1 Allergen Control Strategies A How To Guide




































- Slides: 46

1

Allergen Control Strategies - A “How To” Guide Hormel Foods Corporation Brent Brehmer Corporate Manager of Regulatory Compliance & HACCP

3

4

5

6

7

8

F/Y 2011 – Consumer Response handled > 140, 000 contacts – Top questions: l l 9 1. 2. 3. 4. Where to purchase products Shelf-Life questions Coupon requests Allergen & Gluten questions

Actual Customer Comments/Questions l l l 10 “Is Chi’s Salsa gluten free? ” “Is there dairy is potassium lactate? ” “I just wanted to thank you for going the extra mile for people w/ Celiac Disease. I noticed you even put “gluten-free” on some of your labels. My 5 yr old has the disease & she loves your pepperoni. I can’t tell you how hard it is to stick to her diet & I am so thankful for companies who have regular food that is gluten-free so our whole family can eat the same foods. I can tell you that as long as you’re taking the precautions to keep them gluten-free, I will be a devoted Hormel customer…”

Allergen Program – What Does One Look Like? l Hormel’s – – – 11 110+ Food Safety Assessments 30 GFSI Audits Annually Dozens of Customer Audits

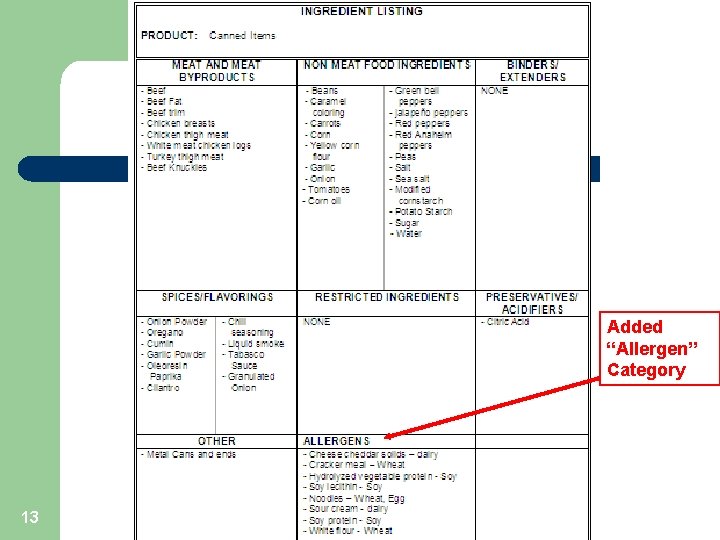
Allergens & HACCP l Allergens are a “Chemical” Hazard – l l Control Point or CCP? Prerequisite Program – l 12 Hazard = presence of an Unlabeled Allergen Hazard Not Likely to Occur Ingredient Usage Page - Added “Allergen” section.

Added “Allergen” Category 13

Possible CCPs l l l 14 Product Changeover Labeling Control of Rework

Starts at “Product Formulation” l 15 R&D

Product Development Strategies l l Determine allergenicity of ingredients; Avoid allergenic ingredients which have no functional effect on the finished product – l 16 Find substitutions for allergenic ingredients! Identify Allergens on Formulas sent to plant.

Hormel’s Allergen Program l “Big 10” – l List on file: – – 17 Big 8 + Sesame Seeds + Molluscan Shellfish At Corporate In Plant New Ingredients HACCP Plan.

Incoming Ingredients – Labeling Requirements of Allergens l “Allergen” Sticker on each Pallet – – l l Supplier Applies If not on, – – l 18 5” x 8” Brightly Colored Apply at Receiving & Notify Supplier Easily readable on walk-throughs.

Storage of Ingredients l l 19 Ideal - Dedicated Room or at Least Rows Realistic – No Allergens stored above nonallergenic product.

Open to contamination or able to cross -contact other products. 20

“Cross Contact” NOT “Cross Contamination” 21

Milk Item Improper Storage 22

Storage of Ingredients - Bulk l l l Containers Labeled Dedicated (Color-coded) Scoops Procedures - Employees in Spice Rooms working w/ Allergens must: – – Wear Disposable outerwear (sleeves, aprons & gloves); Change outerwear & wipe down helmet at: l l 23 Each break When changing to other non-allergen product.

Product Scheduling l l l Huge More important than “color” issues Order must be: 1. 2. 3. 4. 5. 24 Non-Allergen first Allergen #1 & Allergen #2 Etc. Allergen Cleanup

Changeovers – Validation of Cleanups l l 3 Separate Runs on our Production Lines Choose Product Based On: – – l l Run Allergenic Product & Pull Control Sample Conduct the planned “Allergen Cleanup” – l Record Procedures Used (Full 4 -Step Cleanup, Water Rinse, Sugar Rinse, etc. ) Run Non-Allergenic Product & Pull Samples: – 25 Highest Percentage in Product Toughest to Clean (i. e. Burn-On) First Unit, after 5 minutes, after 10 minutes

Changeovers – Validation of Cleanups – (continued) l Test Control Product & Changeover Product – – – University of Nebraska (Lincoln) – FARRP or On-Site using approved test kits (i. e. Neogen® - pending Corp. approval) Must choose Level l HOLD ALL PRODUCT PENDING RESULTS!!!!! Disposition – – 26 l i. e. 10 ppm, 5 ppm, 2. 5 ppm “Negative” – Release Product “Positive” – Contact Corp. QC for disposition Record Results on “Allergen Validation Testing Record”

Neogen. com 27

28

Rework Requirements l l l Label Store Properly Exact Into Exact – l l 29 NOT Like Into Like Record Code Dates Implement Rework Cycle Breaks.

Procedures for “Suspect” Product l l 30 Place all suspect product on HOLD; Call Brent

Label Verification Program 31

Incoming Labels l l Pull 1 from each incoming lot - Place on Hold until: Compare the Big 9 (Hormel Term) l l l l l 32 Ingredient Listing Est. # Nutrition Facts Panel Net Wgt Address Line Safe Handling Instructions (Raw items) Product of Identity Manufacturer ID (37600 on Bar Code Label) SI # + Version – S 0044208 -24 (15 oz. CWB – 24 th version)

Incoming Multiple-Component Ingredients l l 33 Place on Hold Compare Ingredient Listing to Approved File.

Formulation Changes l Line Trial System – – – l If Approved – 34 New Product Reformulation Unique Number Assigned to Each Hold & Release Program System in place to convert

Formulation Verification (Batching) l l l Record Item / SI numbers Record Lot Numbers In-Process Labels – l l Verify amounts used Restricted Ingredients – 35 Must have Ingredients listed Stored under Lock & Key

Packaging Line Procedures l l 36 Save 1 label per Roll (or case); Verify Big 9; Corrugate (save 1 box / pallet); 3 Individuals (min. of 2 mgt. ) Verify

Ingredient / Product Spills l 37 Cleaned in a manner as to prevent any crosscontact.

Packaging “Leakers” l l 38 Dispose of Properly clean line.

Allergen Training l l l 39 Annual Training of all employees (30 minutes) Record

Obsolete Labels l l 40 Isolated Destroyed

Hold Requirements l l Normal Hold for “Quality” Issues Category 1 Hold – Food Safety Issues – – – Pathogen Testing Allergen Testing “Daily Inventory” l l 41 Electronic (minimum) Physical (when possible)

Internal Audits Conducted by Corporate – “Quality Cup” l If score > 90 in each – – – l HACCP Sanitation Quality No Product Recalls or Market Withdrawals l AND > 80 in each “Quality” Category: – – – – 42 Foreign Material Food Defense Wgt Control Label Verification Thermal Processing Minimizing the Window Ingredient Receiving

Additional Site Specific Actions 43

High-Speed Packaging Lines l Red & Yellow Hormel® Chili – – l l 500+ cans / min. Bar-code reading systems – 44 CNB – Soy & Wheat CWB – Soy only (See next slides)

SI # 45

Questions? 46