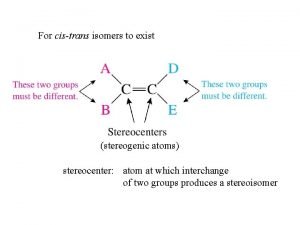
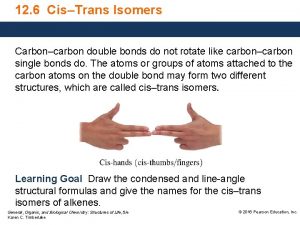
For cistrans isomers to exist stereogenic atoms stereocenter





- Slides: 19

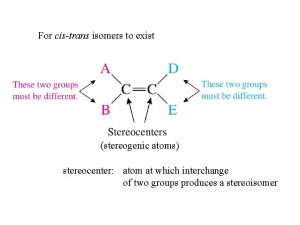
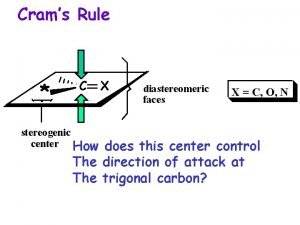
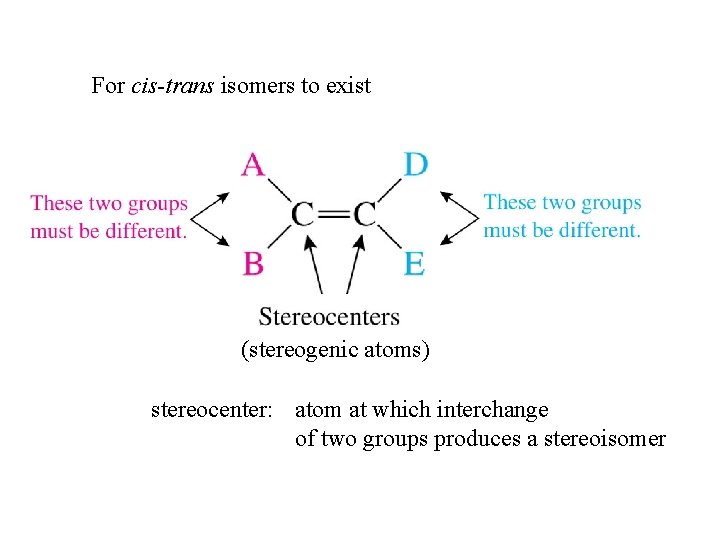
For cis-trans isomers to exist (stereogenic atoms) stereocenter: atom at which interchange of two groups produces a stereoisomer

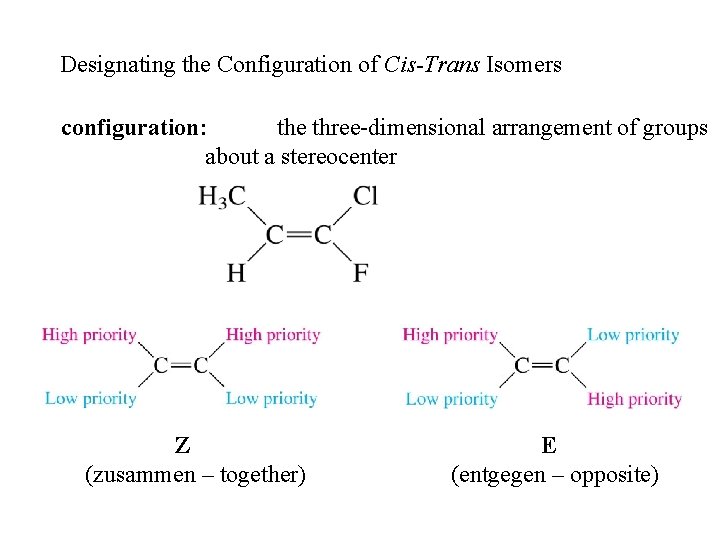
Designating the Configuration of Cis-Trans Isomers configuration: the three-dimensional arrangement of groups about a stereocenter Z (zusammen – together) E (entgegen – opposite)


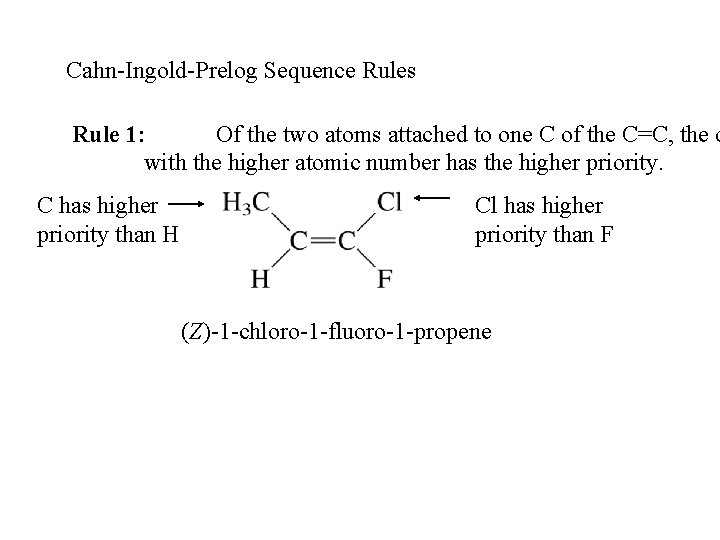
Cahn-Ingold-Prelog Sequence Rules Rule 1: Of the two atoms attached to one C of the C=C, the o with the higher atomic number has the higher priority. C has higher priority than H Cl has higher priority than F (Z)-1 -chloro-1 -fluoro-1 -propene

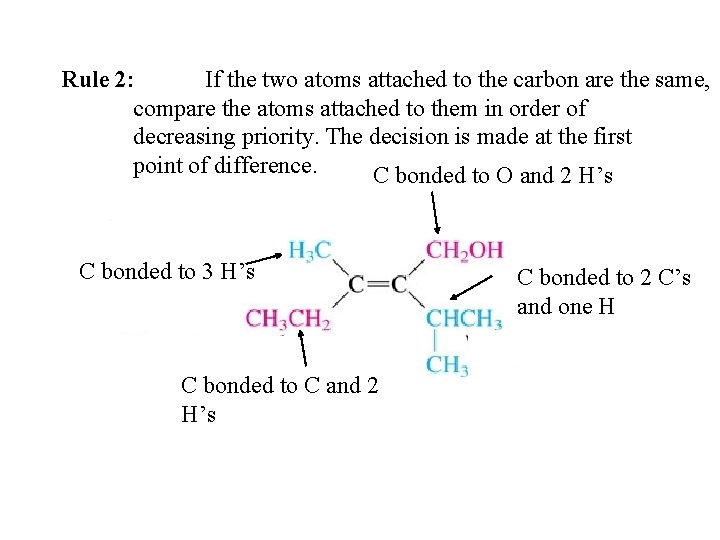
Rule 2: If the two atoms attached to the carbon are the same, compare the atoms attached to them in order of decreasing priority. The decision is made at the first point of difference. C bonded to O and 2 H’s C bonded to 3 H’s C bonded to C and 2 H’s C bonded to 2 C’s and one H

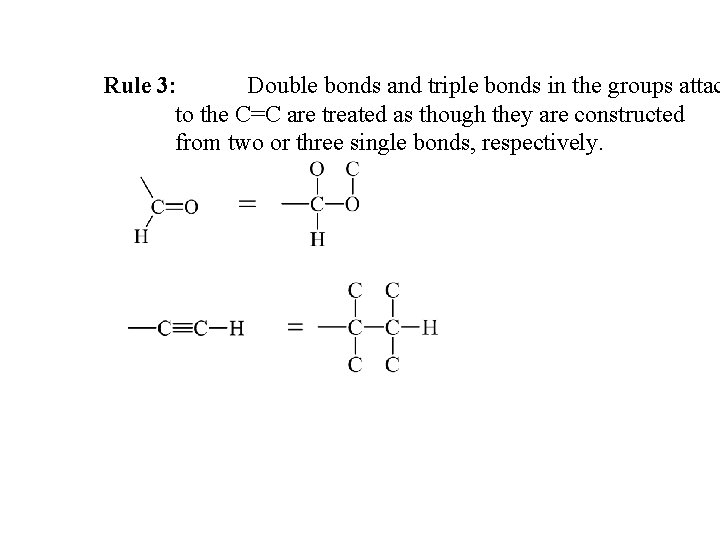
Rule 3: Double bonds and triple bonds in the groups attac to the C=C are treated as though they are constructed from two or three single bonds, respectively.

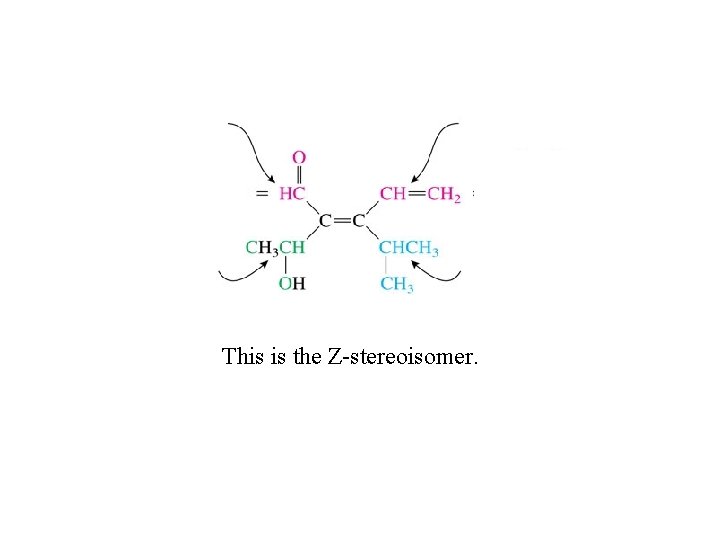
This is the Z-stereoisomer.

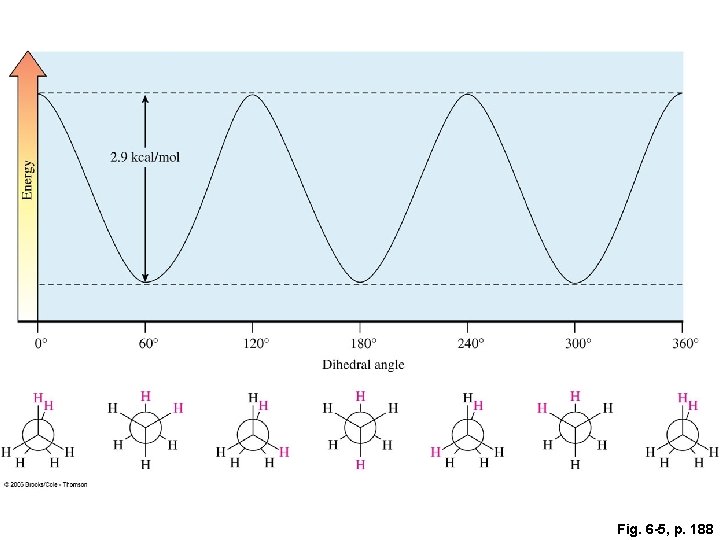
Fig. 6 -5, p. 188


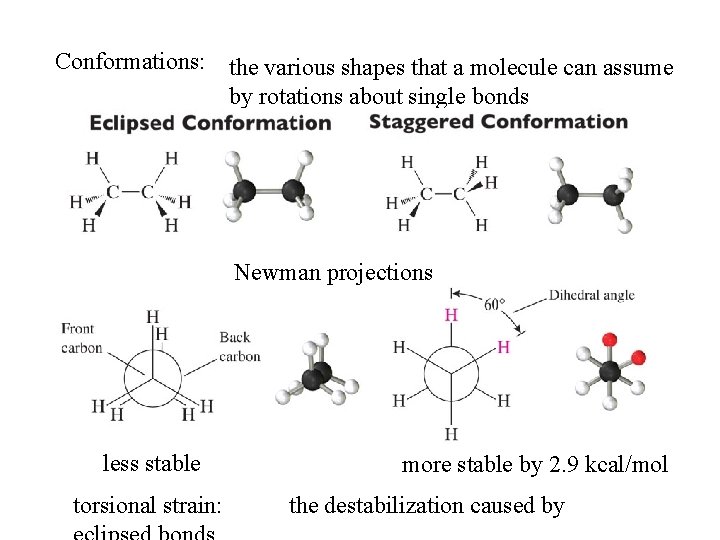
Conformations: the various shapes that a molecule can assume by rotations about single bonds Newman projections less stable torsional strain: more stable by 2. 9 kcal/mol the destabilization caused by

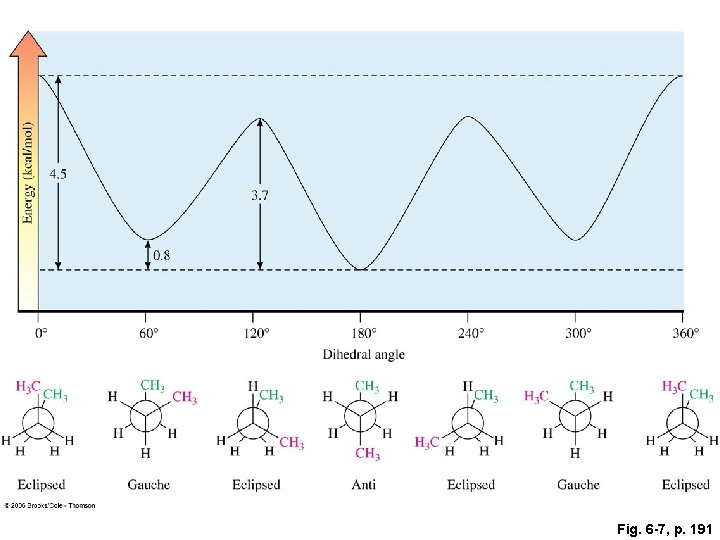
Fig. 6 -7, p. 191

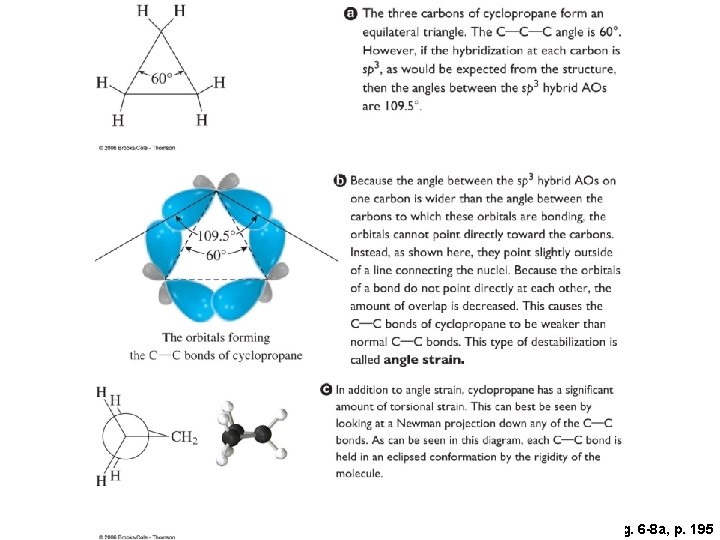
Fig. 6 -8 a, p. 195

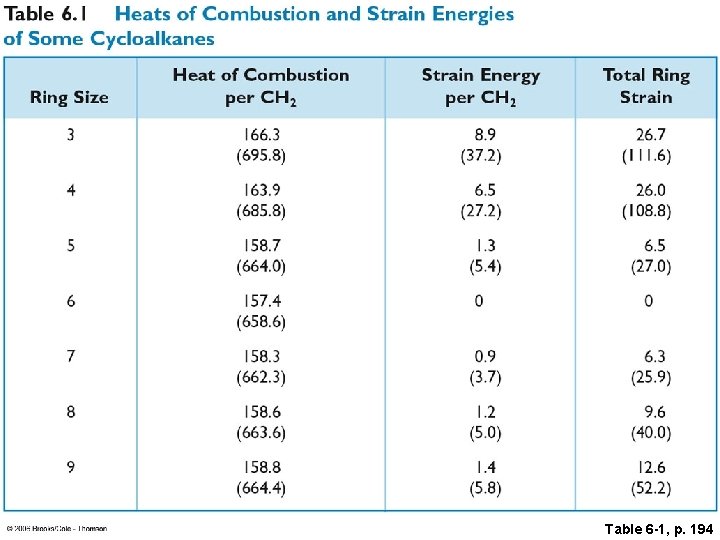
Table 6 -1, p. 194

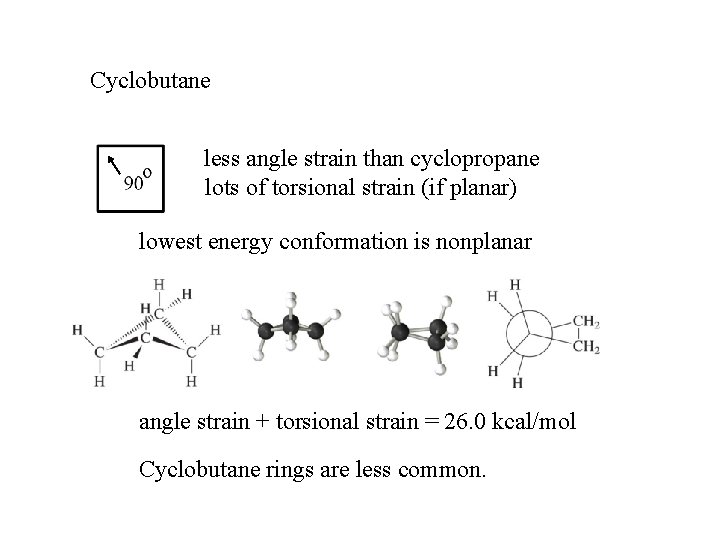
Cyclobutane less angle strain than cyclopropane lots of torsional strain (if planar) lowest energy conformation is nonplanar angle strain + torsional strain = 26. 0 kcal/mol Cyclobutane rings are less common.


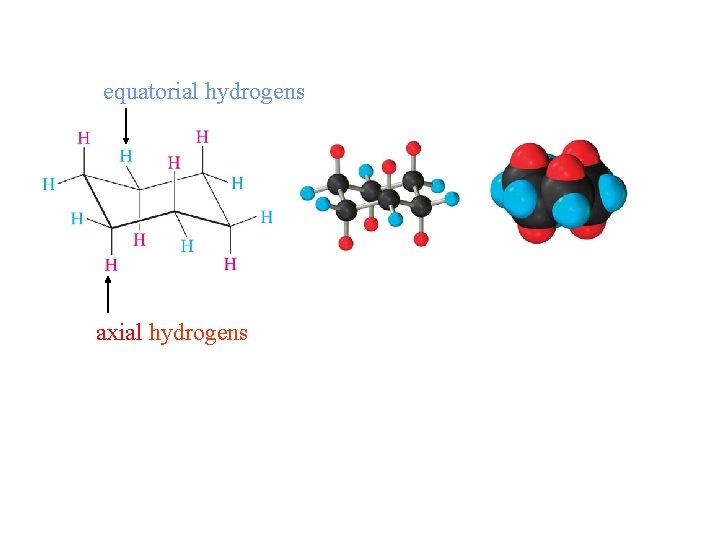
equatorial hydrogens axial hydrogens


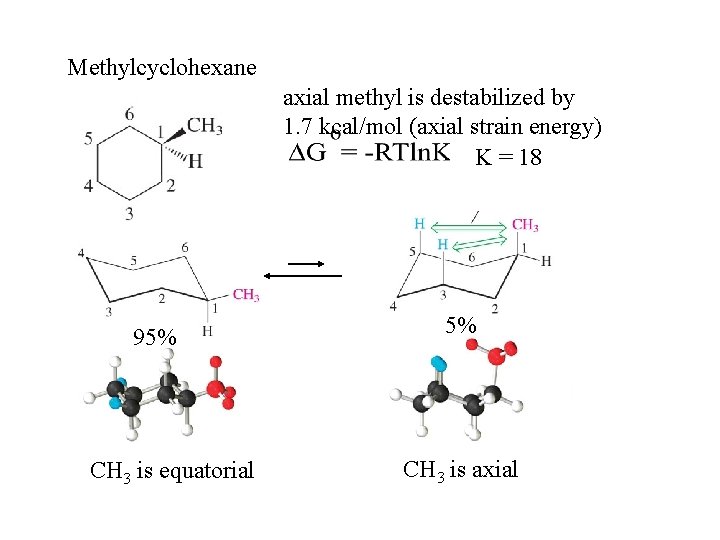
Methylcyclohexane axial methyl is destabilized by 1. 7 kcal/mol (axial strain energy) K = 18 95% CH 3 is equatorial 5% CH 3 is axial

 Stereogenic atoms
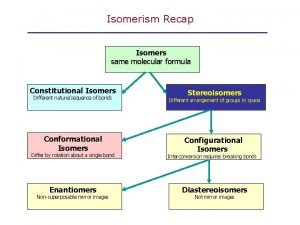
Stereogenic atoms Stereoisomer vs constitutional isomer
Stereoisomer vs constitutional isomer Subtle changes
Subtle changes What is a stereocenter vs chiral center
What is a stereocenter vs chiral center Disulfur decabromide formula
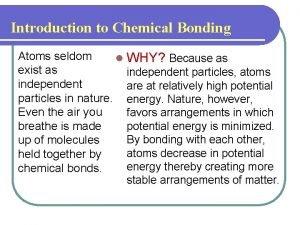
Disulfur decabromide formula Atoms seldom exist as independent particles
Atoms seldom exist as independent particles Atoms seldom exist as independent particles
Atoms seldom exist as independent particles Atoms seldom exist as independent particles
Atoms seldom exist as independent particles At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering A gastrica
A gastrica Egg för emanuel
Egg för emanuel Stickprovsvarians
Stickprovsvarians Rutin för avvikelsehantering
Rutin för avvikelsehantering Presentera för publik crossboss
Presentera för publik crossboss Klassificeringsstruktur för kommunala verksamheter
Klassificeringsstruktur för kommunala verksamheter Myndigheten för delaktighet
Myndigheten för delaktighet Debatt mall
Debatt mall Var 1721 för stormaktssverige
Var 1721 för stormaktssverige Tack för att ni lyssnade
Tack för att ni lyssnade