Specific Allergen Immunotherapy Safety World Allergy Organization 2011

















- Slides: 67

Specific Allergen Immunotherapy Safety World Allergy Organization 2011 Congress Cancun, Mexico Linda Cox, MD, FAAAAI, FACAAI Associate Clinical Professor of Medicine Nova Southeastern University Ft. Lauderdale, Florida

Allergen Immunotherapy Safety Subcutaneous immunotherapy Sublingual Immunotherapy Adverse reactions: types and incidence per published studies , post marketing surveillance & surveys Lessons learned from the literature: risk factors WAO Grading system for SCIT/SLIT systemic reactions and SLIT local reactions Other forms of immunotherapy Oral immunotherapy (foods) Adjuvants/peptides Epicutaneous/intralympahatic

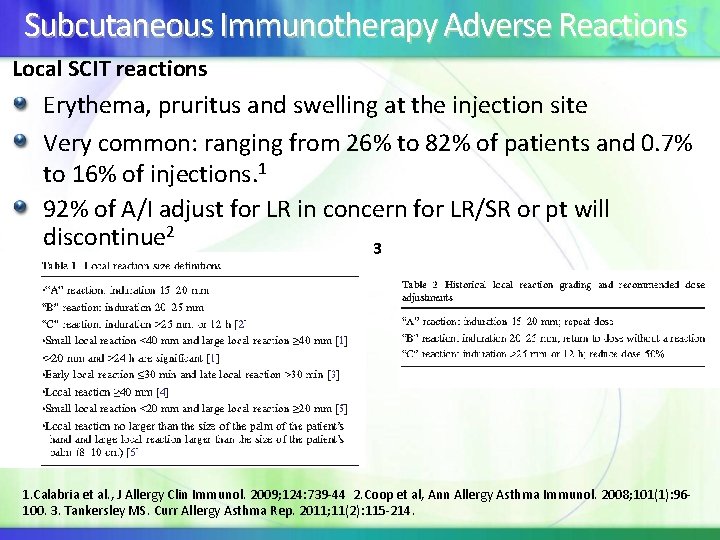
Subcutaneous Immunotherapy Adverse Reactions Local SCIT reactions Erythema, pruritus and swelling at the injection site Very common: ranging from 26% to 82% of patients and 0. 7% to 16% of injections. 1 92% of A/I adjust for LR in concern for LR/SR or pt will discontinue 2 3 1. Calabria et al. , J Allergy Clin Immunol. 2009; 124: 739 -44 2. Coop et al, Ann Allergy Asthma Immunol. 2008; 101(1): 96100. 3. Tankersley MS. Curr Allergy Asthma Rep. 2011; 11(2): 115 -214.

Subcutaneous Immunotherapy Adverse Reactions Local reactions ‘pearls/myths’ Small or large LR rate defined as ≤ or > palm of hand). 1 Not related to glycerin content but Small LR rate higher with increasing allergen content. LLR found not to be predictive of local or systemic reactions with subsequent injections 2 -4 Survey of 249 SCIT patients-those who experienced LR 5 81. 9% deemed LR not to be bothersome. 96. 0% stated they would not stop SCIT because of these LR 1. Calabria et al. , J Allergy Clin Immunol. 2008; 121: 222 -6. 2. Calabria et al. , J Allergy Clin Immunol. 2009; 124: 739 -44. 3. Tankersley et al, J Allergy Clin Immunol. 2000; 106(5): 840 -3. 4. Kelso Ann Allergy Asthma Immunol. 2004; 92(2): 225 -7. 5. Coop et al, Ann Allergy Asthma Immunol. 2008; 101(1): 96 -100

Individuals with a greater frequency of LLR may be a greater risk for SR Methods: Retrospective review of a database: comparing LLR rate in pts who had SRs with pts who did not have SRs LLR= redness & swelling ≥ 25 mm Results: 258 pts had 283 SRs in 108, 621 injections LLR rate 4 times higher in pts with SRs than pts with no SRs SR group: LLR rate: 35. 2% of visits and 19. 5% of injections No SR group: LLR rate: 8. 9% of visits and 5. 3% of injections (P <. 001 each). Conclusions: Patients with increased frequency of LLR may have increased risk for future SR. Roy et al. , Ann Allergy Asthma Immunol 2007; 99: 82 -6.

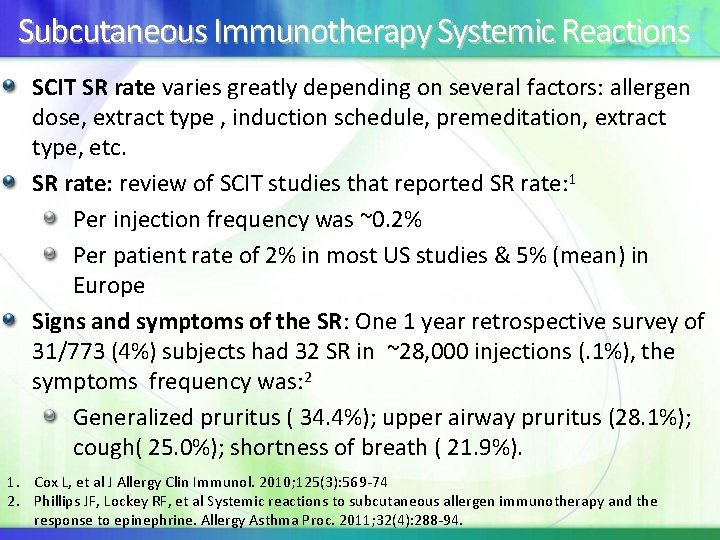
Subcutaneous Immunotherapy Systemic Reactions SCIT SR rate varies greatly depending on several factors: allergen dose, extract type , induction schedule, premeditation, extract type, etc. SR rate: review of SCIT studies that reported SR rate: 1 Per injection frequency was ~0. 2% Per patient rate of 2% in most US studies & 5% (mean) in Europe Signs and symptoms of the SR: One 1 year retrospective survey of 31/773 (4%) subjects had 32 SR in ~28, 000 injections (. 1%), the symptoms frequency was: 2 Generalized pruritus ( 34. 4%); upper airway pruritus (28. 1%); cough( 25. 0%); shortness of breath ( 21. 9%). 1. Cox L, et al J Allergy Clin Immunol. 2010; 125(3): 569 -74 2. Phillips JF, Lockey RF, et al Systemic reactions to subcutaneous allergen immunotherapy and the response to epinephrine. Allergy Asthma Proc. 2011; 32(4): 288 -94.

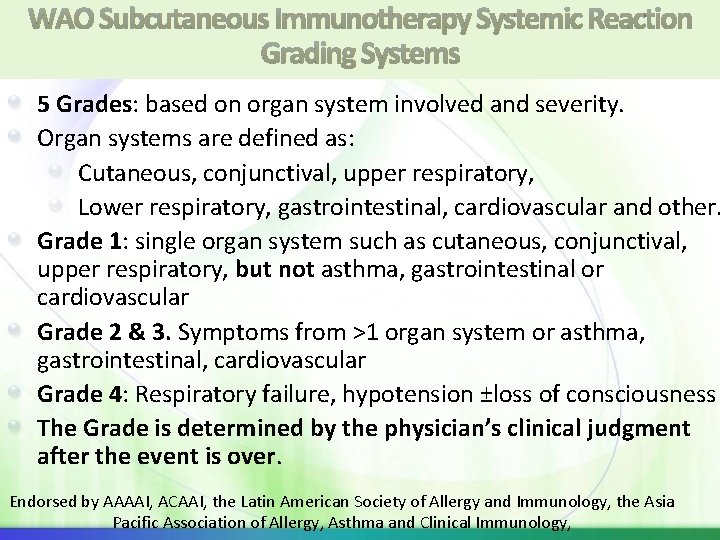
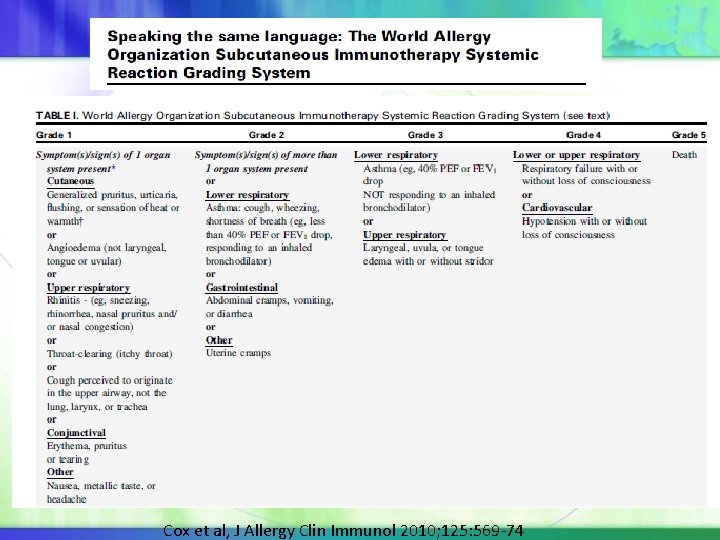
WAO Subcutaneous Immunotherapy Systemic Reaction Grading Systems 5 Grades: based on organ system involved and severity. Organ systems are defined as: Cutaneous, conjunctival, upper respiratory, Lower respiratory, gastrointestinal, cardiovascular and other. Grade 1: single organ system such as cutaneous, conjunctival, upper respiratory, but not asthma, gastrointestinal or cardiovascular Grade 2 & 3. Symptoms from >1 organ system or asthma, gastrointestinal, cardiovascular Grade 4: Respiratory failure, hypotension ±loss of consciousness The Grade is determined by the physician’s clinical judgment after the event is over. Endorsed by AAAAI, ACAAI, the Latin American Society of Allergy and Immunology, the Asia Pacific Association of Allergy, Asthma and Clinical Immunology,

Cox et al, J Allergy Clin Immunol 2010; 125: 569 -74

The final reaction grade will not be determined until the event is over, regardless of the medication administered. The final report should include the first symptom(s)/sign(s) and the time of onset after the SCIT injection and A suffix that denotes if and when epinephrine is or is not administered in relationship to symptom(s)/sign(s) of the SR: a. b. c. d. z. ≤ 5 minutes; >5 minutes to ≤ 10 minutes; >10 to ≤ 20 minutes; >20 minutes; epinephrine not administered.

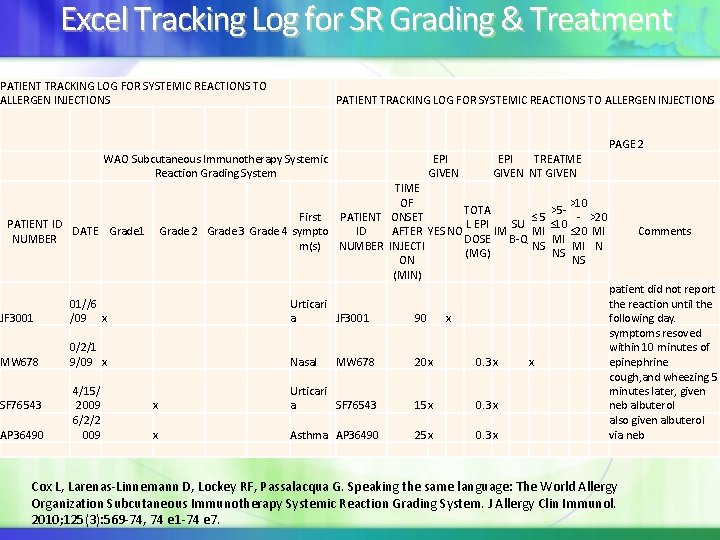
Excel Tracking Log for SR Grading & Treatment PATIENT TRACKING LOG FOR SYSTEMIC REACTIONS TO ALLERGEN INJECTIONS WAO Subcutaneous Immunotherapy Systemic Reaction Grading System First Grade 2 Grade 3 Grade 4 sympto m(s) PATIENT ID DATE Grade 1 NUMBER JF 3001 01//6 /09 x Urticari a MW 678 0/2/1 9/09 x Nasal SF 76543 AP 36490 4/15/ 2009 6/2/2 009 x Urticari a x Asthma EPI GIVEN EPI TREATME GIVEN NT GIVEN PAGE 2 TIME OF >10 TOTA >5 - - >20 PATIENT ONSET ≤ 5 L EPI SU ≤ 10 AFTER YES NO IM ≤ 20 MI Comments ID MI DOSE B-Q MI NUMBER INJECTI NS MI N (MG) NS ON NS (MIN) patient did not report the reaction until the JF 3001 90 x following day. symptoms resoved within 10 minutes of MW 678 20 x 0. 3 x x epinephrine cough, and wheezing 5 minutes later, given SF 76543 15 x 0. 3 x neb albuterol also given albuterol AP 36490 25 x 0. 3 x via neb Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010; 125(3): 569 -74, 74 e 1 -74 e 7.

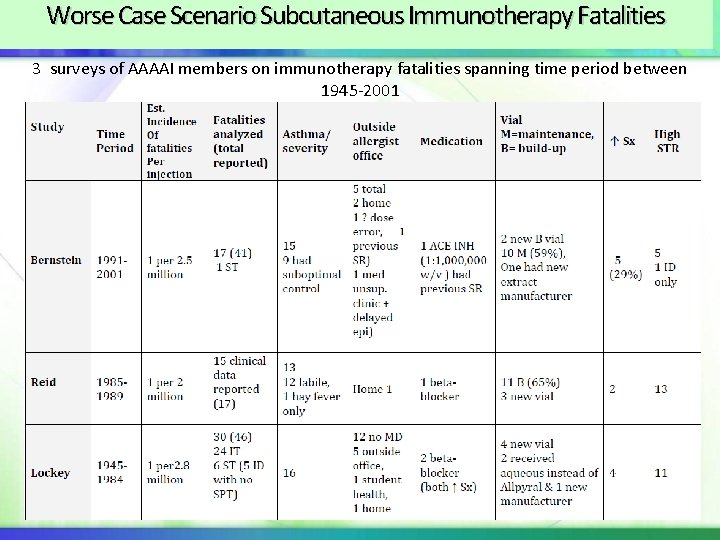
Worse Case Scenario Subcutaneous Immunotherapy Fatalities 3 surveys of AAAAI members on immunotherapy fatalities spanning time period between 1945 -2001

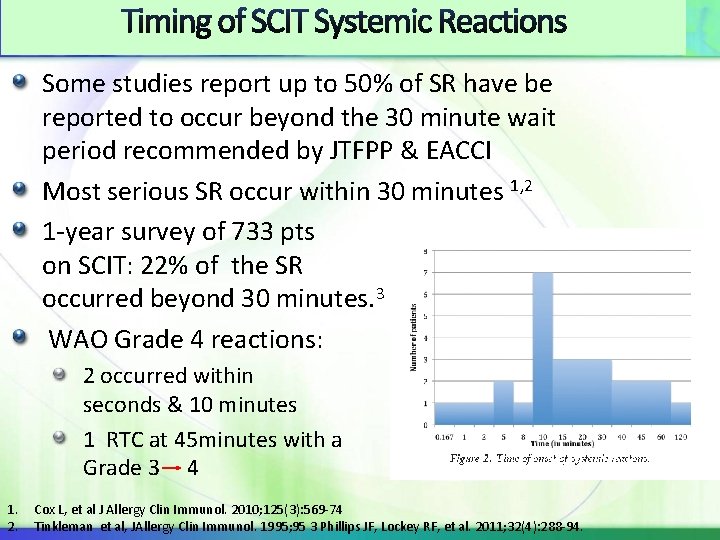
Timing of SCIT Systemic Reactions Some studies report up to 50% of SR have be reported to occur beyond the 30 minute wait period recommended by JTFPP & EACCI Most serious SR occur within 30 minutes 1, 2 1 -year survey of 733 pts on SCIT: 22% of the SR occurred beyond 30 minutes. 3 WAO Grade 4 reactions: 2 occurred within seconds & 10 minutes 1 RTC at 45 minutes with a Grade 3 4 1. 2. Cox L, et al J Allergy Clin Immunol. 2010; 125(3): 569 -74 Tinkleman et al, JAllergy Clin Immunol. 1995; 95 3 Phillips JF, Lockey RF, et al. 2011; 32(4): 288 -94.

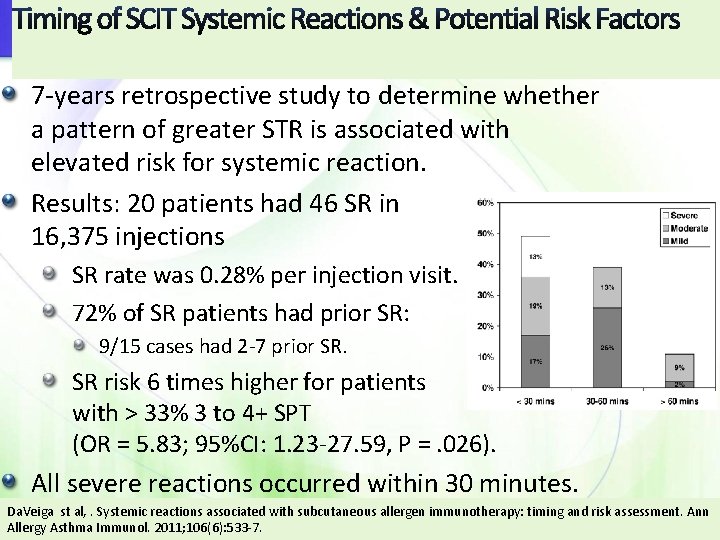
Timing of SCIT Systemic Reactions & Potential Risk Factors 7 -years retrospective study to determine whether a pattern of greater STR is associated with elevated risk for systemic reaction. Results: 20 patients had 46 SR in 16, 375 injections SR rate was 0. 28% per injection visit. 72% of SR patients had prior SR: 9/15 cases had 2 -7 prior SR. SR risk 6 times higher for patients with > 33% 3 to 4+ SPT (OR = 5. 83; 95%CI: 1. 23 -27. 59, P =. 026). All severe reactions occurred within 30 minutes. Da. Veiga st al, . Systemic reactions associated with subcutaneous allergen immunotherapy: timing and risk assessment. Ann Allergy Asthma Immunol. 2011; 106(6): 533 -7.

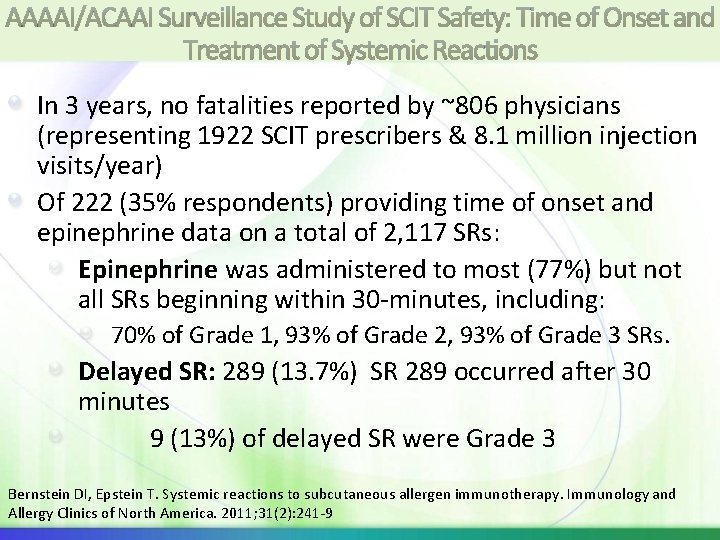
AAAAI/ACAAI Surveillance Study of SCIT Safety: Time of Onset and Treatment of Systemic Reactions In 3 years, no fatalities reported by ~806 physicians (representing 1922 SCIT prescribers & 8. 1 million injection visits/year) Of 222 (35% respondents) providing time of onset and epinephrine data on a total of 2, 117 SRs: Epinephrine was administered to most (77%) but not all SRs beginning within 30 -minutes, including: 70% of Grade 1, 93% of Grade 2, 93% of Grade 3 SRs. Delayed SR: 289 (13. 7%) SR 289 occurred after 30 minutes 9 (13%) of delayed SR were Grade 3 Bernstein DI, Epstein T. Systemic reactions to subcutaneous allergen immunotherapy. Immunology and Allergy Clinics of North America. 2011; 31(2): 241 -9

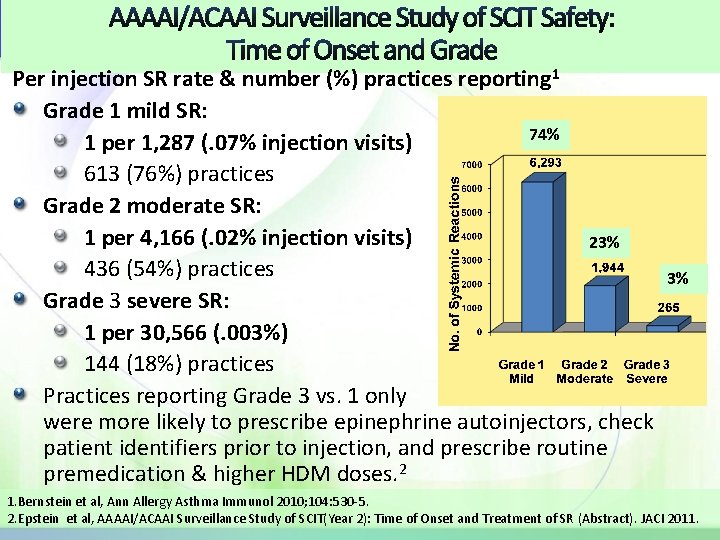
AAAAI/ACAAI Surveillance Study of SCIT Safety: Time of Onset and Grade Per injection SR rate & number (%) practices reporting 1 Grade 1 mild SR: 74% 1 per 1, 287 (. 07% injection visits) 613 (76%) practices Grade 2 moderate SR: 1 per 4, 166 (. 02% injection visits) 23% 436 (54%) practices 3% Grade 3 severe SR: 1 per 30, 566 (. 003%) 144 (18%) practices Practices reporting Grade 3 vs. 1 only were more likely to prescribe epinephrine autoinjectors, check patient identifiers prior to injection, and prescribe routine premedication & higher HDM doses. 2 1. Bernstein et al, Ann Allergy Asthma Immunol 2010; 104: 530 -5. 2. Epstein et al, AAAAI/ACAAI Surveillance Study of SCIT(Year 2): Time of Onset and Treatment of SR (Abstract). JACI 2011.

AAAAI/ACAAI IT Safety Survey July 2010 -July 2011 Response rates were down with 518 respondents representing 1, 135 prescribers in 2010 -11 (630 in previous year) Some important novel findings discovered in Year 3 include: Practices reporting routinely antihistamine premedication were also more likely to have reported injection related SR. Practices never or sometimes reducing allergen doses following LLR were no more likely to experience injection related SR than practices who adjusted doses. Practices who always adjusted allergen doses during peak pollen seasons were significantly less likely to experience moderate or severe SR to allergen injections. Personal communication David Bernstein; publication in preparation

Biphasic Systemic Reactions Summary Statement 35: Biphasic immunotherapy reactions, defined as resolution of the initial reaction with recurrence 2 to 24 hours, were reported in up to 23% of patients, who experienced a SR after SCIT in one study. Biphasic reactions were typically less severe than the initial reaction. C 2 prospective studies found 10%1 and 23%2 of IT SRs were biphasic No specific symptoms during the initial reaction predicted a biphasic reaction. Biphasic reactors more likely to be female , older and require >1 dose of epinephrine during initial SR Biphasic reactions were typically less severe than the initial reaction and none required additional epinephrine 1. Confino-Cohen et al, Ann Allergy Asthma Immunol 2010; 104: 73 -8. 2. Scranton et al, J Allergy Clin Immunol 2009; 123: 493 -8. 17

Wait Period & Delayed reactions The recommendation that a patient should remain in the physician’s office/medical clinic for 30 minutes after the injection is unchanged from the previous update. It is recommended that at the onset of immunotherapy, patients should be counseled on the possibility of immediate and delayed systemic reactions during risk communication; an action plan for such an event should be discussed. The decision to prescribe epinephrine autoinjectors to patients receiving immunotherapy should be at the physician’s discretion. Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127(1 Suppl): S 1 -55. 18

Accelerated Immunotherapy Schedules , Premedication and Medications to be Used with Caution

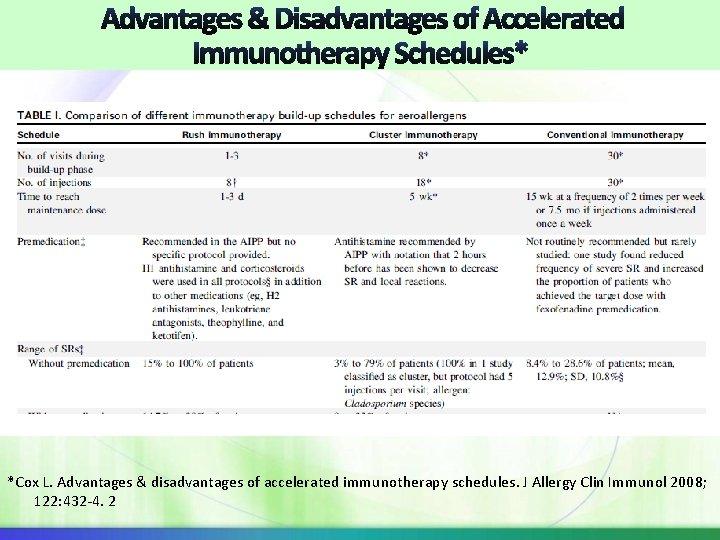
* *Cox L. Advantages & disadvantages of accelerated immunotherapy schedules. J Allergy Clin Immunol 2008; 122: 432 -4. 2

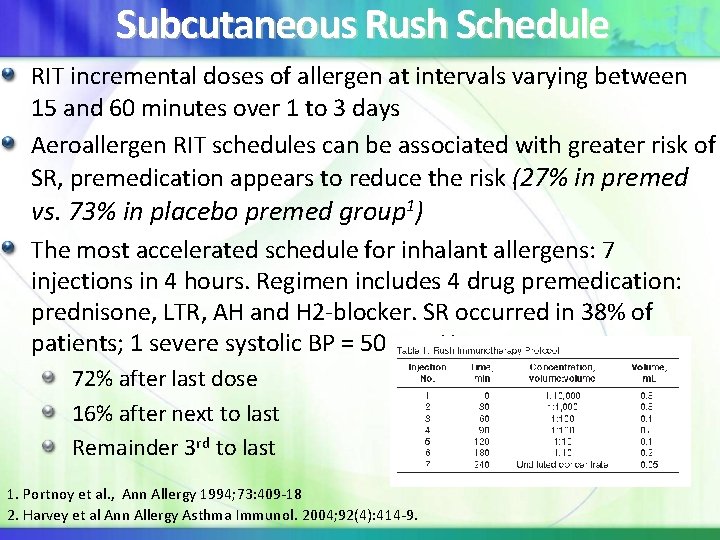
Subcutaneous Rush Schedule RIT incremental doses of allergen at intervals varying between 15 and 60 minutes over 1 to 3 days Aeroallergen RIT schedules can be associated with greater risk of SR, premedication appears to reduce the risk (27% in premed vs. 73% in placebo premed group 1) The most accelerated schedule for inhalant allergens: 7 injections in 4 hours. Regimen includes 4 drug premedication: prednisone, LTR, AH and H 2 -blocker. SR occurred in 38% of patients; 1 severe systolic BP = 50 mm Hg 72% after last dose 16% after next to last Remainder 3 rd to last 1. Portnoy et al. , Ann Allergy 1994; 73: 409 -18 2. Harvey et al Ann Allergy Asthma Immunol. 2004; 92(4): 414 -9.

Subcutaneous Rush Schedule Ultrarush stinging insect protocols achieve the maintenance dose in 2. 5 to 4 hours RIT for administration of Hymenoptera have not been associated with a similar high incidence of systemic reactions. Conflicting data on safety of fire ant (FA) RIT without premedication 1 -day FA RIT without premedication reported 24. 3% of the 37 patients experienced SR most being urticaria and pruritus “Further studies are needed to clarify the risk of fire ant rush immunotherapy, and premedication might be considered. ” (from the 2011 Allergen Immunotherapy Practice Parameter 3 rd Update) 1. Tankersley J Allergy Clin Immunol. 2002; 109(3): 556 -62. 2. Dietrich et al, Ann Allergy Asthma Immunol. 2009; 103(6): 535 -6.

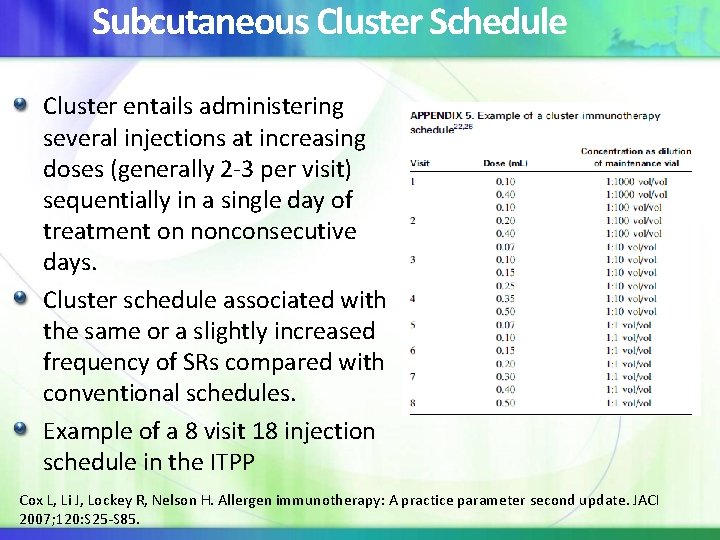
Subcutaneous Cluster Schedule Cluster entails administering several injections at increasing doses (generally 2 -3 per visit) sequentially in a single day of treatment on nonconsecutive days. Cluster schedule associated with the same or a slightly increased frequency of SRs compared with conventional schedules. Example of a 8 visit 18 injection schedule in the ITPP Cox L, Li J, Lockey R, Nelson H. Allergen immunotherapy: A practice parameter second update. JACI 2007; 120: S 25 -S 85.

Studies Comparing Cluster and Conventional Immunotherapy Schedule DBPC study of 239 pts with dust mite AR ± asthma comparing 6 -week with a 12 -week conventional schedule found: 1 No differences between the 2 schedules in terms of AEs Improved clinical and objective parameters in the cluster 6 weeks before conventional group Randomized study of 96 patients with dust mite AR comparing 6 week cluster with 14 week conventional found: Cluster reduced time to maintenance dose by 57%. No differences in SRs compared with conventional schedule. 2 1. Taber et al. , J Allergy Clin Immunol 2005; 116: 109 -18 2. Zhang et al. , Int Arch Allergy Immunol 2009; 148: 161 -9.

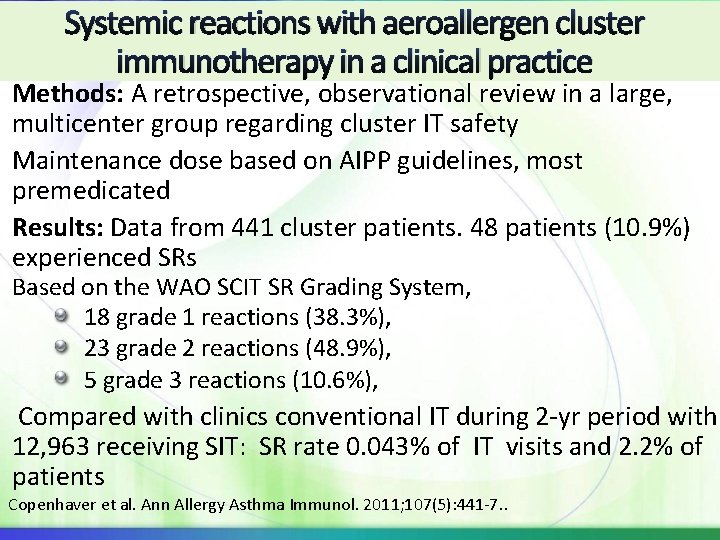
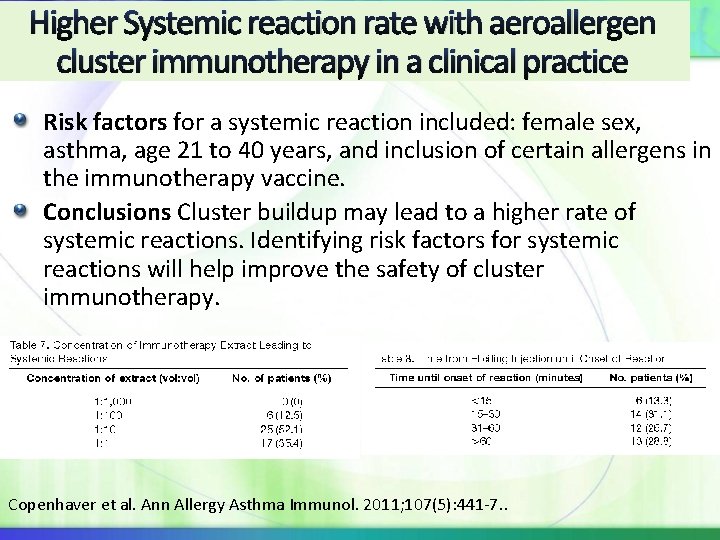
Systemic reactions with aeroallergen cluster immunotherapy in a clinical practice Methods: A retrospective, observational review in a large, multicenter group regarding cluster IT safety Maintenance dose based on AIPP guidelines, most premedicated Results: Data from 441 cluster patients. 48 patients (10. 9%) experienced SRs Based on the WAO SCIT SR Grading System, 18 grade 1 reactions (38. 3%), 23 grade 2 reactions (48. 9%), 5 grade 3 reactions (10. 6%), Compared with clinics conventional IT during 2 -yr period with 12, 963 receiving SIT: SR rate 0. 043% of IT visits and 2. 2% of patients Copenhaver et al. Ann Allergy Asthma Immunol. 2011; 107(5): 441 -7. .

Higher Systemic reaction rate with aeroallergen cluster immunotherapy in a clinical practice Risk factors for a systemic reaction included: female sex, asthma, age 21 to 40 years, and inclusion of certain allergens in the immunotherapy vaccine. Conclusions Cluster buildup may lead to a higher rate of systemic reactions. Identifying risk factors for systemic reactions will help improve the safety of cluster immunotherapy. Copenhaver et al. Ann Allergy Asthma Immunol. 2011; 107(5): 441 -7. .

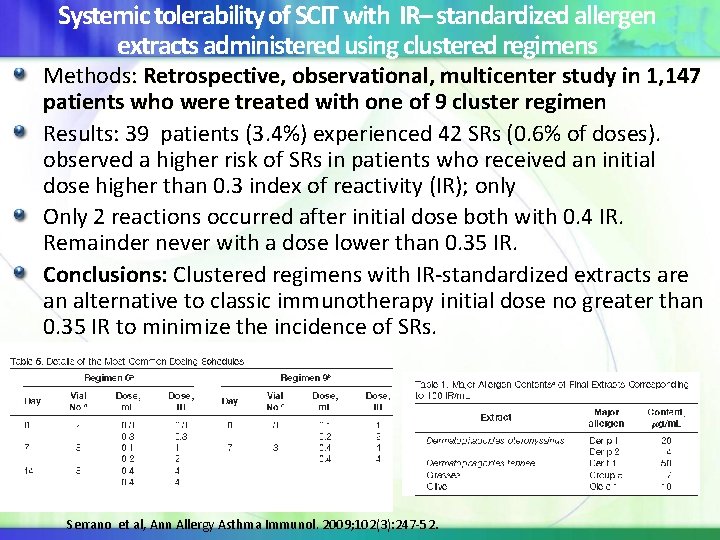
Systemic tolerability of SCIT with IR– standardized allergen extracts administered using clustered regimens Methods: Retrospective, observational, multicenter study in 1, 147 patients who were treated with one of 9 cluster regimen who were treated Results: 39 patients (3. 4%) experienced 42 SRs (0. 6% of doses). observed a higher risk of SRs in patients who received an initial dose higher than 0. 3 index of reactivity (IR); only Only 2 reactions occurred after initial dose both with 0. 4 IR. Remainder never with a dose lower than 0. 35 IR. Conclusions: Clustered regimens with IR-standardized extracts are an alternative to classic immunotherapy initial dose no greater than 0. 35 IR to minimize the incidence of SRs. Serrano et al, Ann Allergy Asthma Immunol. 2009; 102(3): 247 -52.

Measures to Improve Safety Premedication Antihistamines Studies with RIT & cluster suggest decreased incidence of local and SRs. Conventional IT: One DBPC study found premedication with fexofenadine reduced # of severe SRs, ↑ number of pts who reached TMD &↓ time to TMD 1 Leukotriene receptor antagonist Anecdotal reports of reductions in SR rates. One DBPC study demonstrated ↓ LLR during venom RIT with moneleukast 2 1. Ohashi et al, Ann Allergy Asthma Immunol 2006; 96 2. Wohrl et al. , Int Arch Allergy Immunol 2007; 144: 137 -42

Omalizumab Premedication and Allergen Immunotherapy Summary Statement 58: Omalizumab pretreatment has been shown to improve the safety and tolerability of cluster and rush immunotherapy schedules in patients with moderate-persistent asthma and allergic rhinitis, respectively. Additionally, omalizumab used in combination with immunotherapy has been shown to be effective in improving symptom scores compared to immunotherapy alone. A Cox L, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127(1 Suppl): S 1 -55.

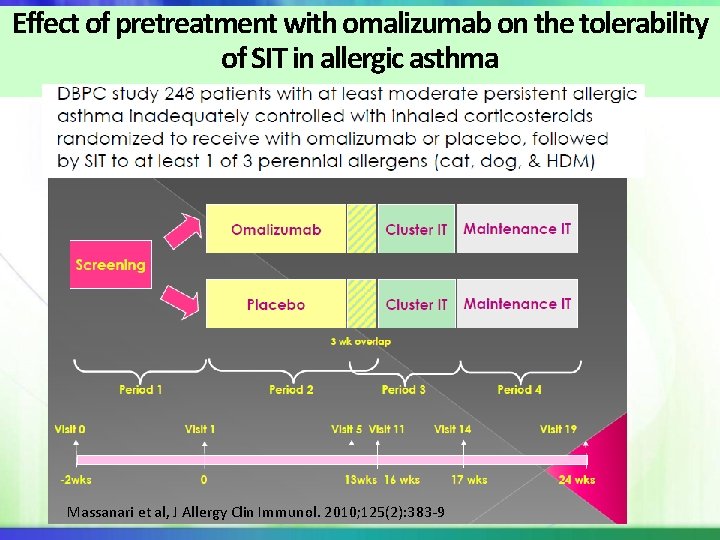
Effect of pretreatment with omalizumab on the tolerability of SIT in allergic asthma Massanari et al, J Allergy Clin Immunol. 2010; 125(2): 383 -9

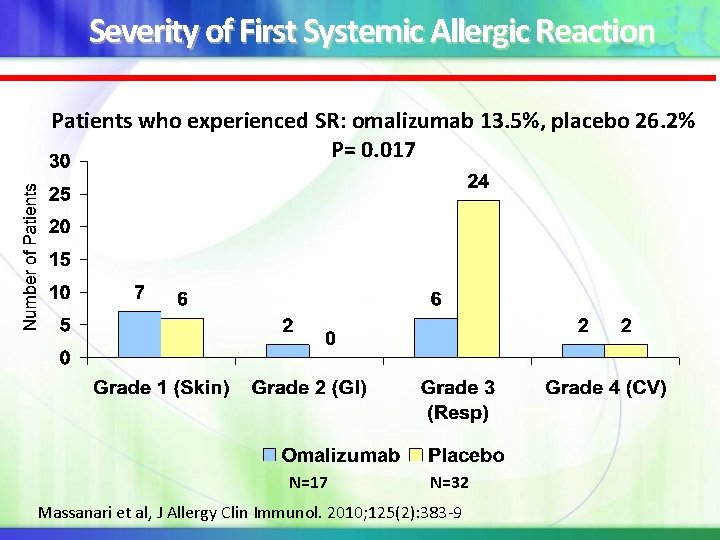
Severity of First Systemic Allergic Reaction Patients who experienced SR: omalizumab 13. 5%, placebo 26. 2% P= 0. 017 N=17 N=32 Massanari et al, J Allergy Clin Immunol. 2010; 125(2): 383 -9

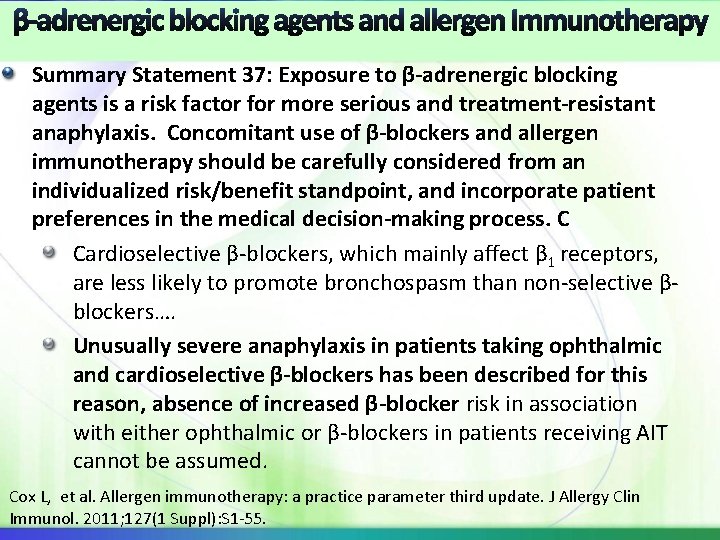
β-adrenergic blocking agents and allergen Immunotherapy Summary Statement 37: Exposure to β-adrenergic blocking agents is a risk factor for more serious and treatment-resistant anaphylaxis. Concomitant use of β-blockers and allergen immunotherapy should be carefully considered from an individualized risk/benefit standpoint, and incorporate patient preferences in the medical decision-making process. C Cardioselective β-blockers, which mainly affect β 1 receptors, are less likely to promote bronchospasm than non-selective βblockers…. Unusually severe anaphylaxis in patients taking ophthalmic and cardioselective β-blockers has been described for this reason, absence of increased β-blocker risk in association with either ophthalmic or β-blockers in patients receiving AIT cannot be assumed. Cox L, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127(1 Suppl): S 1 -55.

ACE inhibitors & Venom Immunotherapy Summary Statement 40: . ACE inhibitors have been associated with greater risk for more severe reaction from VIT as well as field stings ACE inhibitor discontinuation should be considered for patients receiving VIT. Concurrent administration of VIT and an ACE inhibitor is warranted in selected cases in which no equally efficacious alternative for an ACE inhibitor exists, and this is judged to be favorable from an individualized risk/benefit standpoint and consideration of patient preferences. No evidence exists that angiotensin receptor blockers are associated with greater risk for anaphylaxis from allergen immunotherapy. C Cox L, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127(1 Suppl): S 1 -55.

ACE Inhibitors Associated with More Severe but not More Frequent Venom Anaphylaxis Retrospective review to evaluate frequency VIT or field sting SRs in 79 pts on angiotensin-converting enzyme inhibitors(ACE-I) did not find an increase SR frequency 1 Cases of anaphylaxis/severe anaphylaxis in pts receiving VIT while on ACE Inhibitors, then none when withheld, with recurrence when ACE Inhibitor restarted. 1 , 2 A large multi-center study found that ACE inhibitors were associated with increased risk for more severe anaphylaxis after venom field sting. 3 These data provide support for the contention that ACE inhibitor use is not associated with increased SR frequency; however, greater risk for more serious reaction may still exist. 1. 2. 3. 4. White KM, England RW. Ann Allergy Asthma Immunol 2008; 101: 426 -30. Tunon-de-Lara et al, Lancet 1992; 340: 908 Ober et al, J Allergy Clin Immunol 2003; 112: 1008 -9 Ruëff et al, J Allergy Clin Immunol 2009; 124: 1047 -54. 34

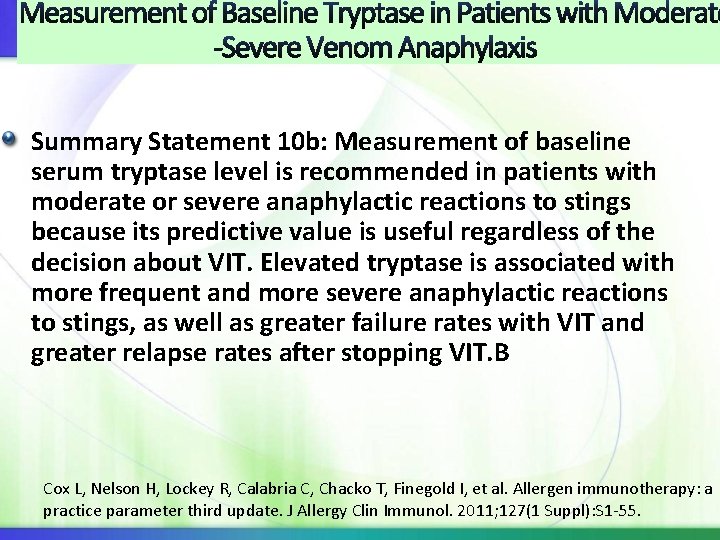
Measurement of Baseline Tryptase in Patients with Moderate -Severe Venom Anaphylaxis Summary Statement 10 b: Measurement of baseline serum tryptase level is recommended in patients with moderate or severe anaphylactic reactions to stings because its predictive value is useful regardless of the decision about VIT. Elevated tryptase is associated with more frequent and more severe anaphylactic reactions to stings, as well as greater failure rates with VIT and greater relapse rates after stopping VIT. B Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127(1 Suppl): S 1 -55.

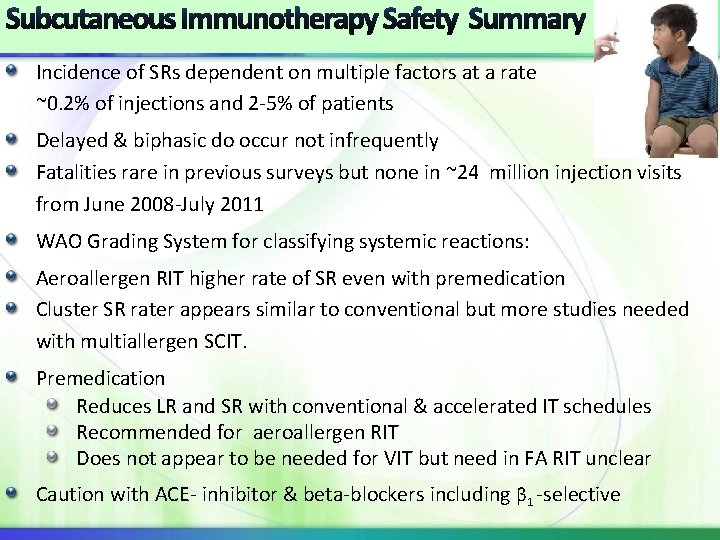
Incidence of SRs dependent on multiple factors at a rate ~0. 2% of injections and 2 -5% of patients Delayed & biphasic do occur not infrequently Fatalities rare in previous surveys but none in ~24 million injection visits from June 2008 -July 2011 WAO Grading System for classifying systemic reactions: Aeroallergen RIT higher rate of SR even with premedication Cluster SR rater appears similar to conventional but more studies needed with multiallergen SCIT. Premedication Reduces LR and SR with conventional & accelerated IT schedules Recommended for aeroallergen RIT Does not appear to be needed for VIT but need in FA RIT unclear Caution with ACE- inhibitor & beta-blockers including β 1 -selective

Some difficulty in evaluating SLIT safety because: Treatment administered at home Thus adverse reactions primarily occur at home, i. e. , unwitnessed and/or not evaluated by a someone with medical training May be significant variability in accuracy and interpretation of patient’s reported AEs

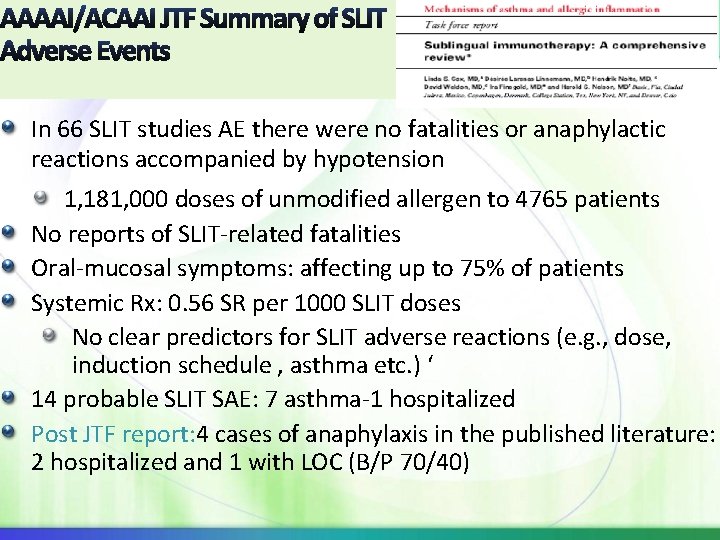
AAAAI/ACAAI JTF Summary of SLIT Adverse Events In 66 SLIT studies AE there were no fatalities or anaphylactic reactions accompanied by hypotension 1, 181, 000 doses of unmodified allergen to 4765 patients No reports of SLIT-related fatalities Oral-mucosal symptoms: affecting up to 75% of patients Systemic Rx: 0. 56 SR per 1000 SLIT doses No clear predictors for SLIT adverse reactions (e. g. , dose, induction schedule , asthma etc. ) ‘ 14 probable SLIT SAE: 7 asthma-1 hospitalized Post JTF report: 4 cases of anaphylaxis in the published literature: 2 hospitalized and 1 with LOC (B/P 70/40)

SLIT Safety in Published Literature No reports of SLIT-related fatalities to date in an estimated ___billion doses Most AE are local and occur in the beginning of treatment Dose-response relationship with AEs in some studies No apparent relationship with updosing schedule and AEs Several large (>400 patients) grass-pollen tablet studies in adults & children demonstrate good safety profile with no updosing Few reported cases of anaphylaxis (at least 6 )

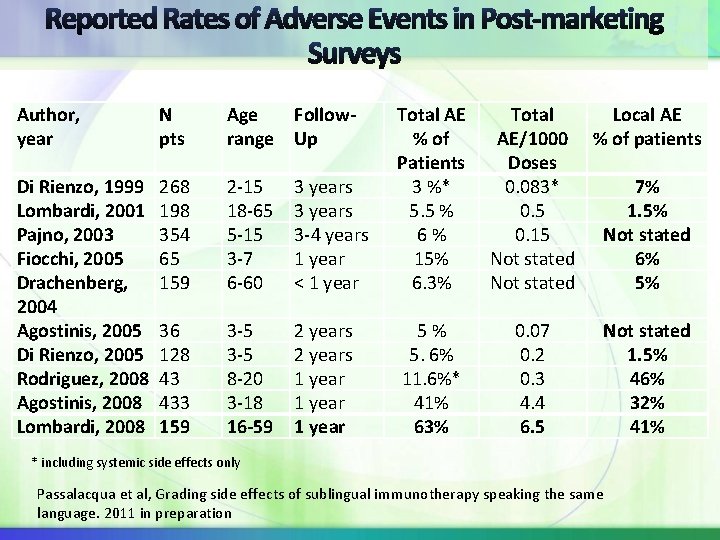
Author, year Di Rienzo, 1999 Lombardi, 2001 Pajno, 2003 Fiocchi, 2005 Drachenberg, 2004 Agostinis, 2005 Di Rienzo, 2005 Rodriguez, 2008 Agostinis, 2008 Lombardi, 2008 N pts Age range Follow. Up 268 198 354 65 159 2 -15 18 -65 5 -15 3 -7 6 -60 3 years 3 -4 years 1 year < 1 year Total AE Total Local AE % of AE/1000 % of patients Patients Doses 3 %* 0. 083* 7% 5. 5 % 0. 5 1. 5% 6 % 0. 15 Not stated 15% Not stated 6% 6. 3% Not stated 5% 36 128 43 433 159 3 -5 8 -20 3 -18 16 -59 2 years 1 year 5 % 5. 6% 11. 6%* 41% 63% 0. 07 0. 2 0. 3 4. 4 6. 5 Not stated 1. 5% 46% 32% 41% * including systemic side effects only Passalacqua et al, Grading side effects of sublingual immunotherapy speaking the same language. 2011 in preparation

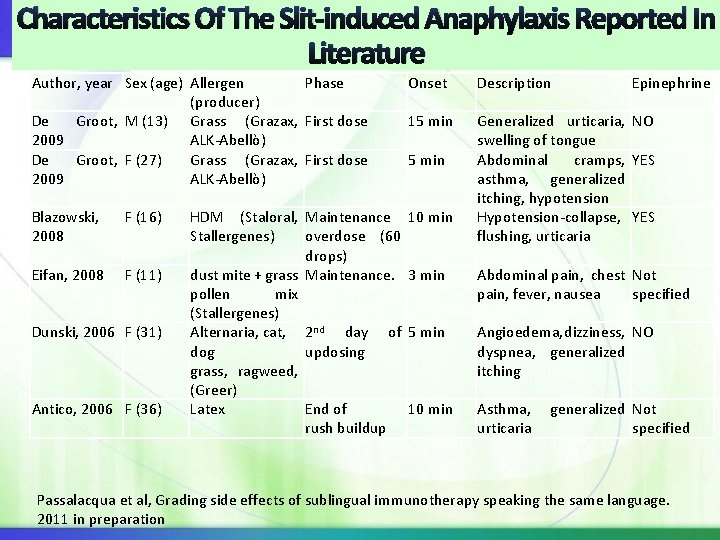
Characteristics Of The Slit-induced Anaphylaxis Reported In Literature Author, year Sex (age) Allergen Phase (producer) De Groot, M (13) Grass (Grazax, First dose 2009 ALK-Abellò) De Groot, F (27) Grass (Grazax, First dose 2009 ALK-Abellò) Blazowski, 2008 F (16) Eifan, 2008 F (11) Dunski, 2006 F (31) Antico, 2006 F (36) Onset Description 15 min Generalized urticaria, NO swelling of tongue Abdominal cramps, YES asthma, generalized itching, hypotension Hypotension-collapse, YES flushing, urticaria 5 min HDM (Staloral, Maintenance 10 min Stallergenes) overdose (60 drops) dust mite + grass Maintenance. 3 min pollen mix (Stallergenes) Alternaria, cat, 2 nd day of 5 min dog updosing grass, ragweed, (Greer) Latex End of 10 min rush buildup Epinephrine Abdominal pain, chest Not pain, fever, nausea specified Angioedema, dizziness, NO dyspnea, generalized itching Asthma, generalized Not urticaria specified Passalacqua et al, Grading side effects of sublingual immunotherapy speaking the same language. 2011 in preparation

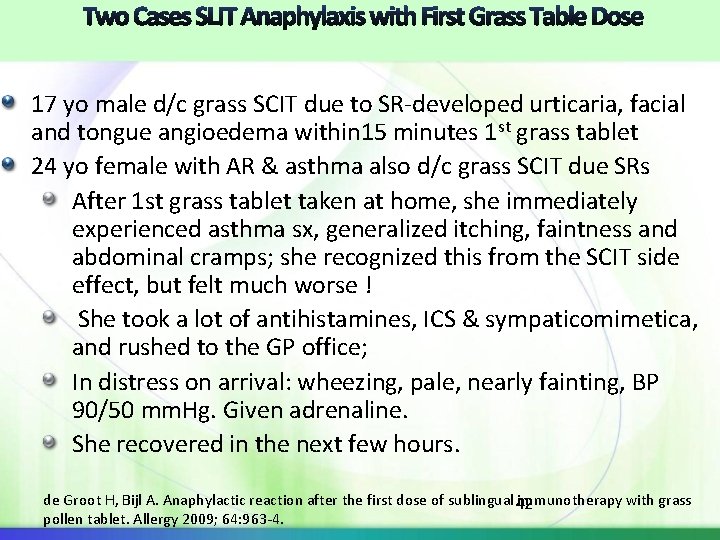
17 yo male d/c grass SCIT due to SR-developed urticaria, facial and tongue angioedema within 15 minutes 1 st grass tablet 24 yo female with AR & asthma also d/c grass SCIT due SRs After 1 st grass tablet taken at home, she immediately experienced asthma sx, generalized itching, faintness and abdominal cramps; she recognized this from the SCIT side effect, but felt much worse ! She took a lot of antihistamines, ICS & sympaticomimetica, and rushed to the GP office; In distress on arrival: wheezing, pale, nearly fainting, BP 90/50 mm. Hg. Given adrenaline. She recovered in the next few hours. de Groot H, Bijl A. Anaphylactic reaction after the first dose of sublingual immunotherapy with grass 42 pollen tablet. Allergy 2009; 64: 963 -4.

Risk factors in these cases? dose, gap in treatment, history of previous SR, updosing phase, multi-allergen treatment, delay in epinephrine, height of season, asthma? ? Multiallergen SLIT Safety: Two postmarketing surveys (1 adult 1, 1 pediatrics 2) found no difference in safety of between single allergen & multiple allergen SLIT. Previous SR: prospective study of 43 pts receiving SLIT : 3/5 pts with SLIT SR had previous SCIT SRs 3 1. Lombardi C Allergy 2008; 63: 375 -6 2. Agostinis F , Allergy 2008; 63: 1637 -9 3. Rodriguez-Perez N Ann Allergy Asthma Immunol 2008; 101: 304 -10 43

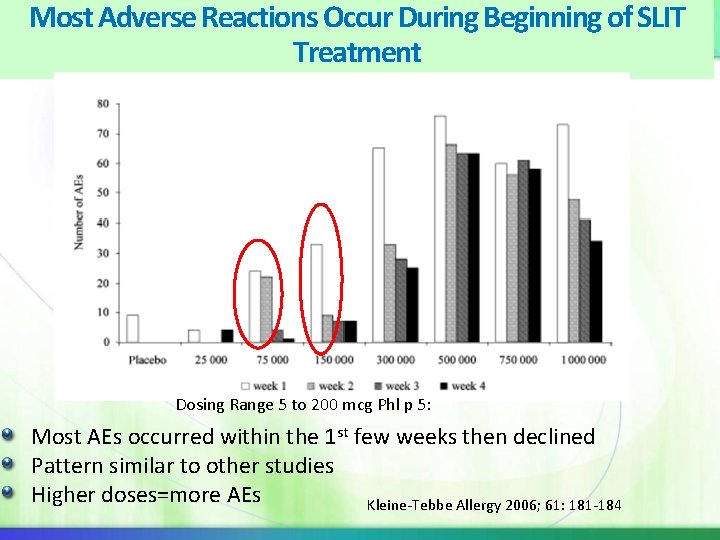
Most Adverse Reactions Occur During Beginning of SLIT Treatment Dosing Range 5 to 200 mcg Phl p 5: Most AEs occurred within the 1 st few weeks then declined Pattern similar to other studies Higher doses=more AEs Kleine-Tebbe Allergy 2006; 61: 181 -184

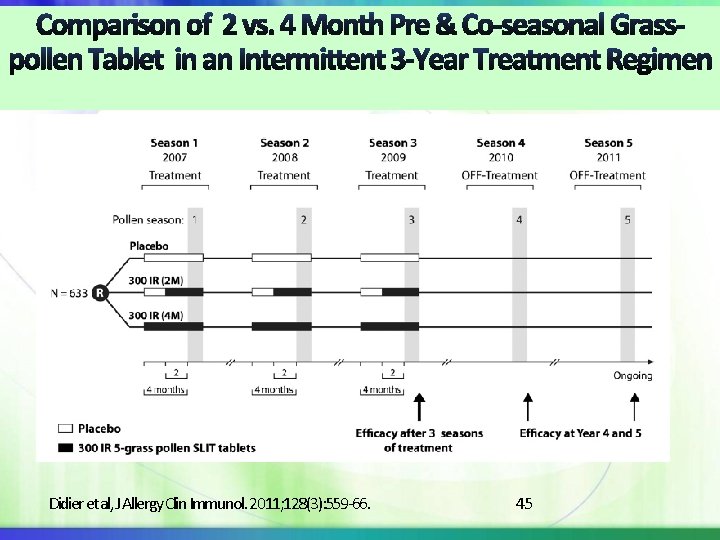
Didier et al, J Allergy Clin Immunol. 2011; 128(3): 559 -66. 45

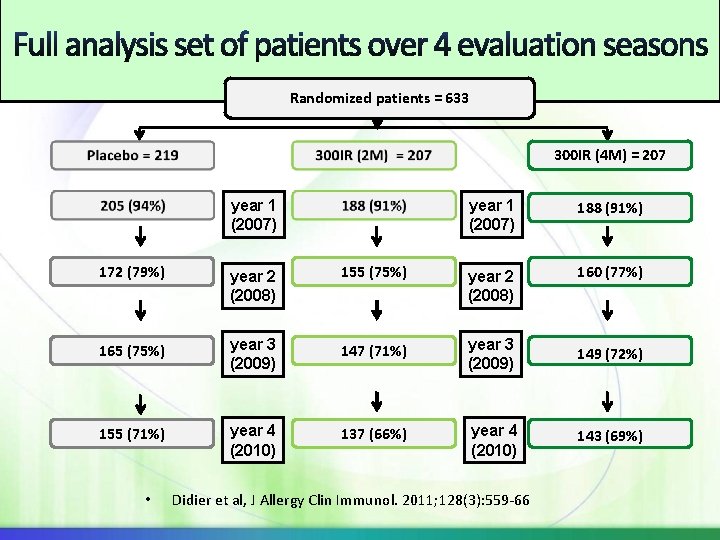
Randomized patients = 633 300 IR (4 M) = 207 year 1 (2007) 188 (91%) 172 (79%) year 2 (2008) 155 (75%) year 2 (2008) 160 (77%) 165 (75%) year 3 (2009) 147 (71%) year 3 (2009) 149 (72%) 155 (71%) year 4 (2010) 137 (66%) year 4 (2010) 143 (69%) • Didier et al, J Allergy Clin Immunol. 2011; 128(3): 559 -66

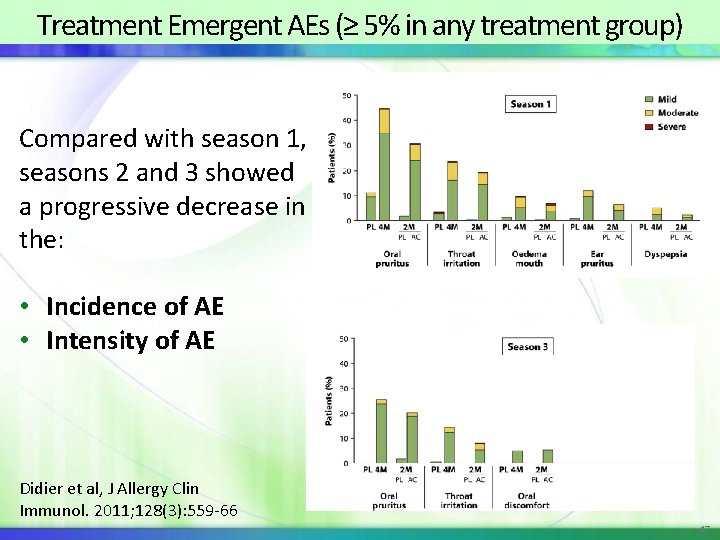
Treatment Emergent AEs (≥ 5% in any treatment group) Compared with season 1, seasons 2 and 3 showed a progressive decrease in the: • Incidence of AE • Intensity of AE Didier et al, J Allergy Clin Immunol. 2011; 128(3): 559 -66 47

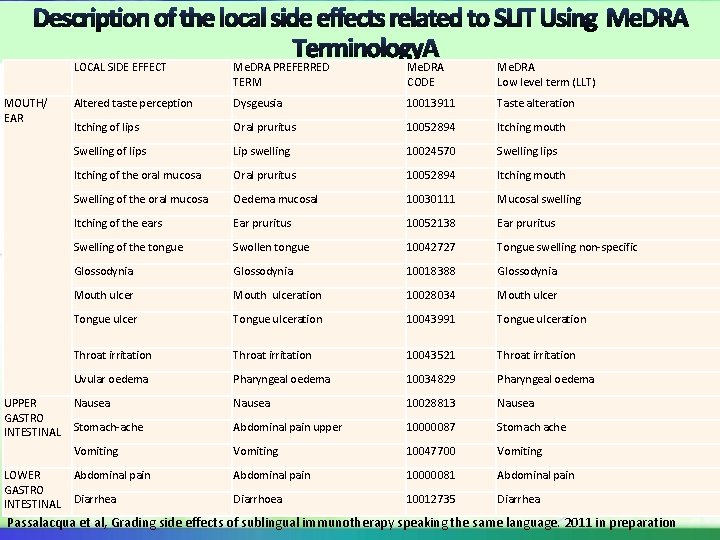
LOCAL SIDE EFFECT Me. DRA PREFERRED TERM Me. DRA CODE Me. DRA Low level term (LLT) MOUTH/ EAR Altered taste perception Dysgeusia 10013911 Taste alteration Itching of lips Oral pruritus 10052894 Itching mouth Swelling of lips Lip swelling 10024570 Swelling lips Itching of the oral mucosa Oral pruritus 10052894 Itching mouth Swelling of the oral mucosa Oedema mucosal 10030111 Mucosal swelling Itching of the ears Ear pruritus 10052138 Ear pruritus Swelling of the tongue Swollen tongue 10042727 Tongue swelling non-specific Glossodynia 10018388 Glossodynia Mouth ulceration 10028034 Mouth ulcer Tongue ulceration 10043991 Tongue ulceration Throat irritation 10043521 Throat irritation Uvular oedema Pharyngeal oedema 10034829 Pharyngeal oedema Nausea 10028813 Nausea Stomach-ache Abdominal pain upper 10000087 Stomach ache Vomiting 10047700 Vomiting Abdominal pain 10000081 Abdominal pain Diarrhea Diarrhoea 10012735 Diarrhea UPPER GASTRO INTESTINAL LOWER GASTRO INTESTINAL Passalacqua et al, Grading side effects of sublingual immunotherapy speaking the same language. 2011 in preparation

Passalacqua et al, Grading side effects of sublingual immunotherapy speaking the same language. 2011 in preparation

EAACI Immunotherapy Task Force Recommendations “The scientific documentation for treatment schedules and dose modifications is limited. For routine treatment following the guidelines from the manufacturers is sensible. • The administration of SLIT must be postponed in the following circumstances: • – In the presence of oro-pharyngeal infection. • – In the case of major dental surgery. • – Acute gastroenteritis. • – Exacerbation of the asthma. • – PEFR <80% of personal best value. • – Simultaneous administration of viral vaccines. ” Alvarez-Cuesta et al. Standards for practical allergen-specific immunotherapy. Allergy 2006; 61 Suppl 82: 1 -20.

Sublingual Immunotherapy Safety Summary Specific instructions should be provided regarding the management of AE, unplanned interruptions in treatment and situations when SLIT should be withheld. SLIT should only be prescribed by allergy -trained physicians Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009; 64 Suppl 91: 1 -59.

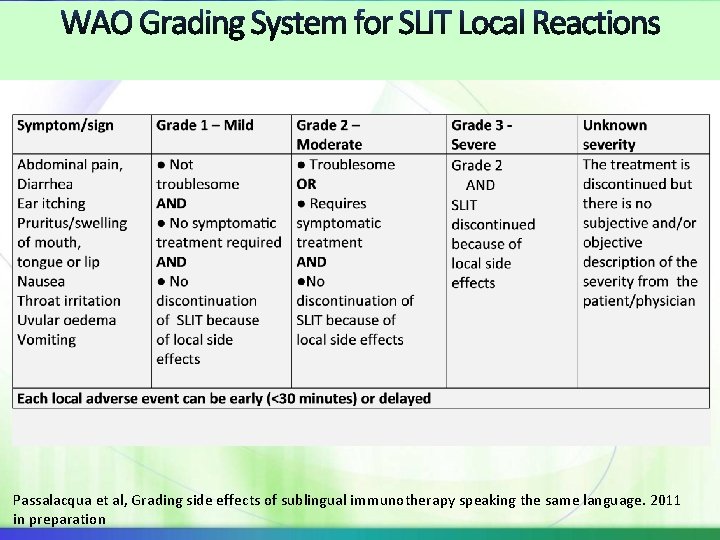
SLIT appears to be better tolerated than SCIT WAO SLIT Position Paper Paris 2009 Majority of SLIT AE’s are oromucosal & occur during the beginning of treatment A few cases of SLIT-related anaphylaxis have been reported but no fatalities Risk factors for the occurrence of SLIT SAE have not yet been established Proposed WAO system of for reporting SLIT adverse local reactions WAO grading system for SCIT systemic reactions to be used for SLIT SR with exception of GI and some upper respiratory symptoms

Sublingual Immunotherapy Safety: Unmet Needs Answered/Partially Answered The safety of SLIT in patients who have had SR with SCIT The safety of SLIT with multiple allergens Interruptions in treatment: how long between doses is it safe to administer usual dose? Is it safe to administer SLIT with no induction with all formulations? Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009; 64 Suppl 91: 1 -59.

Sublingual Immunotherapy Safety: Still Unmet Needs The safety of SLIT in moderate to severe asthmatics. ? Are oropharyngeal infections or lesions risk factors for SLIT SRs? Under which clinical situations should an SLIT dose be withheld? The safety of SLIT in pregnant or breast or feeding women. The safety of SLIT in patients on beta-blockers. Are there any risk factors that identify which patients may experience a SR with SLIT? Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009; 64 Suppl 91: 1 -59.

Novel Immunotherapy Formulations And Routes: Looking Toward The Future

TOLAMBA: ISS of DNA Cp. G motif covalently linked to Amb a 1 TLR 9 agonist: shifts immune response toward TH 1 Protocol: 6 injections-highest dose 30 mcg Amb a 1 PC trial of 738 subjects with AR reported that treatment “was well tolerated in all groups” and no TOLAMBA-related SAEs 2 Pollinex® Quattro/MATA MPL + modified allergen TLR 4 agonist: shown to induce TH 1 cytokines Protocol: 4 injections; highest dose 24 mcg of Php p 1 One-year postmarketing surveillance of 1736 pt, given 8512 injections, SR were reported by 1. 6% of the patients. 14 patients reported severe reactions but there no instances of anaphylactic shock. 2 DBPC study of 1028 pts found MPL (514 pts) was “well tolerated” 1. . Bernstein et al. , JACI 2007; 119: S 78 -S 9 2. Drachenberg et al. , Int Rev Allergol Clin Immunol 2002; 8: 219 -23 3. Du. Buske Allergy Asthma Proc. 2011; 32(3): 239 -47.

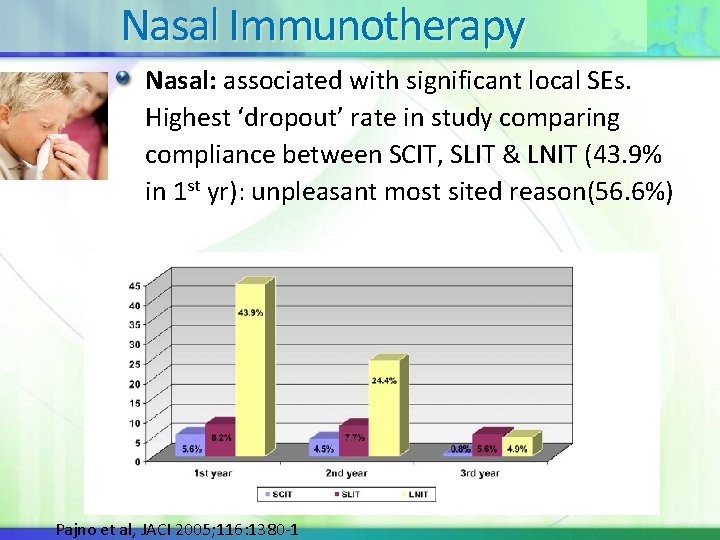
Nasal Immunotherapy Nasal: associated with significant local SEs. Highest ‘dropout’ rate in study comparing compliance between SCIT, SLIT & LNIT (43. 9% in 1 st yr): unpleasant most sited reason(56. 6%) Pajno et al, JACI 2005; 116: 1380 -1

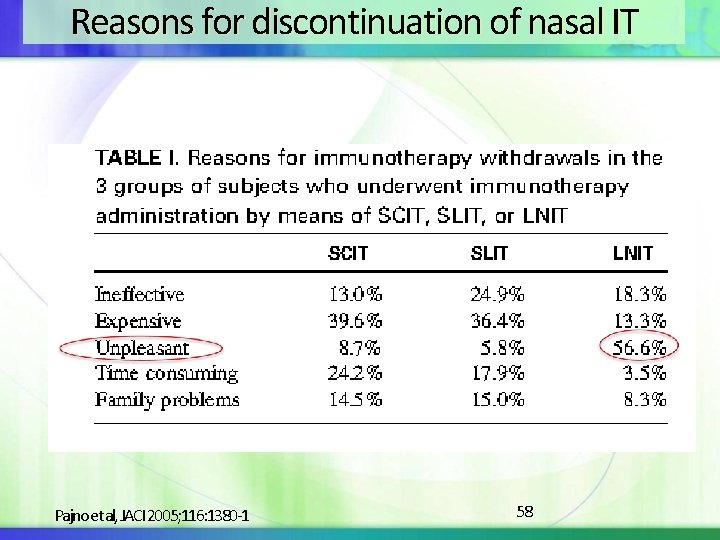
Reasons for discontinuation of nasal IT Pajno et al, JACI 2005; 116: 1380 -1 58

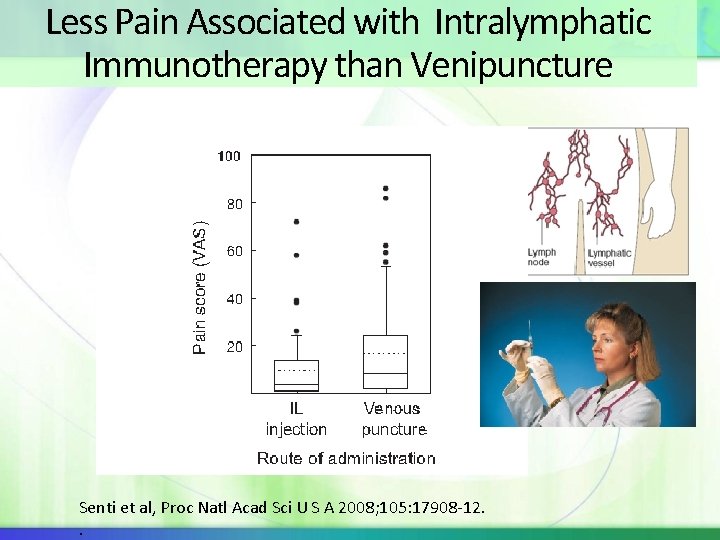
Intralymphatic Immunotherapy Intralymphatic (ILIT): non-controlled study was conducted with 165 grass-pollen allergic subjects comparing 3 injections of grass allergen extract into the inguinal lymph nodes at 4 week intervals to 3 years SCIT 3 Results: The total extract dose was more than 1000 -fold less with ILIT. Systemic reactions were less frequent, but nasal tolerance to allergen increased more rapidly with ILIT. After three years, there were no clinical differences in outcomes between the two treatments. Senti et al, Proc Natl Acad Sci U S A 2008; 105: 17908 -12. .

Less Pain Associated with Intralymphatic Immunotherapy than Venipuncture Senti et al, Proc Natl Acad Sci U S A 2008; 105: 17908 -12. .

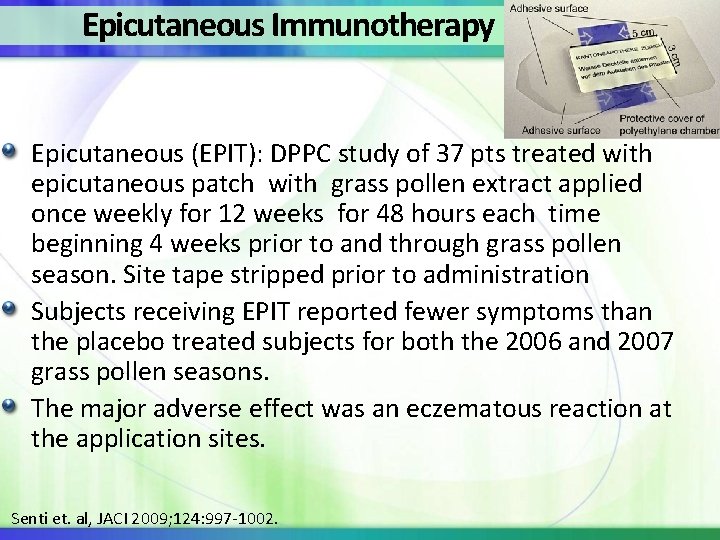
Epicutaneous Immunotherapy Epicutaneous (EPIT): DPPC study of 37 pts treated with epicutaneous patch with grass pollen extract applied once weekly for 12 weeks for 48 hours each time beginning 4 weeks prior to and through grass pollen season. Site tape stripped prior to administration Subjects receiving EPIT reported fewer symptoms than the placebo treated subjects for both the 2006 and 2007 grass pollen seasons. The major adverse effect was an eczematous reaction at the application sites. Senti et. al, JACI 2009; 124: 997 -1002.

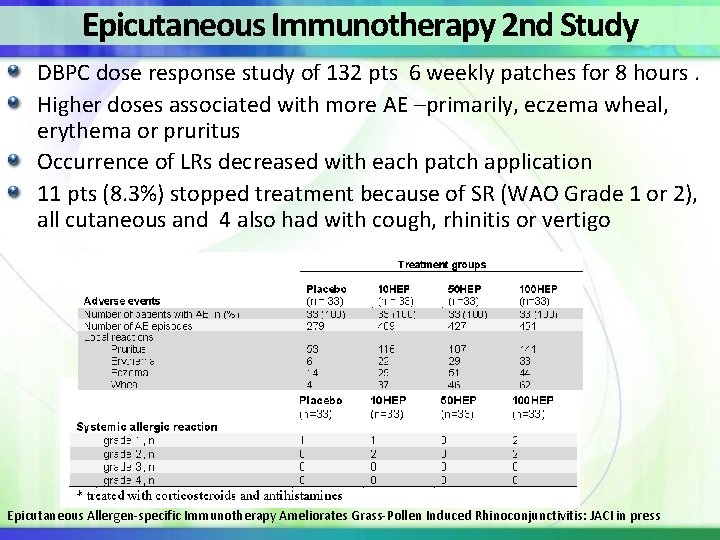
Epicutaneous Immunotherapy 2 nd Study DBPC dose response study of 132 pts 6 weekly patches for 8 hours. Higher doses associated with more AE –primarily, eczema wheal, erythema or pruritus Occurrence of LRs decreased with each patch application 11 pts (8. 3%) stopped treatment because of SR (WAO Grade 1 or 2), all cutaneous and 4 also had with cough, rhinitis or vertigo Epicutaneous Allergen-specific Immunotherapy Ameliorates Grass-Pollen Induced Rhinoconjunctivitis: JACI in press

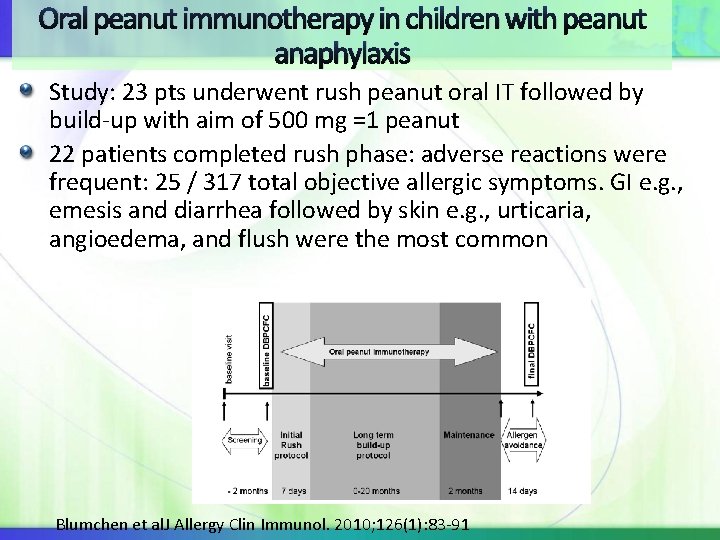
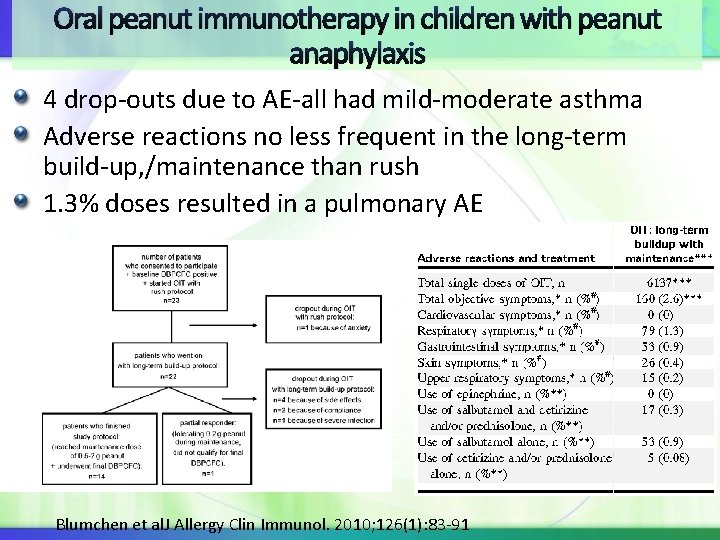
Oral peanut immunotherapy in children with peanut anaphylaxis Study: 23 pts underwent rush peanut oral IT followed by build-up with aim of 500 mg =1 peanut 22 patients completed rush phase: adverse reactions were frequent: 25 / 317 total objective allergic symptoms. GI e. g. , emesis and diarrhea followed by skin e. g. , urticaria, angioedema, and flush were the most common Blumchen et al. J Allergy Clin Immunol. 2010; 126(1): 83 -91

Oral peanut immunotherapy in children with peanut anaphylaxis 4 drop-outs due to AE-all had mild-moderate asthma Adverse reactions no less frequent in the long-term build-up, /maintenance than rush 1. 3% doses resulted in a pulmonary AE Blumchen et al. J Allergy Clin Immunol. 2010; 126(1): 83 -91

ty Objective: To investigate the association of SCIT with the incidence of autoimmune disease ischemic heart disease (IHD) and all-cause mortality. Methods: All Danish citizens , age 18 by 1997 , without other known diseases followed through central registries on medications and hospital admissions. Study period: 1997 -2006. Compared persons receiving SCIT and persons receiving conventional allergy treatment (CAT; nasal steroids or oral antihistamines) with regard to mortality and development of autoimmune diseases, acute myocardial infarction (AMI), and IHD. Linneberg A, et al, Association of subcutaneous allergen-specific immunotherapy with incidence of autoimmune disease, ischemic heart disease, and mortality. J Allergy Clin Immunol. 2011 in press

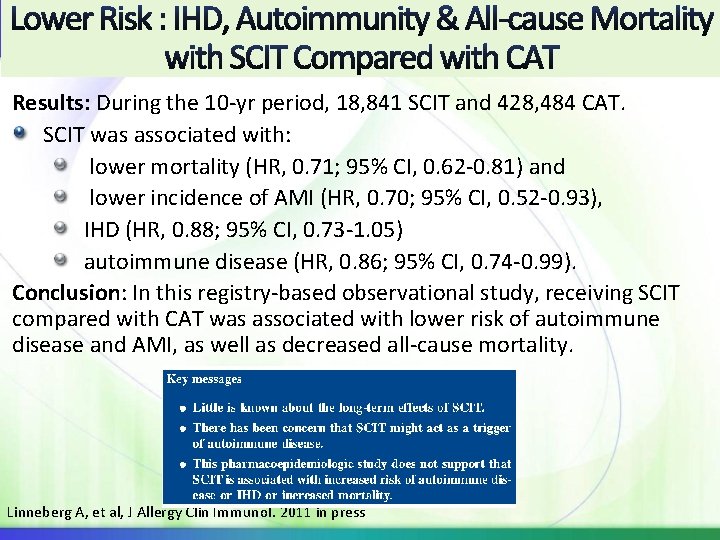
Results: During the 10 -yr period, 18, 841 SCIT and 428, 484 CAT. SCIT was associated with: lower mortality (HR, 0. 71; 95% CI, 0. 62 -0. 81) and lower incidence of AMI (HR, 0. 70; 95% CI, 0. 52 -0. 93), IHD (HR, 0. 88; 95% CI, 0. 73 -1. 05) autoimmune disease (HR, 0. 86; 95% CI, 0. 74 -0. 99). Conclusion: In this registry-based observational study, receiving SCIT compared with CAT was associated with lower risk of autoimmune disease and AMI, as well as decreased all-cause mortality. Linneberg A, et al, J Allergy Clin Immunol. 2011 in press

Immunotherapy Safety Take Home Points SCIT safe but requires medical supervision SLIT appears to have safer profile, home administration standard of care with appropriate patient education Adjuvants offer ultra-short course with some systemic AE but administered Nasal-significant local AEs Epicutaneous skin AE common and some SR reported Intralymphatic small study number but signifcant safety signal Food oral IT: frequent AEs WAO Grading System for Systemic Reactions and SLIT Local Reactions. . please use!!!!
 Immunotherapy for pots
Immunotherapy for pots How to draw a motor bike
How to draw a motor bike Ukons immunotherapy guidelines
Ukons immunotherapy guidelines World allergy organ j
World allergy organ j Avoid the offending allergen that
Avoid the offending allergen that Control ctrl allergen
Control ctrl allergen Joneja allergen chart
Joneja allergen chart Joneja allergen chart
Joneja allergen chart Idph allergen awareness
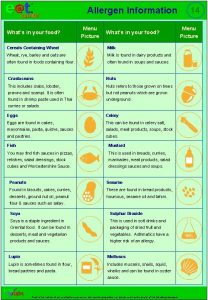
Idph allergen awareness Allergen checklist for food suppliers and manufacturers
Allergen checklist for food suppliers and manufacturers Menu with allergen information
Menu with allergen information Plain english allergen labelling
Plain english allergen labelling Fsa allergy chef cards
Fsa allergy chef cards Specific weight glycerin
Specific weight glycerin Specific gravity pharmacy
Specific gravity pharmacy World kidney day 2011
World kidney day 2011 Dr fox allergy
Dr fox allergy Shellfish allergy and chloraprep
Shellfish allergy and chloraprep Latex allergy rash pictures
Latex allergy rash pictures Latex allergy
Latex allergy Allergy wise
Allergy wise Allergy therapy makes bees go away
Allergy therapy makes bees go away Latex fruit syndrome
Latex fruit syndrome Macaulay allergy
Macaulay allergy Loratadine pregnancy category
Loratadine pregnancy category Ipratropium (atrovent) 0.03 nasal spray
Ipratropium (atrovent) 0.03 nasal spray Allergy shiners
Allergy shiners Allergy
Allergy Anegdotal
Anegdotal Allergy
Allergy Icd 10 code for allergic reaction to peanuts
Icd 10 code for allergic reaction to peanuts Allergy rates
Allergy rates Rhinitis allergy
Rhinitis allergy Allergy board review course
Allergy board review course Rhinitis allergy
Rhinitis allergy Allergy
Allergy Acr contrast reaction card
Acr contrast reaction card Anaphylactoid vs anaphylaxis
Anaphylactoid vs anaphylaxis Penicillin allergy cme
Penicillin allergy cme Keva wellness products
Keva wellness products Food allergy testing auckland
Food allergy testing auckland Capers allergy
Capers allergy American academy of allergy asthma and immunology 2018
American academy of allergy asthma and immunology 2018 Allergy asthma immunol res impact factor
Allergy asthma immunol res impact factor Process organization in computer organization
Process organization in computer organization Block essay
Block essay Hse advisor adalah
Hse advisor adalah Ecdis safety settings
Ecdis safety settings Qbs safety care
Qbs safety care Process safety vs personal safety
Process safety vs personal safety Ind safety report
Ind safety report Basic safety (construction site safety orientation)
Basic safety (construction site safety orientation) Basic safety construction site safety orientation
Basic safety construction site safety orientation Function of world trade organization
Function of world trade organization Wno world nature organization
Wno world nature organization Largest sports organization in the world
Largest sports organization in the world Functions of the world trade organization
Functions of the world trade organization Health promotion and maintenance
Health promotion and maintenance World animal health information system
World animal health information system World health organization
World health organization World health organization
World health organization World health organization
World health organization World trade organization
World trade organization World trade organization
World trade organization Old world monkey vs new world monkey
Old world monkey vs new world monkey Coffee new world or old world
Coffee new world or old world Real world vs digital world
Real world vs digital world The world of the forms
The world of the forms