







- Slides: 20

Gas Chromatography

Gas chromatography q one of most widely used techniques for qualitative & quantitative analysis. q benchtop gas chromatograph-mass spectrometer system (Fig. 1) that provides high-resolution separations & identification of compounds separated. q such systems are invaluable in industrial, biomedical, and forensic laboratories.

Fig. 1. Gas Chromatography

Gas chromatography including q columns and stationary phases most widely used q Various detection systems, including mass spectrometry q Primary concern with gas-liquid chromatography q Brief concern of gas-solid chromatography

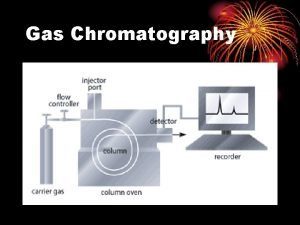
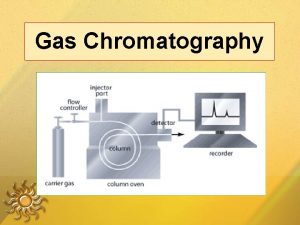
In gas chromatography, q components of vaporized sample are separated by being distributed between a mobile gaseous phase and liquid or solid stationary phase held in a column q in performing a gas chromatographic separation, sample is vaporized and injected onto the head of a chromatographic column q elution is brought about by the flow of an inert gaseous mobile phase

q In contrast to most other types of chromatography, the mobile phase does not interact with molecules of the analyte. q The only function of the mobile phase is to transport the analyte through the column. q Mobile phase is sometimes called a carrier gas

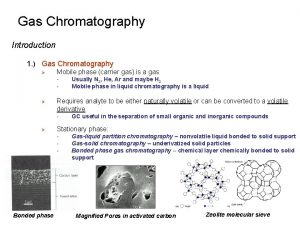
q Two types of gas chromatography are encountered: gas-liquid chromatography (GLC) and gas-solid chromatography (GSC). q Gas-liquid chromatography finds widespread use in all fields of science where its name is usually shortened to gas chromatography (GC). q Gas-solid chromatography is based on a solid stationary phase in which retention of analytes occurs because of physical adsorption.

q Gas-solid chromatography has limited application because of semipermanent retention of active or polar molecules and severe tailing of elution peaks q Tailing is due to the nonlinear character of adsorption process q This technique has not found wide application except for the separation of certain low-molecular-mass gaseous species

q mobile phase is a gas, and the stationary phase is a liquid that is retained on the surface of an inert solid by adsorption or chemical bonding. q In gas-solid chromatography, the mobile phase is a gas, and the stationary phase is a solid that retains the analytes by physical adsorption. q Gas-solid chromatography permits the separation and determination of low-molecular-mass gases, such as air components, hydrogen sulfide, carbon monoxide, and nitrogen oxides.

q Gas-liquid chromatography is based on partitioning of the analyte between a gaseous mobile phase and a liquid phase immobilized on the surface of an inert solid packing or on the walls of capillary tubing. q The concept of gas-liquid chromatography was first pronounced in 1941 by Martin and Synge, who were also responsible for the development of liquid-liquid partition chromatography.

q More than a decade was to elapse, however, before the value of gas-liquid chromatography was demonstrated experimentally and this technique began to be used as a routine laboratory tool. q In 1955, the first commercial apparatus for gas-liquid chromatography appeared on the market. q Since that time, the growth in applications of this technique has been phenomenal. q Currently, several hundred thousand gas chromatographs are in use throughout the world.

Instruments for Gas-Liquid Chromatography Many changes and improvements in gas chromatographic instruments have appeared in the marketplace since their commercial introduction. In the 1970 s, electronic integrators and computer-based dataprocessing equipment became common. The 1980 s saw computers being used for automatic control of such instrument parameters as column temperature, flow rates, and sample injection.

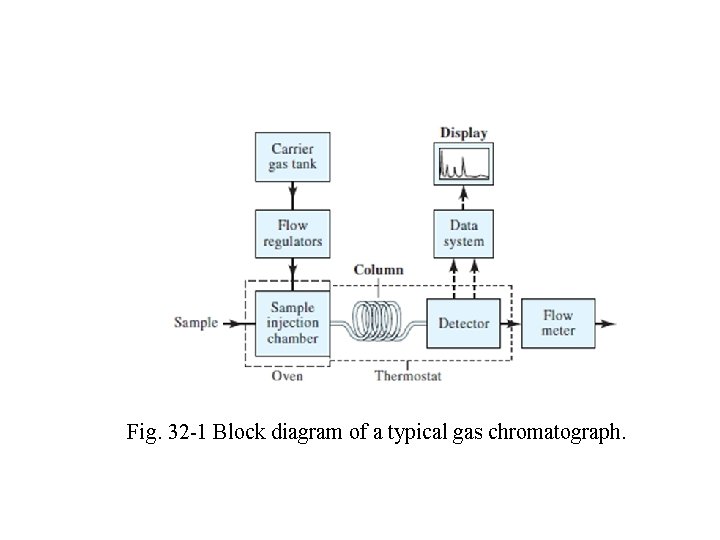
This same decade also saw the development of very highperformance instruments at moderate costs Perhaps most important, the introduction of open tubular columns that are capable of separating components of complex mixtures in relatively short times. Today, some 50 instrument manufacturers offer about 150 different models of gas chromatographic equipment at costs that vary from $1000 to over $50, 000. The basic components of a typical instrument for performing gas chromatography are shown in Fig. 32 -1

Fig. 32 -1 Block diagram of a typical gas chromatograph.

32 A-1 Carrier Gas System q The mobile phase gas in gas chromatography is called the carrier gas and must be chemically inert. q Helium is the most common mobile phase, although argon, nitrogen, and hydrogen are also used. These gases are available in pressurized tanks. q Pressure regulators, gauges, and flow meters are required to control the flow rate of the gas. q Classically, flow rates in gas chromatographs were regulated by controlling the gas inlet pressure.

q A two-stage pressure regulator at the gas cylinder and some sort of pressure regulator or flow regulator mounted in the chromatograph were used. q Inlet pressures usually range from 10 to 50 psi (lb/in 2) above room pressure, yielding flow rates of 25 to 150 m. L/min with packed columns and 1 to 25 m. L/min for open tubular capillary columns. q With pressure-controlled devices, it is assumed that flow rates are constant if the inlet pressure remains constant. q Newer chromatographs use electronic pressure controllers both for packed and for capillary columns.

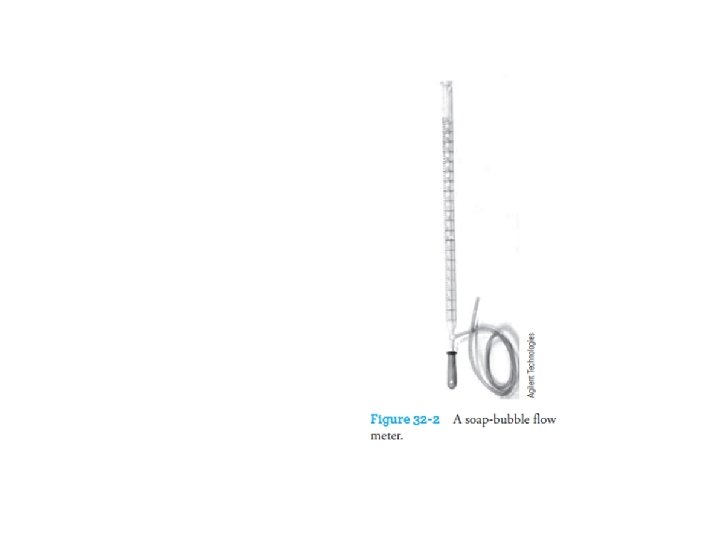
q With any chromatograph, it is desirable to measure the flow through the column. q The classical soap-bubble meter shown in Figure 32 -2 is still widely used. q A soap film is formed in the path of the gas when a rubber bulb containing an aqueous solution of soap or detergent is squeezed; q The time required for this film to move between two graduations on the burette is measured and converted to volumetric flow rate.


q Volumetric flow rates and linear flow velocities are related by Eqs. 31 -12 or 31 -13. q Bubble flow meters are now available with digital readouts that eliminate some human reading errors. q Usually, the flow meter is located at the end of the column, as shown in Fig. 32 -1. q The use of electronic flow meters has become increasingly common.

q Digital flow meters are available that measure mass flow, volume flow, or both. q Volumetric flow measurements are independent of the gas composition. q Mass flow meters are calibrated for specific gas compositions, but, unlike volumetric meters, they are independent of temperature and pressure.
 One empire one god one emperor
One empire one god one emperor One one little dog run
One one little dog run One king one law one faith
One king one law one faith One god one empire one emperor
One god one empire one emperor One ford plan
One ford plan See one do one teach one
See one do one teach one See one, do one, teach one
See one, do one, teach one Structure of twelfth night
Structure of twelfth night See one do one teach one
See one do one teach one One vision one identity one community
One vision one identity one community One vision one identity one community
One vision one identity one community Van deemter graph
Van deemter graph Gradient vs isocratic elution
Gradient vs isocratic elution Stationary phase in gas chromatography
Stationary phase in gas chromatography Gas chromatography basic principle
Gas chromatography basic principle Gas liquid chromatography
Gas liquid chromatography Percent composition gas chromatography
Percent composition gas chromatography Mobile phase vs stationary phase
Mobile phase vs stationary phase Basic gas chromatography
Basic gas chromatography Permanent gases
Permanent gases Gas chromatography youtube
Gas chromatography youtube