Gas Chromatography Gas chromatography q one of most














- Slides: 39

Gas Chromatography

Gas chromatography q one of most widely used techniques for qualitative & quantitative analysis. q benchtop gas chromatograph-mass spectrometer system (Fig. 1) that provides high-resolution separations & identification of compounds separated. q such systems are invaluable in industrial, biomedical, and forensic laboratories.

Fig. 1. Gas Chromatography

Gas chromatography including q columns and stationary phases most widely used q Various detection systems, including mass spectrometry q Primary concern with gas-liquid chromatography q Brief concern of gas-solid chromatography

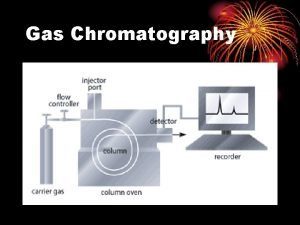
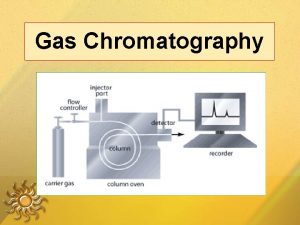
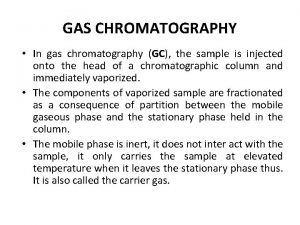
In gas chromatography, q components of vaporized sample are separated by being distributed between a mobile gaseous phase and liquid or solid stationary phase held in a column q in performing a gas chromatographic separation, sample is vaporized and injected onto the head of a chromatographic column q elution is brought about by the flow of an inert gaseous mobile phase

q In contrast to most other types of chromatography, the mobile phase does not interact with molecules of the analyte. q The only function of the mobile phase is to transport the analyte through the column. q Mobile phase is sometimes called a carrier gas

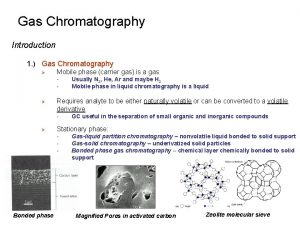
q Two types of gas chromatography are encountered: gas-liquid chromatography (GLC) and gas-solid chromatography (GSC). q Gas-liquid chromatography finds widespread use in all fields of science where its name is usually shortened to gas chromatography (GC). q Gas-solid chromatography is based on a solid stationary phase in which retention of analytes occurs because of physical adsorption.

q Gas-solid chromatography has limited application because of semipermanent retention of active or polar molecules and severe tailing of elution peaks q Tailing is due to the nonlinear character of adsorption process q This technique has not found wide application except for the separation of certain low-molecular-mass gaseous species

q mobile phase is a gas, and the stationary phase is a liquid that is retained on the surface of an inert solid by adsorption or chemical bonding. q In gas-solid chromatography, the mobile phase is a gas, and the stationary phase is a solid that retains the analytes by physical adsorption. q Gas-solid chromatography permits the separation and determination of low-molecular-mass gases, such as air components, hydrogen sulfide, carbon monoxide, and nitrogen oxides.

q Gas-liquid chromatography is based on partitioning of the analyte between a gaseous mobile phase and a liquid phase immobilized on the surface of an inert solid packing or on the walls of capillary tubing. q The concept of gas-liquid chromatography was first pronounced in 1941 by Martin and Synge, who were also responsible for the development of liquid-liquid partition chromatography.

q More than a decade was to elapse, however, before the value of gas-liquid chromatography was demonstrated experimentally and this technique began to be used as a routine laboratory tool. q In 1955, the first commercial apparatus for gas-liquid chromatography appeared on the market. q Since that time, the growth in applications of this technique has been phenomenal. q Currently, several hundred thousand gas chromatographs are in use throughout the world.

Instruments for Gas-Liquid Chromatography Many changes and improvements in gas chromatographic instruments have appeared in the marketplace since their commercial introduction. In the 1970 s, electronic integrators and computer-based dataprocessing equipment became common. The 1980 s saw computers being used for automatic control of such instrument parameters as column temperature, flow rates, and sample injection.

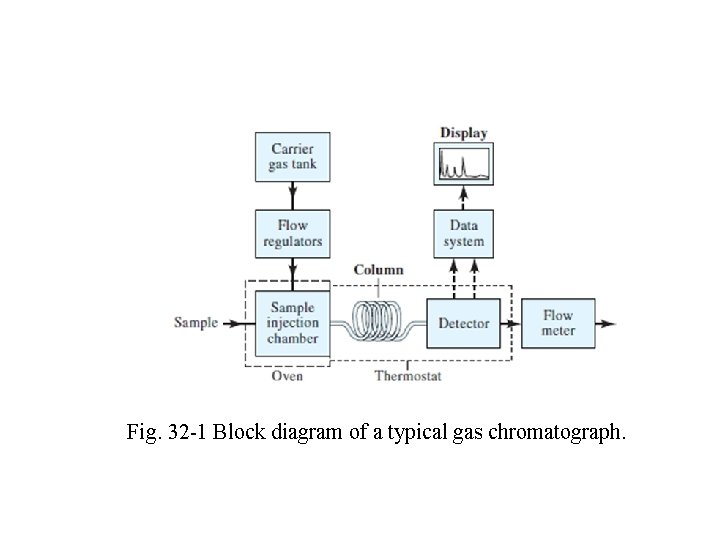
This same decade also saw the development of very highperformance instruments at moderate costs Perhaps most important, the introduction of open tubular columns that are capable of separating components of complex mixtures in relatively short times. Today, some 50 instrument manufacturers offer about 150 different models of gas chromatographic equipment at costs that vary from $1000 to over $50, 000. The basic components of a typical instrument for performing gas chromatography are shown in Fig. 32 -1

Fig. 32 -1 Block diagram of a typical gas chromatograph.

32 A-1 Carrier Gas System q The mobile phase gas in gas chromatography is called the carrier gas and must be chemically inert. q Helium is the most common mobile phase, although argon, nitrogen, and hydrogen are also used. These gases are available in pressurized tanks. q Pressure regulators, gauges, and flow meters are required to control the flow rate of the gas. q Classically, flow rates in gas chromatographs were regulated by controlling the gas inlet pressure.

q A two-stage pressure regulator at the gas cylinder and some sort of pressure regulator or flow regulator mounted in the chromatograph were used. q Inlet pressures usually range from 10 to 50 psi (lb/in 2) above room pressure, yielding flow rates of 25 to 150 m. L/min with packed columns and 1 to 25 m. L/min for open tubular capillary columns. q With pressure-controlled devices, it is assumed that flow rates are constant if the inlet pressure remains constant. q Newer chromatographs use electronic pressure controllers both for packed and for capillary columns.

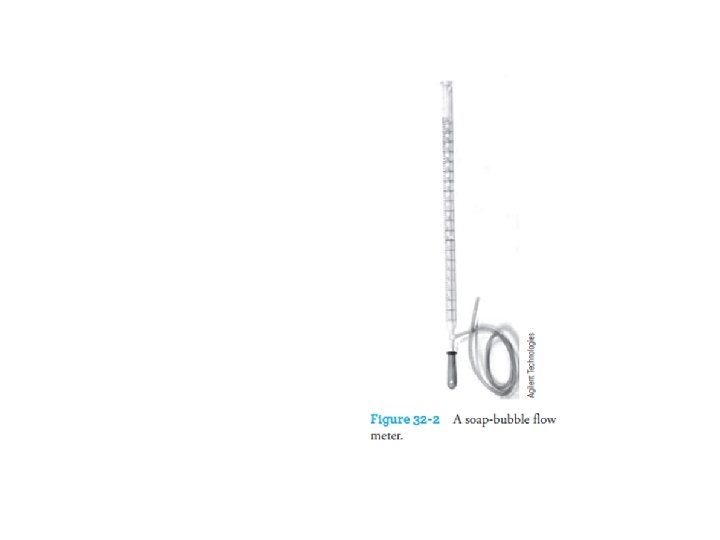
q With any chromatograph, it is desirable to measure the flow through the column. q The classical soap-bubble meter shown in Figure 32 -2 is still widely used. q A soap film is formed in the path of the gas when a rubber bulb containing an aqueous solution of soap or detergent is squeezed; q The time required for this film to move between two graduations on the burette is measured and converted to volumetric flow rate.


q Volumetric flow rates and linear flow velocities are related by Eqs. 31 -12 or 31 -13. q Bubble flow meters are now available with digital readouts that eliminate some human reading errors. q Usually, the flow meter is located at the end of the column, as shown in Fig. 32 -1. q The use of electronic flow meters has become increasingly common.

q Digital flow meters are available that measure mass flow, volume flow, or both. q Volumetric flow measurements are independent of the gas composition. q Mass flow meters are calibrated for specific gas compositions, but, unlike volumetric meters, they are independent of temperature and pressure.

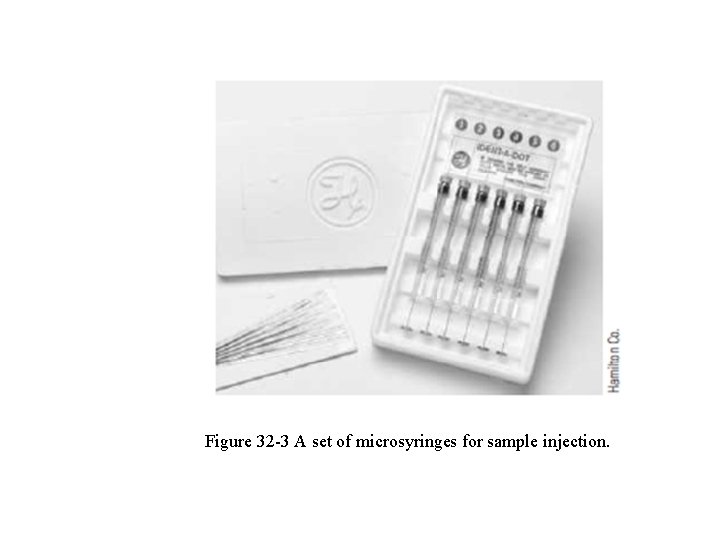
32 A-2 Sample Injection system q For high column efficiency, a suitably sized sample should be introduced as a “plug” of vapor. q Slow injection or oversized samples cause band spreading and poor resolution. q Calibrated microsyringes, such as those shown in Fig. 32 -3, are used to inject liquid samples through a rubber or silicone diaphragm, or septum, into a heated sample port located at head of the column.

Figure 32 -3 A set of microsyringes for sample injection.

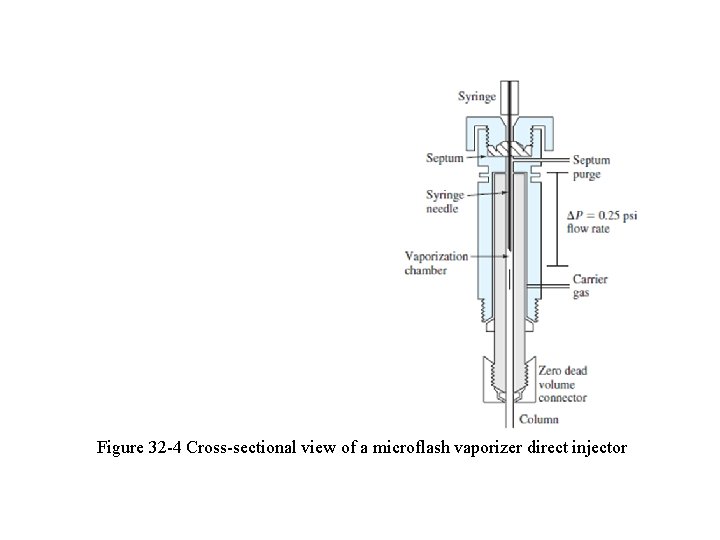
q The sample port (Fig. 32 -4) is usually kept at about 50 o. C greater than the boiling point of the least volatile component of the sample. q For ordinary packed analytical columns, sample sizes range from a few tenths of a microliter to 20 m. L. q Capillary columns require samples that are smaller by a factor of 100 or more. q For these columns, a sample splitter is often needed to deliver a small known fraction (1: 100 to 1: 500) of the injected sample, with the remainder going to waste.

Figure 32 -4 Cross-sectional view of a microflash vaporizer direct injector

Commercial gas chromatographs intended for use with capillary columns incorporate such splitters, and they also allow for splitless injection when packed columns are used.

For the most reproducible sample injection, newer gas chromatographs use autoinjectors and autosamplers, such as the system shown in Fig. 32 -5. With such autoinjectors, syringes are filled, and the sample injected into the chromatograph automatically.

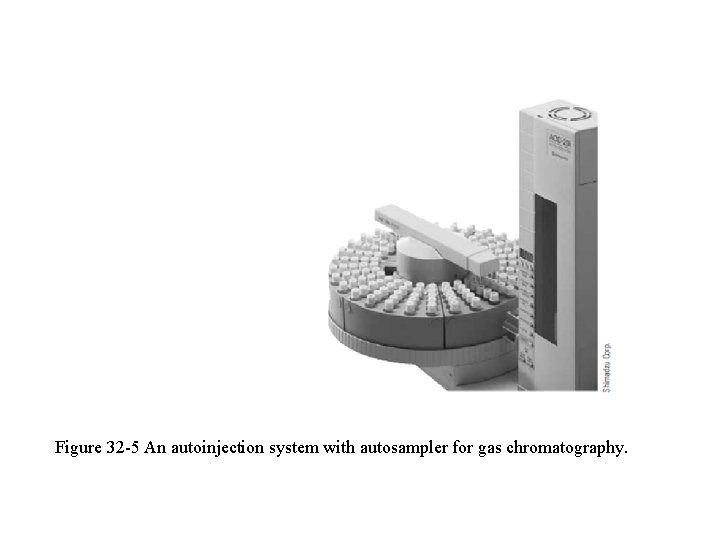
Figure 32 -5 An autoinjection system with autosampler for gas chromatography.

q In the autosampler, samples are contained in vials on a sample turntable. q The autoinjector syringe picks up the sample through a septum on the vial and injects the sample through a septum on the chromatograph. q With the unit shown, up to 150 sample vials can be placed on the turntable. q Injection volumes can vary from 0. 1 m. L with a 10 -m. L syringe to 200 m. L with a 200 -ml syringe.

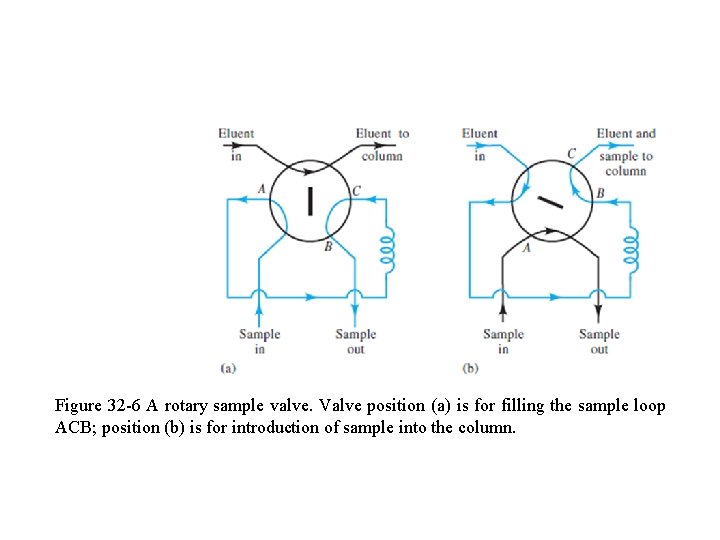
q Standard deviations as low as 0. 3% are common with autoinjection systems. q For introducing gases, a sample valve, such as that shown in Fig. 32 -6, is often used instead of a syringe. q With such devices, sample sizes can be reproduced to better than 0. 5% relative.

Figure 32 -6 A rotary sample valve. Valve position (a) is for filling the sample loop ACB; position (b) is for introduction of sample into the column.

Liquid samples can also be introduced through a sampling valve. Solid samples are introduced as solutions or alternatively are sealed into thin-walled vials that can be inserted at the head of the column and punctured or crushed from the outside.

32 A-3 Column Configurations and Column Ovens q The columns in gas chromatography are of two general types: packed columns or capillary columns. q In the past, the vast majority of gas chromatographic analyses used packed columns. q For most current applications, packed columns have been replaced by more efficient and faster capillary columns.

Chromatographic columns vary in length from less than 2 m to 60 m or more. They are constructed of stainless steel, glass, fused silica, or Teflon. In order to fit into an oven for thermostating, they are usually formed as coils having diameters of 10 to 30 cm (Fig. 32 -7).

Figure 32 -7 Fused-silica capillary columns.

q Column temperature is an important variable that must be controlled to a few tenths of a degree for precise work. q Thus, the column is normally housed in a thermostated oven. q The optimum column temperature depends on the boiling point of the sample and the degree of separation required. q Roughly, temperature equal to or slightly above average boiling point of sample results in reasonable elution time (2 - 30 min).

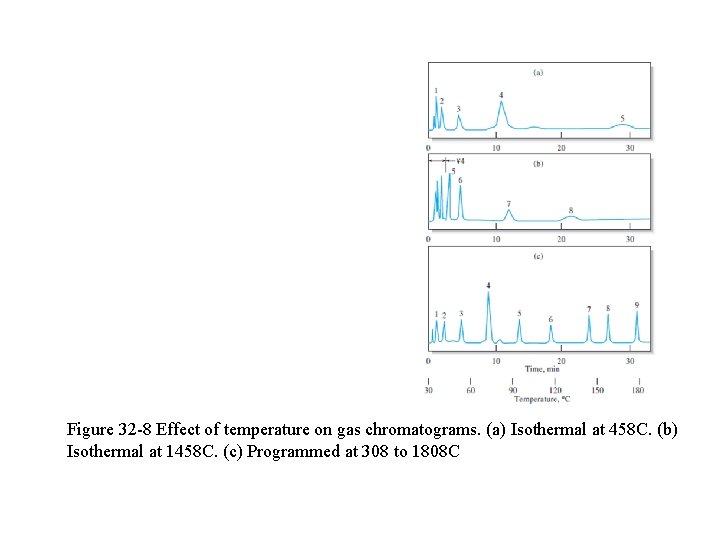
q For samples with a broad boiling range, it is often desirable to use temperature programming whereby the column temperature is increased either continuously or in steps as the separation proceeds. q Fig. 32 -8 shows the improvement in a chromatogram brought about by temperature programming. q In general, optimum resolution is associated with minimal temperature.

Figure 32 -8 Effect of temperature on gas chromatograms. (a) Isothermal at 458 C. (b) Isothermal at 1458 C. (c) Programmed at 308 to 1808 C

q The cost of lowered temperature, however, is an increase in elution time and, therefore, the time required to complete an analysis (Fig. 32 -8 a & b) q Analytes of limited volatility can sometimes be determined by forming derivatives that are more volatile

Temperature programming in gas chromatography is achieved by increasing the column temperature continuously or in steps during elution.
 One god one empire one religion
One god one empire one religion One one little dog run
One one little dog run One king one law one faith
One king one law one faith Byzantine definition
Byzantine definition Ford one plan
Ford one plan See one do one teach one
See one do one teach one One price policy
One price policy Structure of twelfth night
Structure of twelfth night See one do one teach one
See one do one teach one Asean tourism strategic plan
Asean tourism strategic plan One vision one identity one community
One vision one identity one community Gc injection
Gc injection Gradient vs isocratic elution
Gradient vs isocratic elution Stationary phase in gas chromatography
Stationary phase in gas chromatography Basic principle of gas chromatography
Basic principle of gas chromatography Gas liquid chromatography
Gas liquid chromatography Percent composition gas chromatography
Percent composition gas chromatography Hplc definition
Hplc definition Basic gas chromatography
Basic gas chromatography Gas chromatography history
Gas chromatography history Gas chromatography youtube
Gas chromatography youtube Flame ionization detector
Flame ionization detector Kromatografi adalah
Kromatografi adalah Gas chromatography
Gas chromatography Gas chromatography
Gas chromatography Hplc slideshare
Hplc slideshare Gas chromatography theory
Gas chromatography theory Most general to most specific classification
Most general to most specific classification Most general to most specific classification
Most general to most specific classification In the name of allah most gracious most merciful
In the name of allah most gracious most merciful The most
The most Ponceau pronunciation
Ponceau pronunciation In the name of allah the most beneficent the most merciful
In the name of allah the most beneficent the most merciful This is the study of grouping and naming organisms
This is the study of grouping and naming organisms Most general to most specific classification
Most general to most specific classification In the name of god most gracious prayer
In the name of god most gracious prayer In the name of allah the most gracious
In the name of allah the most gracious In the name of allah most gracious most merciful in arabic
In the name of allah most gracious most merciful in arabic The most gracious
The most gracious Aqidah
Aqidah