Future trends in formulations of Allergen Immunotherapy Dr














- Slides: 38

Future trends in formulations of Allergen Immunotherapy Dr. Paranjothy M. Pharm. , Ph. D Project Director

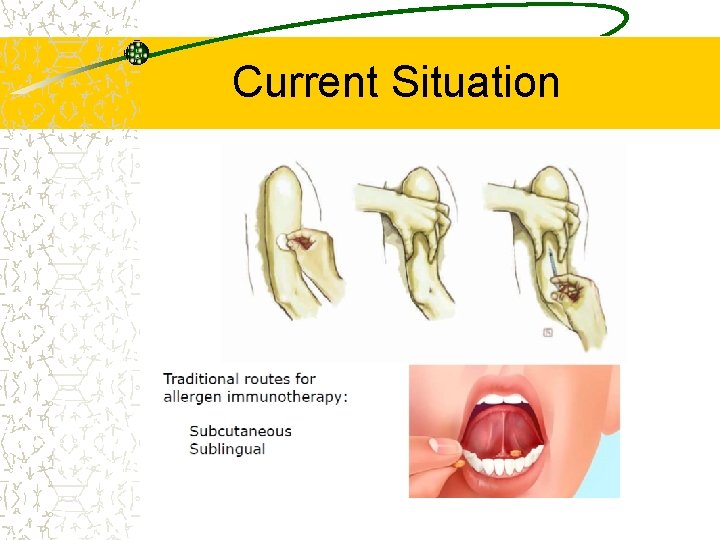
Current Situation

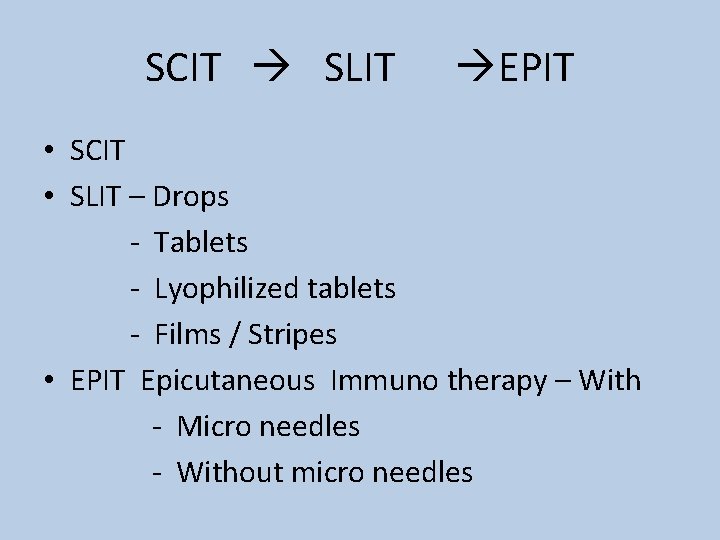
SCIT SLIT EPIT • SCIT • SLIT – Drops - Tablets - Lyophilized tablets - Films / Stripes • EPIT Epicutaneous Immuno therapy – With - Micro needles - Without micro needles

Future trends ----- EPIT ( EPicutanepous Immuno Therapy) EPIT with Micro needles (Trans dermal patches) EPIT without micro needles ( Occlusion patches)

EPIT is not new to India !! Concept of EPIT was first described 1000 years back in India. Placing allergen / vaccine on scarified skin ( Small pox vaccination was given by scratch or multiple insertion technique bifurcated needle) First report of EPIT for treating allergic disease was published in 1921. Then there was a silence in EPIT. Now again there is faster development with EPIT all over world. Recently EDS - Viaskin® developed by DMV technologies from France and they are the leaders

WAO Symposium on Food Allergy and Microbiome On 5 -6 December 2015, Miami, FL, USA Anna Nowak Wegrzn at Mount Sinai Hospital New York made a presentation on SLIT and EPIT.

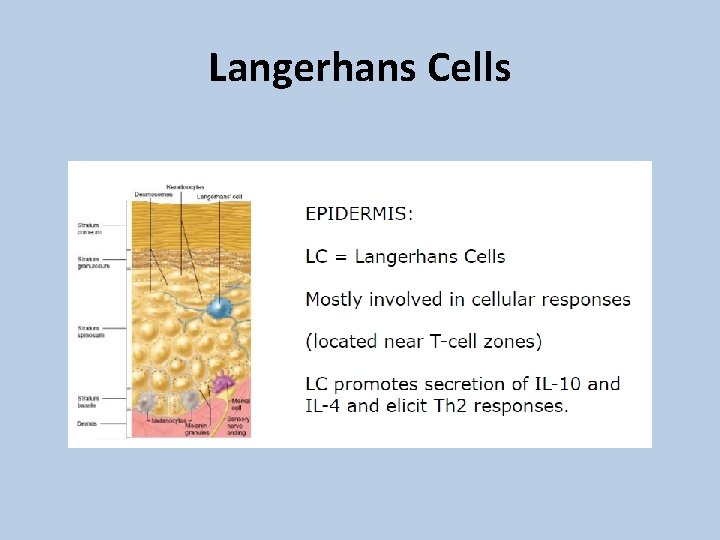
EPIT targets the outermost layer of the skin, the epidermis, which is characterized by three unique features: 1. Barrier function provided by keratinocytes; 2. Potent immune surveillance afforded by keratinocytes and Langerhans Cells (LCs); 3. Absence of vascularization, markedly reducing the risk of systemic reactions.

Mechanism There is only mechanical disruption of the skin and placement of the drug or vaccine with in the epidermis where it can more readily reach the site of action The micro needles dissolves with in minutes releasing the cargo at the intended delivery site. Micro needle patches covered by hundreds of micro needles that pierce only the stratum corneum ( uppermost 50 µm of the skin thus allowing the drug to by pass this barrier )

EPIT Antigen is taken-up by local LCs in the epidermis and transported to local draining lymph nodes where induction of Ig. G responses occur.


EPIT

SKIN

Langerhans Cells

EPIT • The skin as an immunological organ in topical vaccination • Allergen-specific immunotherapy by topical epicutaneous administration • Epidermis has Langerhans cells – Antigen Presenting Cells ( APCs) • Biodegradable microneedle patches offer a real advance for certain applications – particularly vaccinations

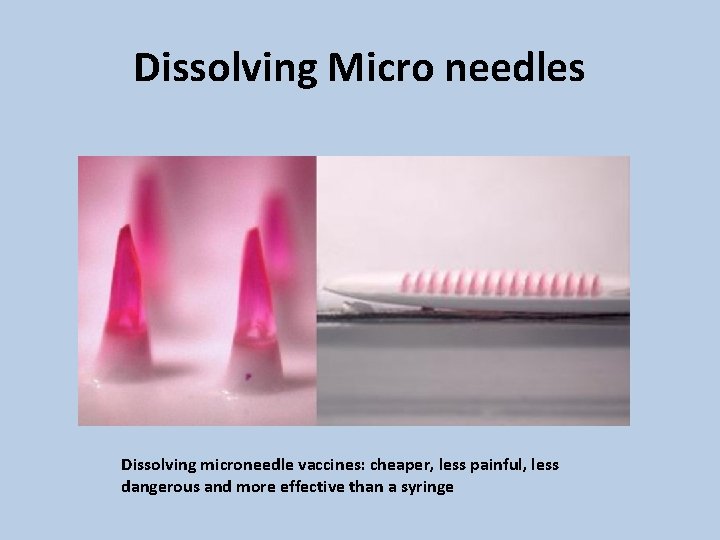
Dissolving Micro needles Dissolving microneedle vaccines: cheaper, less painful, less dangerous and more effective than a syringe

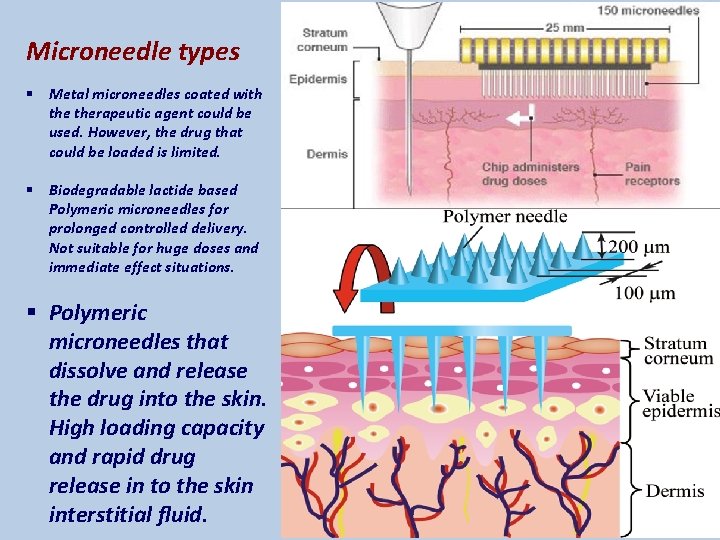
Microneedle types § Metal microneedles coated with therapeutic agent could be used. However, the drug that could be loaded is limited. § Biodegradable lactide based Polymeric microneedles for prolonged controlled delivery. Not suitable for huge doses and immediate effect situations. § Polymeric microneedles that dissolve and release the drug into the skin. High loading capacity and rapid drug release in to the skin interstitial fluid.

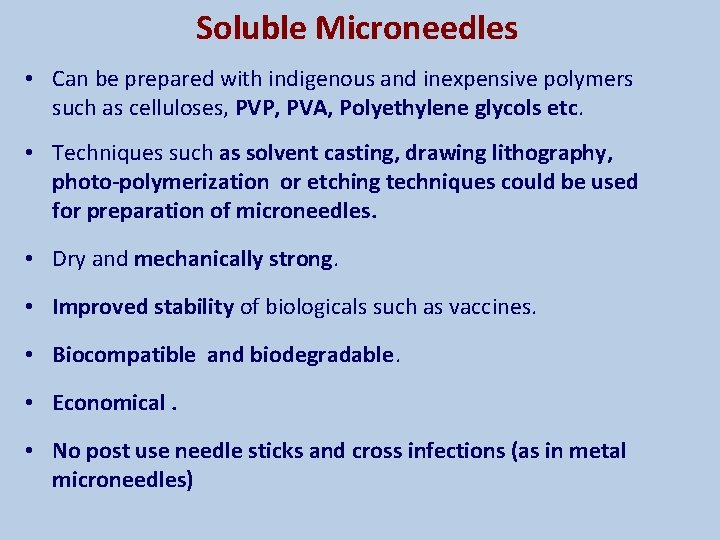
Soluble Microneedles • Can be prepared with indigenous and inexpensive polymers such as celluloses, PVP, PVA, Polyethylene glycols etc. • Techniques such as solvent casting, drawing lithography, photo-polymerization or etching techniques could be used for preparation of microneedles. • Dry and mechanically strong. • Improved stability of biologicals such as vaccines. • Biocompatible and biodegradable. • Economical. • No post use needle sticks and cross infections (as in metal microneedles)

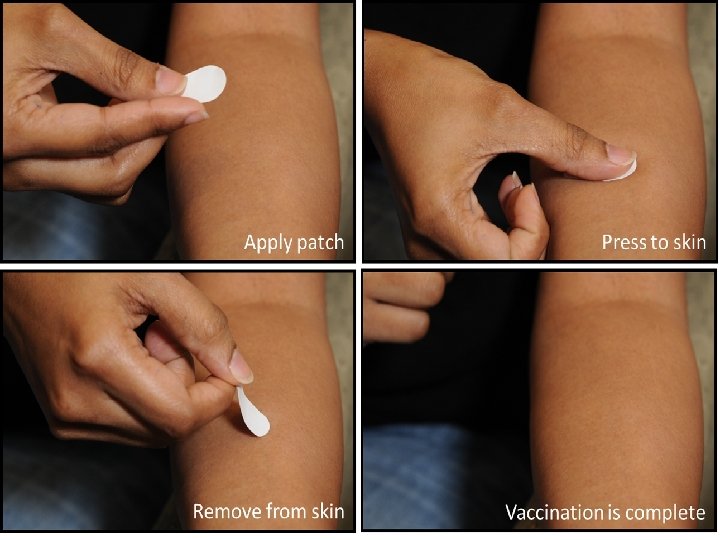
Micro needle patches • Microneedle patches are self-applied bandaid style adhesives that can be applied to any area of the skin. The active area is an array of tiny needles, just 650 microns in length, or 0. 65 mm. As the patch is applied to the skin, the microneedles penetrate the top layer of skin, but not deeply enough to activate any pain receptors

Polymer based • The microneedles are made from a harmless dissolving polymer that's mixed with a freezedried vaccine. So instead of the needles injecting a fluid, the needles quickly dissolve to become the fluid. When you pull the patch off, there's nothing sharp left on it, you can throw it straight in the bin without worrying that it'll be a hazard to others.

Advantages The advantages in a nutshell would appear to be: • 1) No painful piercing of the skin • 2) They can be self-applied, sold through the mail or over the counter • 3) No sharp hazardous waste after immunization • 4) More effective vaccinations

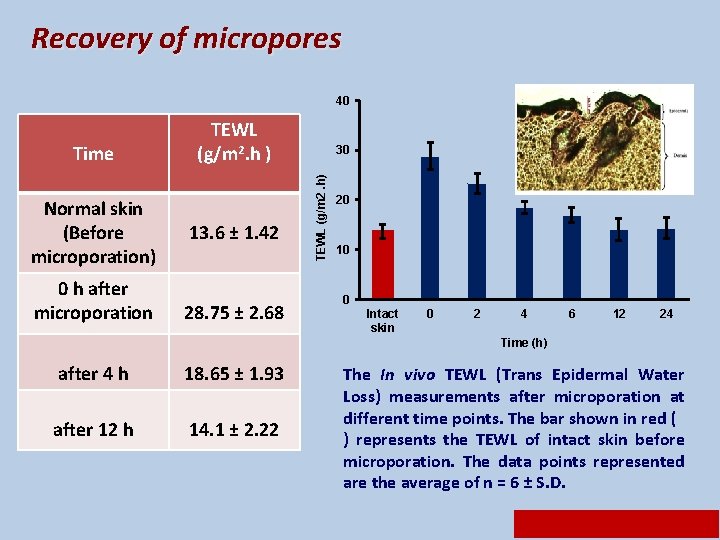
Recovery of micropores 40 Normal skin (Before microporation) 0 h after microporation 13. 6 ± 1. 42 28. 75 ± 2. 68 30 TEWL (g/m 2. h) Time TEWL (g/m 2. h ) 20 10 0 Intact skin 0 2 4 6 12 24 Time (h) after 4 h 18. 65 ± 1. 93 after 12 h 14. 1 ± 2. 22 The In vivo TEWL (Trans Epidermal Water Loss) measurements after microporation at different time points. The bar shown in red ( ) represents the TEWL of intact skin before microporation. The data points represented are the average of n = 6 ± S. D.

Safety of Micro needle patches Safe without dangers posed by hypodermic needles ( Injections Shots !) ( Immunogenicity) Minimally invasive Flu shot as simple as a band aid Self administration Replaces painful syringe No risk of anaphylactic shocks

Micro needles Ice knife : Kill and Escape !! Soluble polymers : Dissolve and vanish. It saves and brings relief. Master – Mold : Ganesh Chaturthi : PDMS ( Poly Di Methyl Siloxane) molds :

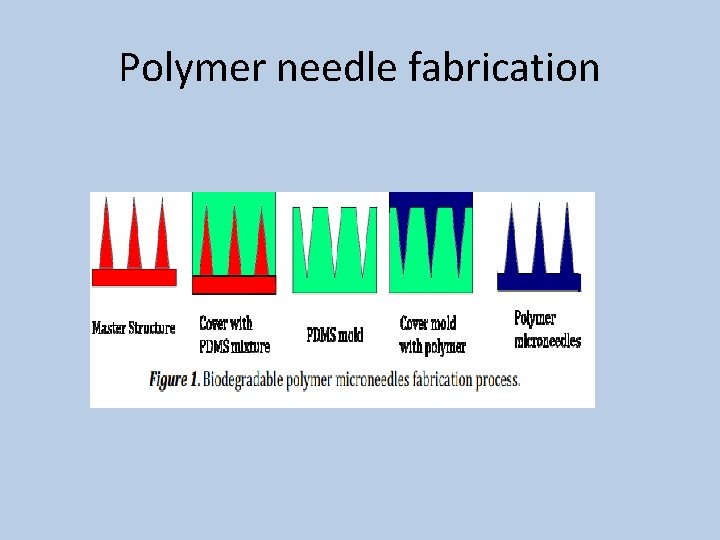
Polymer needle fabrication

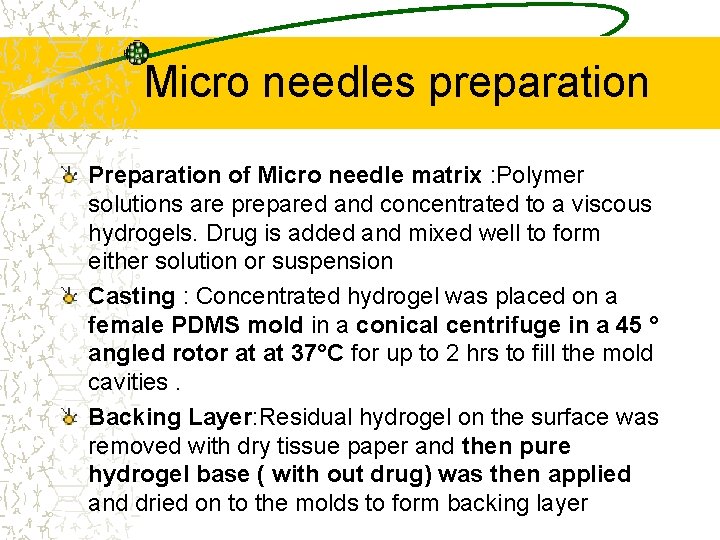
Micro needles preparation Preparation of Micro needle matrix : Polymer solutions are prepared and concentrated to a viscous hydrogels. Drug is added and mixed well to form either solution or suspension Casting : Concentrated hydrogel was placed on a female PDMS mold in a conical centrifuge in a 45 ° angled rotor at at 37°C for up to 2 hrs to fill the mold cavities. Backing Layer: Residual hydrogel on the surface was removed with dry tissue paper and then pure hydrogel base ( with out drug) was then applied and dried on to the molds to form backing layer

Formulation design parameters Capable enough to insert in to the skin without breakage Polymers should have sufficient mechanical strength Polymers should be compatible with the drug chosen Should not produce any pain The geometry of the micro needle is important where sharpness of the tip strongly effects insertion in to the skin

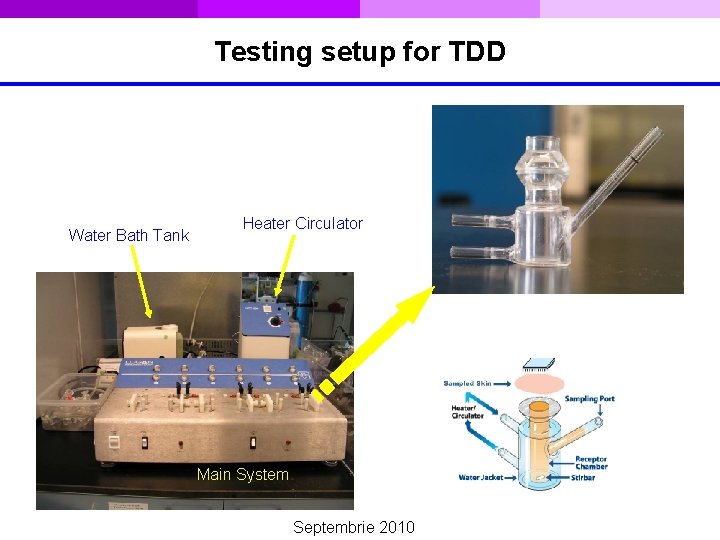
Testing setup for TDD Water Bath Tank Heater Circulator Main System Septembrie 2010

Skin preparation Skin Preparation Protocol: 1. Excise the skin from abdominal area of rats/pigs; 2. Remove the hair from the sampled skins; 3. Remove the adhering fat and other visceral debris by tweezers; 4. Scrape off the underlying subcutaneous fat to leave the skin to be 1. 5 mmthick; 5. Wash the skin with physiological saline; 6. Wrap the skin in aluminum foil; 7. Store at -80 o. C Rat Skin Pig Skin Septembrie 2010

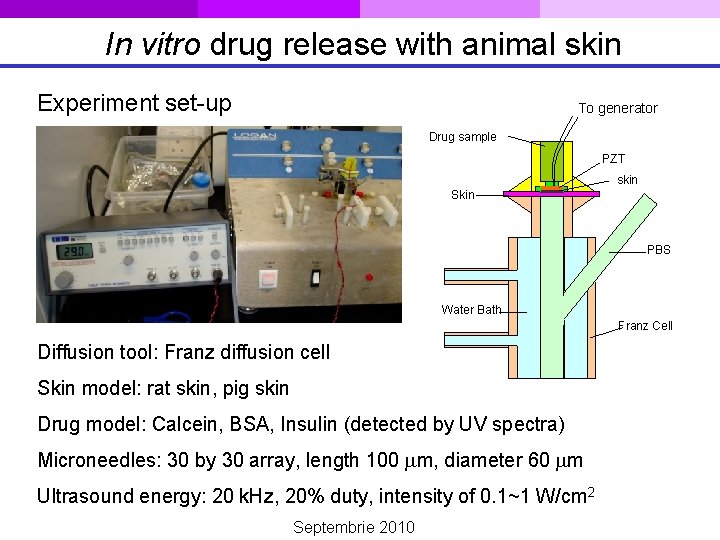
In vitro drug release with animal skin Experiment set-up To generator Drug sample PZT skin Skin PBS Water Bath Franz Cell Diffusion tool: Franz diffusion cell Skin model: rat skin, pig skin Drug model: Calcein, BSA, Insulin (detected by UV spectra) Microneedles: 30 by 30 array, length 100 m, diameter 60 m Ultrasound energy: 20 k. Hz, 20% duty, intensity of 0. 1~1 W/cm 2 Septembrie 2010

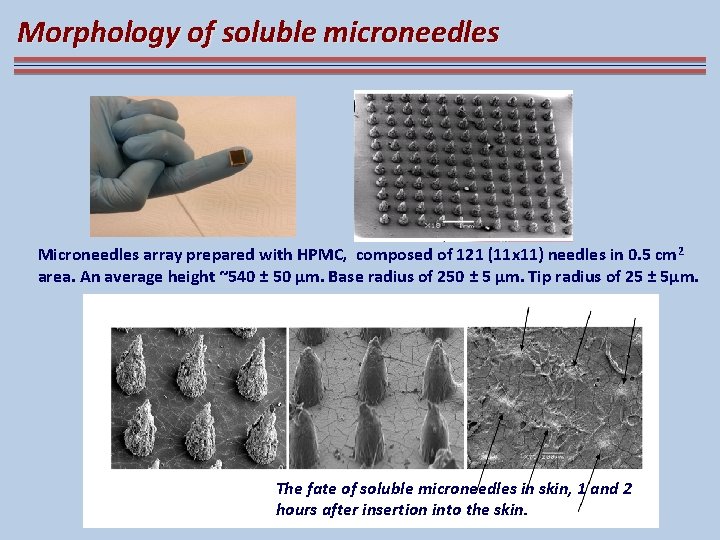
Morphology of soluble microneedles Microneedles array prepared with HPMC, composed of 121 (11 x 11) needles in 0. 5 cm 2 area. An average height ~540 ± 50 μm. Base radius of 250 ± 5 μm. Tip radius of 25 ± 5μm. The fate of soluble microneedles in skin, 1 and 2 hours after insertion into the skin.


Needle less DDS • Uses Electrostatic forces to drive the drug / Biologic • Occlusion of the skin promotes drug delivery

Viaskin –Epicutaneous Delivery System (EDS)

Viaskin: Peanut Cows milk and HDM

EPIT Without micro needles ! Research is in early stage Proteins are loaded on the central polyethylene membrane and are retained on this support owing to electrostatic forces. The EDS creates an occlusive chamber on the skin that generates moisture and releases proteins from its support. Proteins are then absorbed through the skin where they interact with epidermal immune cells. (b) Viaskins EDS applied on the back of BALB/cmice. Hair was removed by shaving and then using a depilator cream.

Issues US FDA wants that Micro needle patches have to be sterile. Suitable methods of sterilization should be investigated and validated

Conclusion • Epidermis contains a number of potent APCs. They are assumed to capture allergen and trigger appropriate immune response • Adjuvants can be topically administered • Epidermis is non-vascularised • Epicutaneous can be administered at Home itself and painless. • EPIT will be of future immunotherapy for allergen administration

Thank You for your Attention! Septembrie 2010 paranjothy 47@gmail. com
 Delmovate
Delmovate Initinib
Initinib Immunotherapy
Immunotherapy As discussed
As discussed Duxone color formula
Duxone color formula Serial polyadic dp formulation
Serial polyadic dp formulation Kant's categorical imperative
Kant's categorical imperative Future continuous and future perfect examples
Future continuous and future perfect examples Future perfect simple vs future perfect continuous
Future perfect simple vs future perfect continuous Fsa allergy chef cards
Fsa allergy chef cards Avoid the offending allergen that
Avoid the offending allergen that Idph allergen awareness
Idph allergen awareness Control ctrl allergen
Control ctrl allergen Menu with allergen information
Menu with allergen information Allergen checklist for food suppliers and manufacturers
Allergen checklist for food suppliers and manufacturers Joneja allergen chart
Joneja allergen chart Plain english allergen labelling
Plain english allergen labelling Joneja allergen chart
Joneja allergen chart Future of ihrm
Future of ihrm Current and future trends of media and information.
Current and future trends of media and information. Future trends
Future trends Future of ihrm
Future of ihrm Gis evolution and future trends
Gis evolution and future trends Present continuous future
Present continuous future Future continuous
Future continuous Future continuous.
Future continuous. Future tenses summary
Future tenses summary Kondicional engleski
Kondicional engleski Future nurse programme
Future nurse programme Future vs future perfect
Future vs future perfect Present past continuous
Present past continuous Future plans and finished future actions
Future plans and finished future actions Future continuous tense vs future simple
Future continuous tense vs future simple Definition of periodic trend
Definition of periodic trend Trends of 1950s
Trends of 1950s Current trends in medical education
Current trends in medical education Electron affinity trend
Electron affinity trend Human resource management global edition
Human resource management global edition Ip transit price trends
Ip transit price trends