EVPP 110 Molecules Processes of Life Activities Microscopy







- Slides: 36

EVPP 110 Molecules & Processes of Life Activities: • Microscopy • Water Properties / Alteration by Pollutants • Diffusion & Osmosis Week of September 17 th, 2018 Version 1. 2. Last updated: 9/23/2018 9: 32: 06 AM


Molecules & Process of Life Activity 1 – Microscopy

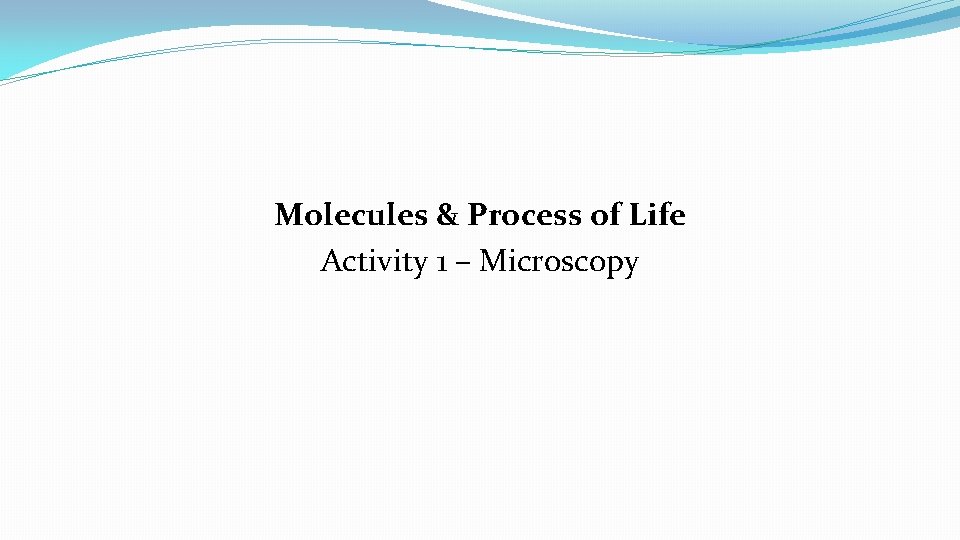
Eyepiece Compound microscope Neck Nosepiece Objectives Stage controls Macrofocus Stage Diaphragm Light source Base Microfocus Light adjustment

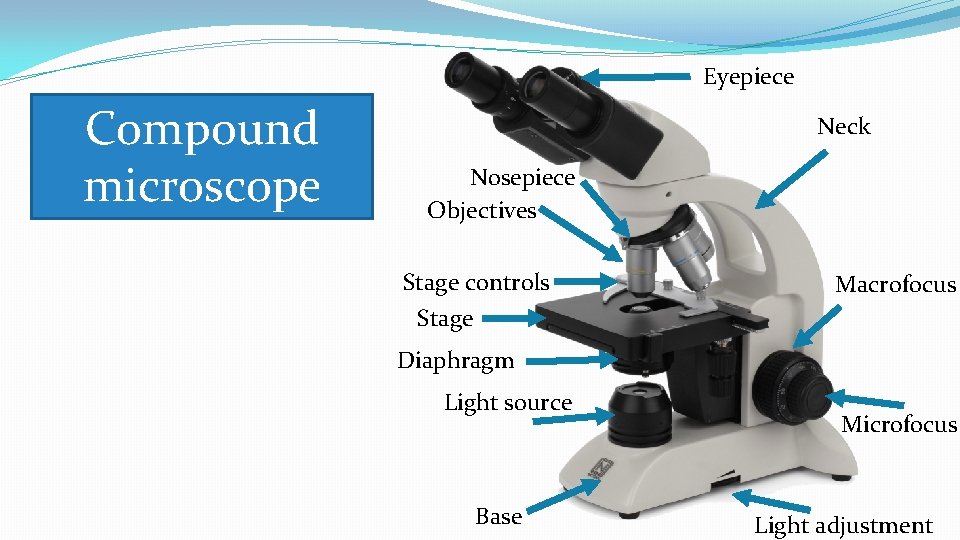
�Example of image seen with compound microscope.

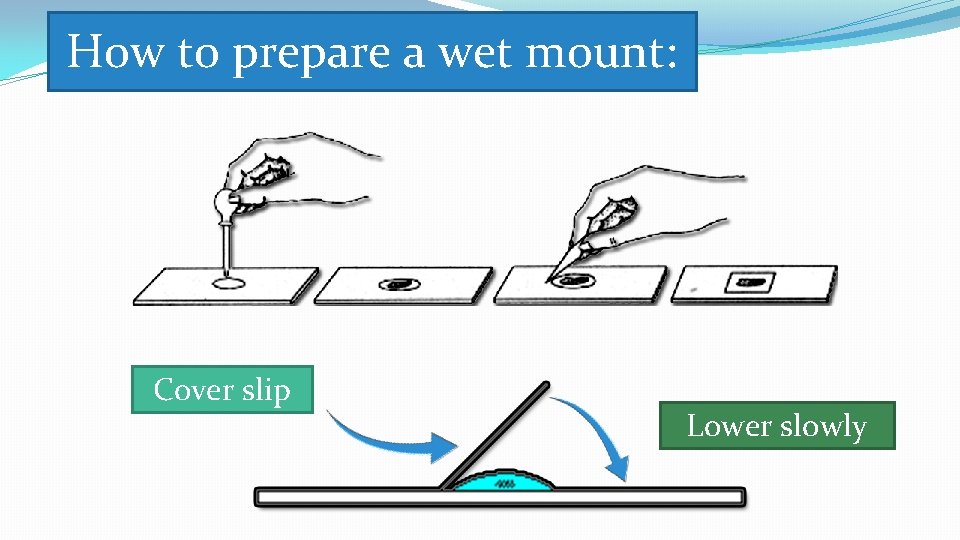
How to prepare a wet mount: Cover slip Lower slowly

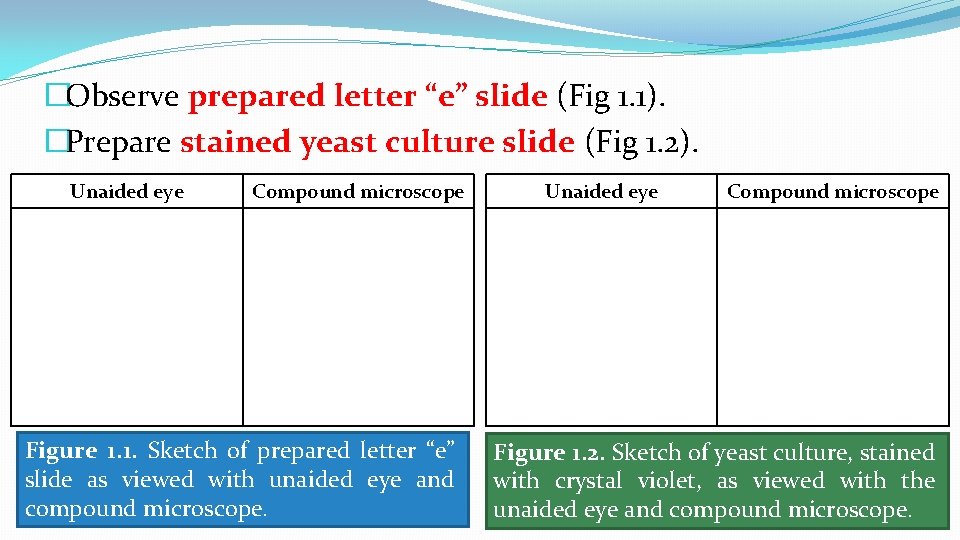
�Observe prepared letter “e” slide (Fig 1. 1). �Prepare stained yeast culture slide (Fig 1. 2). Unaided eye Compound microscope Figure 1. 1. Sketch of prepared letter “e” slide as viewed with unaided eye and compound microscope. Unaided eye Compound microscope Figure 1. 2. Sketch of yeast culture, stained with crystal violet, as viewed with the unaided eye and compound microscope.

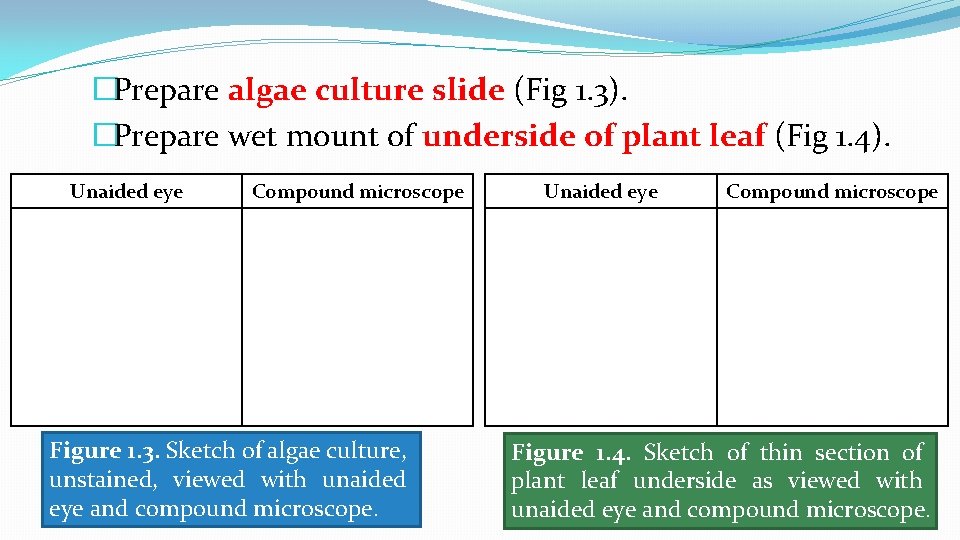
�Prepare algae culture slide (Fig 1. 3). �Prepare wet mount of underside of plant leaf (Fig 1. 4). Unaided eye Compound microscope Figure 1. 3. Sketch of algae culture, unstained, viewed with unaided eye and compound microscope. Unaided eye Compound microscope Figure 1. 4. Sketch of thin section of plant leaf underside as viewed with unaided eye and compound microscope.

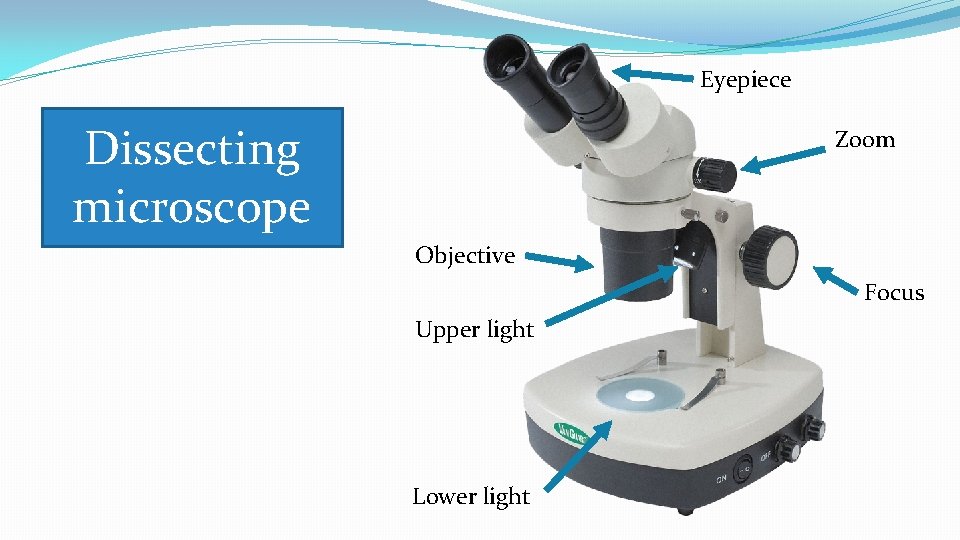
Eyepiece Dissecting microscope Zoom Objective Focus Upper light Lower light

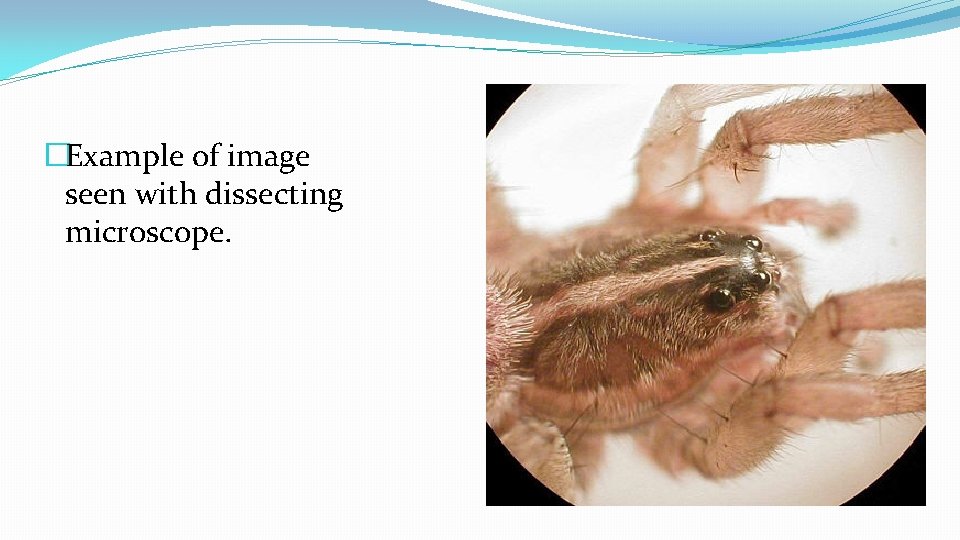
�Example of image seen with dissecting microscope.

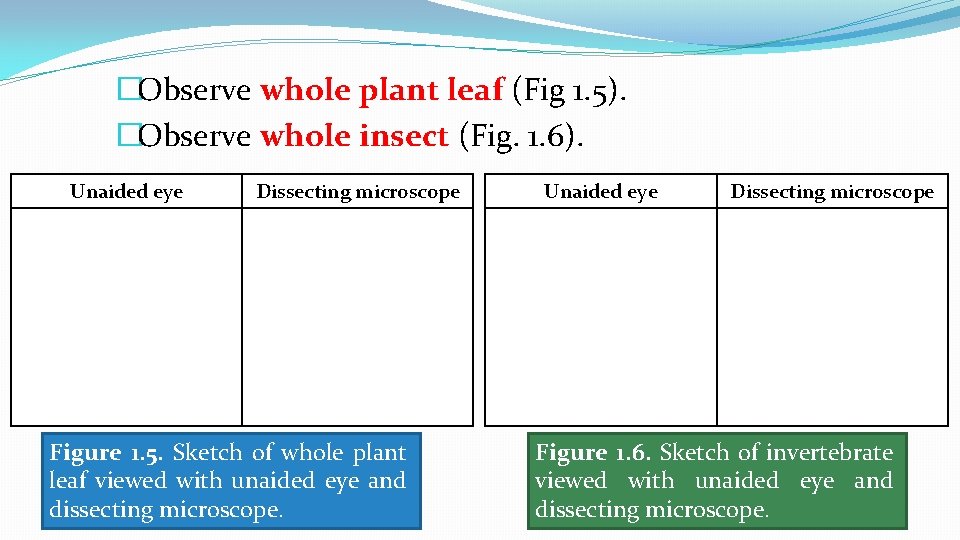
�Observe whole plant leaf (Fig 1. 5). �Observe whole insect (Fig. 1. 6). Unaided eye Dissecting microscope Figure 1. 5. Sketch of whole plant leaf viewed with unaided eye and dissecting microscope. Unaided eye Dissecting microscope Figure 1. 6. Sketch of invertebrate viewed with unaided eye and dissecting microscope.

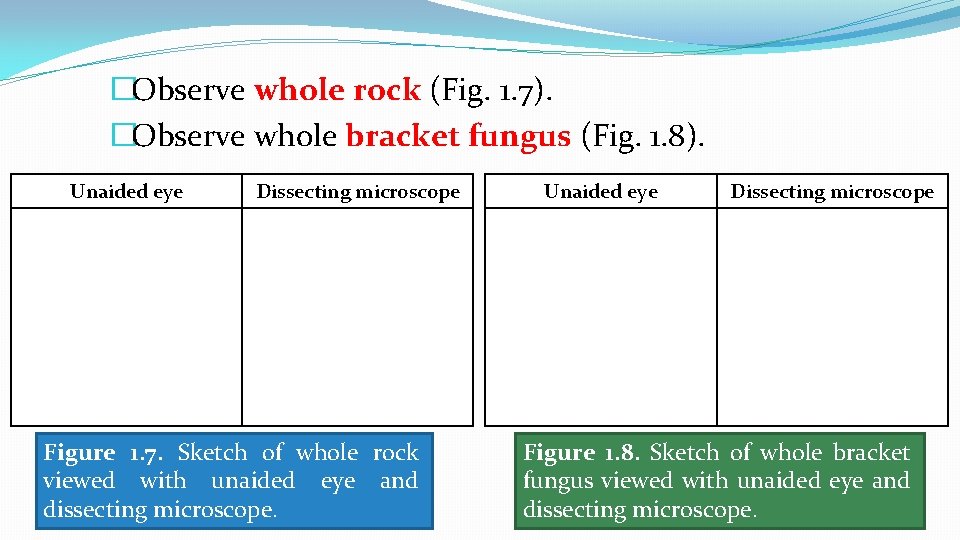
�Observe whole rock (Fig. 1. 7). �Observe whole bracket fungus (Fig. 1. 8). Unaided eye Dissecting microscope Figure 1. 7. Sketch of whole rock viewed with unaided eye and dissecting microscope. Unaided eye Dissecting microscope Figure 1. 8. Sketch of whole bracket fungus viewed with unaided eye and dissecting microscope.

Molecules & Process of Life Activity 2 – Properties of Water and Alteration by Pollutants

Table 2. 1 Part A. # of 1. 25 m. L spoons of powdered sugar added to 140 m. L container before water spills: Explanation: Water properties demonstrated: Unpolluted Water Predicted Actual �Fill 140 m. L plastic container with tap water (unpolluted) until bulging. �Predict number of 1. 25 m. L spoons of powdered sugar that can be added to the water before it spills. �Add sugar one spoon at a time until water spills. �Repeat procedure with simulated polluted water. Polluted Water Predicted Actual

Table 2. 2 # seconds until air balloon pops after contact with flame: Explanation: # seconds until water balloon pops after contact with flame: Explanation: Water properties demonstrated: �Fill one balloon with tap water. �Fill 2 nd balloon from air pump until same size as 1 st balloon. �Predict number seconds it will take for air and water balloons to pop. �Hold air balloon over candle, record number of seconds until it pops. �Repeat procedure with water balloon. Prediction Actual

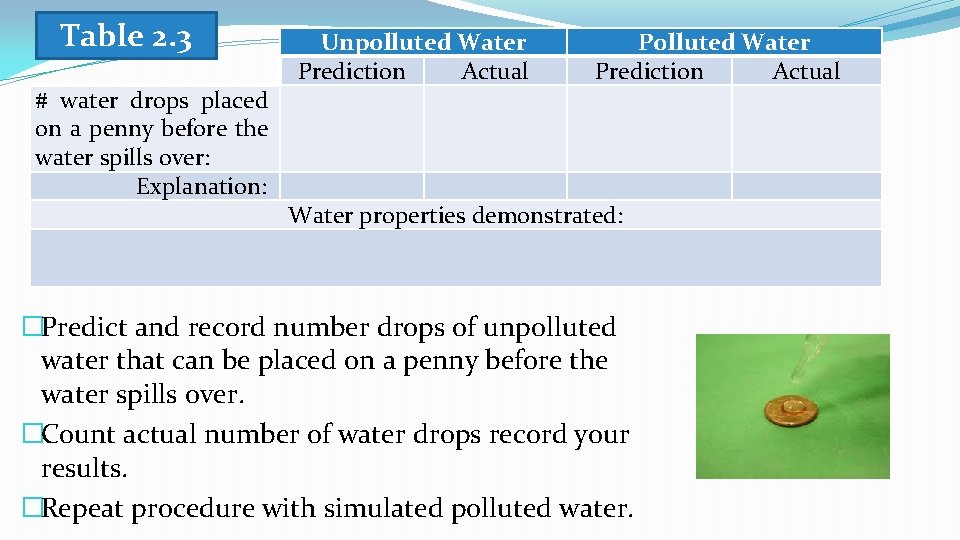
Table 2. 3 # water drops placed on a penny before the water spills over: Explanation: Unpolluted Water Prediction Actual Polluted Water Prediction Actual Water properties demonstrated: �Predict and record number drops of unpolluted water that can be placed on a penny before the water spills over. �Count actual number of water drops record your results. �Repeat procedure with simulated polluted water.

Table 2. 4 Prior to Pollutant Appearance of water surface adjacent to sewing pin: Behavior of the sewing pin on/in water: Explanation of behavior: Water properties demonstrated: After Pollutant �Fill weigh boat with tap water until water is level with (but not bulging above) rim. �Gently place sewing pin on water surface. �Observe and record appearance of water surface adjacent to pin. �Dip tip of a toothpick in liquid soap (pollutant), touch soapy tip to water surface.

Molecules & Process of Life – Activity 4 – Diffusion and Osmosis – Part A

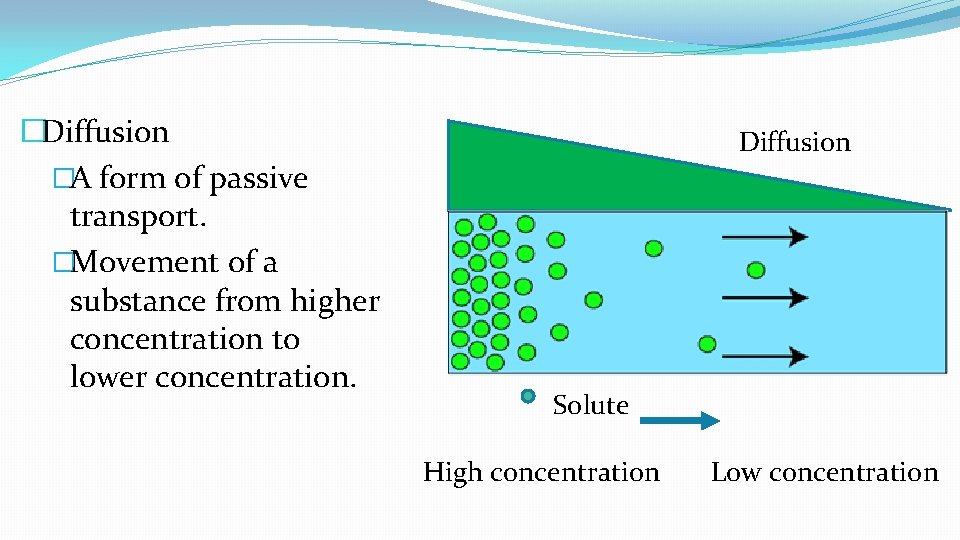
�Diffusion �A form of passive transport. �Movement of a substance from higher concentration to lower concentration. Diffusion Solute High concentration Low concentration

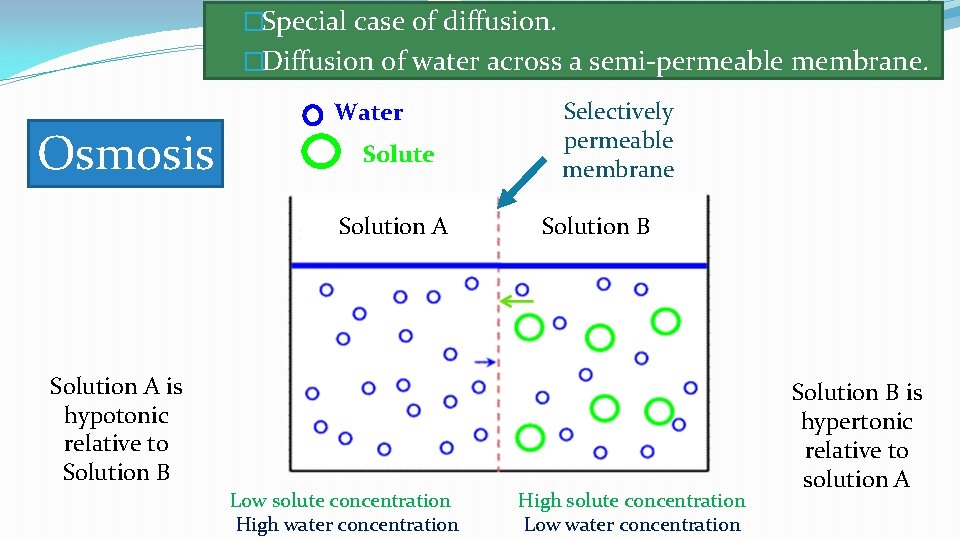
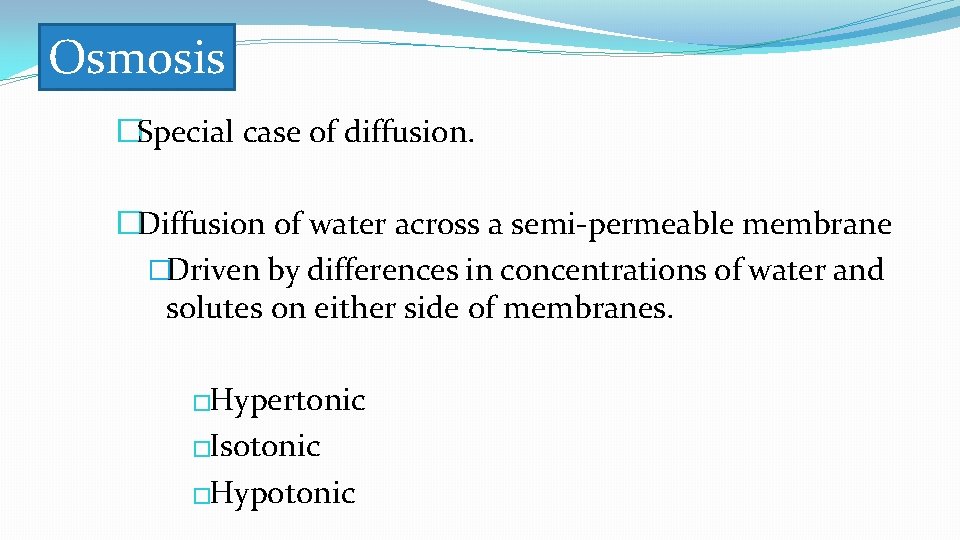
�Special case of diffusion. �Diffusion of water across a semi-permeable membrane. Osmosis Water Solute Solution A Selectively permeable membrane Solution B Solution A is hypotonic relative to Solution B Low solute concentration High water concentration High solute concentration Low water concentration Solution B is hypertonic relative to solution A

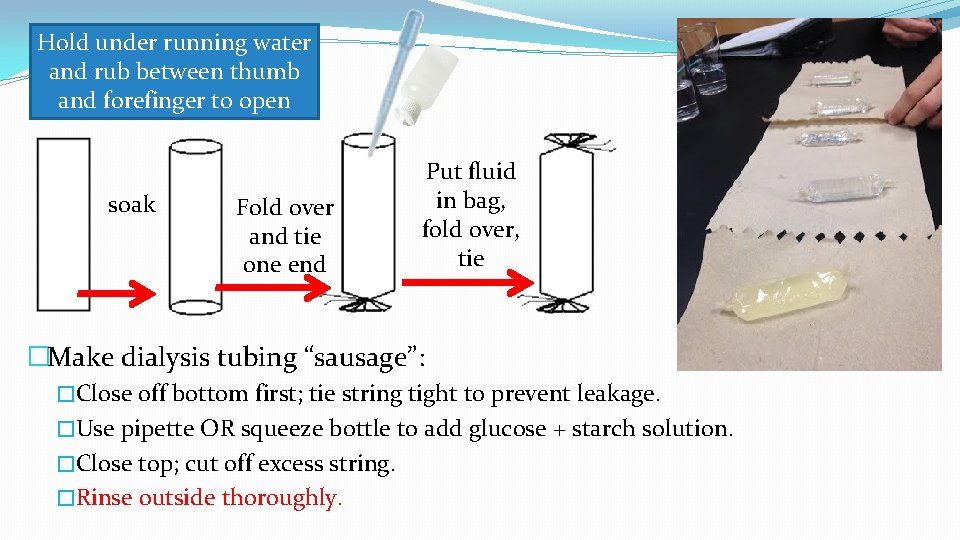
�Dialysis tubing �Comes flat in a roll. �To “open” your piece into a tube, hold it under running water and rub it between thumb and index finger.

�Starch indicator solution �Iodine-based. �In absence of starch = “-” �Amber/yellowish color. �In presence of starch = “+” �Inky/blue-black color.

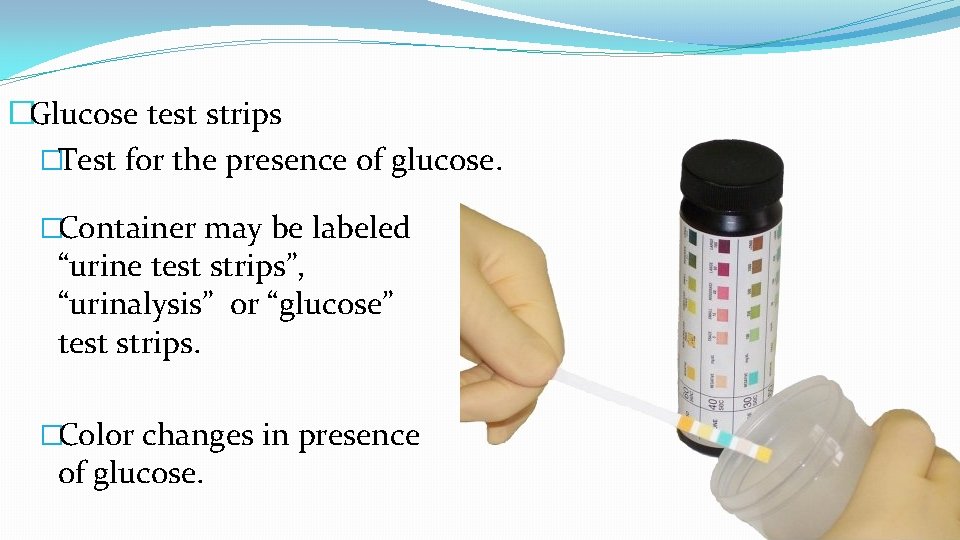
�Glucose test strips �Test for the presence of glucose. �Container may be labeled “urine test strips”, “urinalysis” or “glucose” test strips. �Color changes in presence of glucose.

Hold under running water and rub between thumb and forefinger to open soak Fold over and tie one end Put fluid in bag, fold over, tie �Make dialysis tubing “sausage”: �Close off bottom first; tie string tight to prevent leakage. �Use pipette OR squeeze bottle to add glucose + starch solution. �Close top; cut off excess string. �Rinse outside thoroughly.

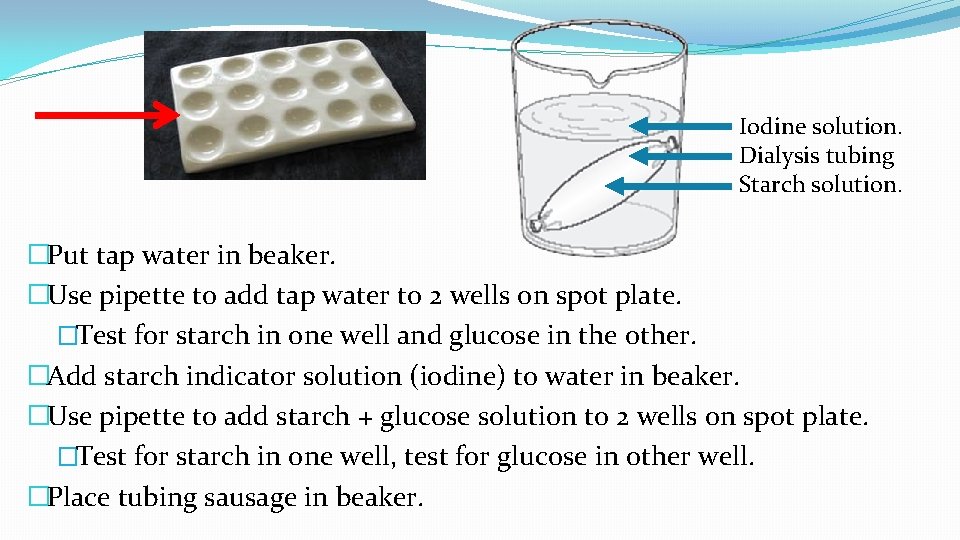
Iodine solution. Dialysis tubing Starch solution. �Put tap water in beaker. �Use pipette to add tap water to 2 wells on spot plate. �Test for starch in one well and glucose in the other. �Add starch indicator solution (iodine) to water in beaker. �Use pipette to add starch + glucose solution to 2 wells on spot plate. �Test for starch in one well, test for glucose in other well. �Place tubing sausage in beaker.

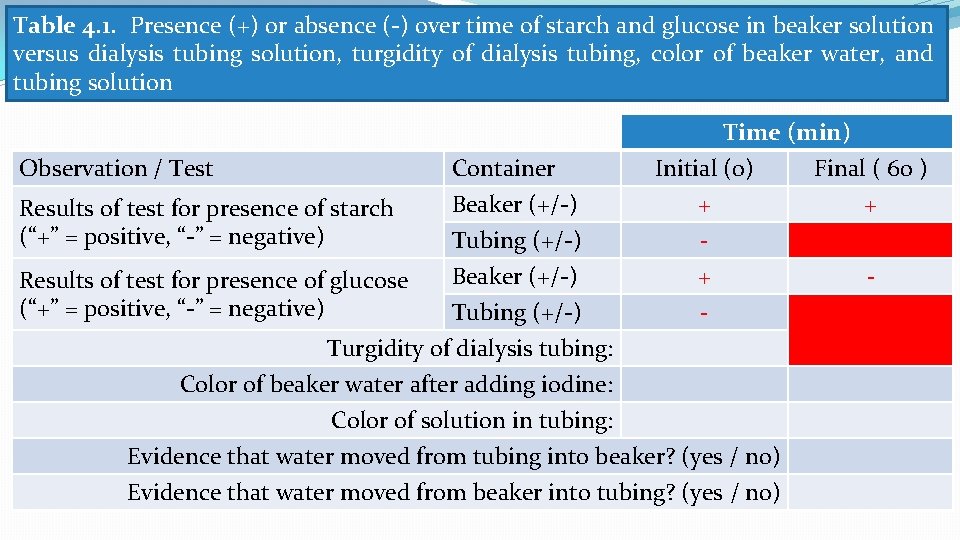
Table 4. 1. Presence (+) or absence (-) over time of starch and glucose in beaker solution versus dialysis tubing solution, turgidity of dialysis tubing, color of beaker water, and tubing solution Observation / Test Results of test for presence of starch (“+” = positive, “-” = negative) Container Beaker (+/-) Tubing (+/-) Time (min) Initial (0) Final ( 60 ) + + - Beaker (+/-) + Tubing (+/-) Turgidity of dialysis tubing: Color of beaker water after adding iodine: Color of solution in tubing: Evidence that water moved from tubing into beaker? (yes / no) Evidence that water moved from beaker into tubing? (yes / no) Results of test for presence of glucose (“+” = positive, “-” = negative) -

Molecules & Process of Life – Activity 4 – Diffusion and Osmosis – Part B

Osmosis �Special case of diffusion. �Diffusion of water across a semi-permeable membrane �Driven by differences in concentrations of water and solutes on either side of membranes. �Hypertonic �Isotonic �Hypotonic

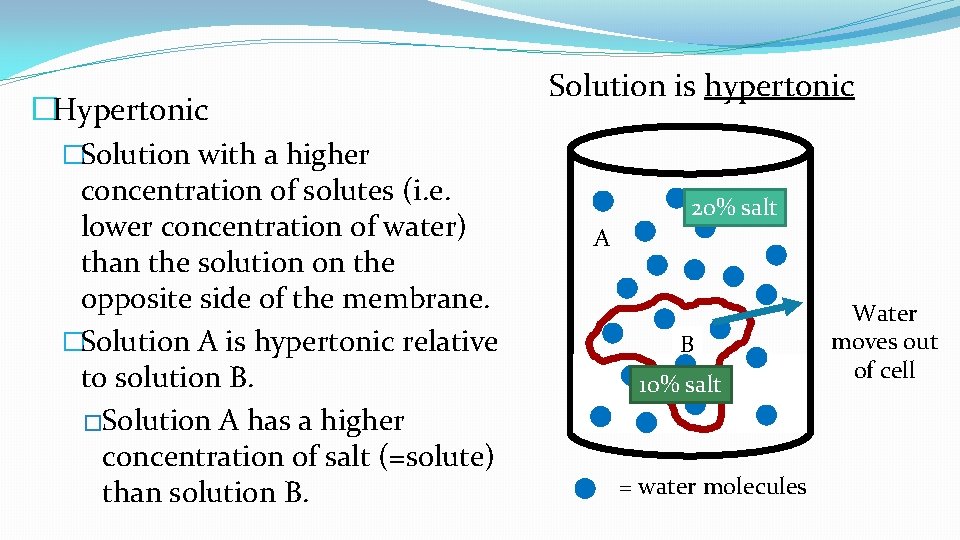
�Hypertonic �Solution with a higher concentration of solutes (i. e. lower concentration of water) than the solution on the opposite side of the membrane. �Solution A is hypertonic relative to solution B. �Solution A has a higher concentration of salt (=solute) than solution B. Solution is hypertonic 20% salt A B 10% salt = water molecules Water moves out of cell

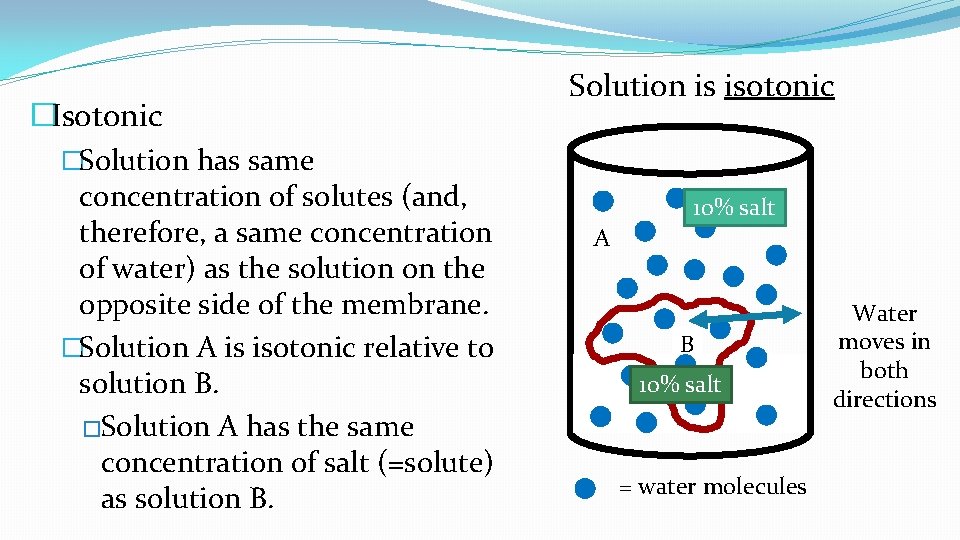
�Isotonic �Solution has same concentration of solutes (and, therefore, a same concentration of water) as the solution on the opposite side of the membrane. �Solution A is isotonic relative to solution B. �Solution A has the same concentration of salt (=solute) as solution B. Solution is isotonic 10% salt A B 10% salt = water molecules Water moves in both directions

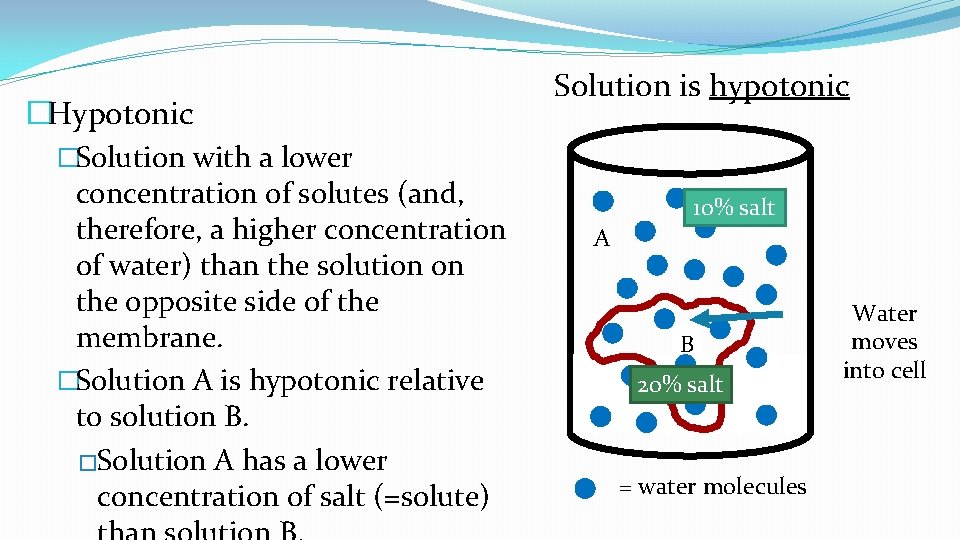
�Hypotonic �Solution with a lower concentration of solutes (and, therefore, a higher concentration of water) than the solution on the opposite side of the membrane. �Solution A is hypotonic relative to solution B. �Solution A has a lower concentration of salt (=solute) Solution is hypotonic 10% salt A B 20% salt = water molecules Water moves into cell

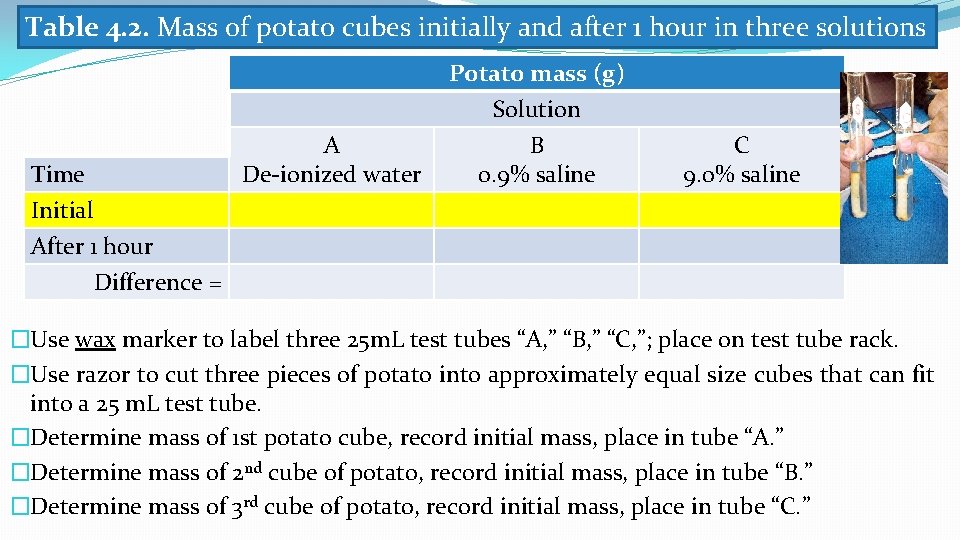
Table 4. 2. Mass of potato cubes initially and after 1 hour in three solutions Time Initial After 1 hour A De-ionized water Potato mass (g) Solution B 0. 9% saline C 9. 0% saline Difference = �Use wax marker to label three 25 m. L test tubes “A, ” “B, ” “C, ”; place on test tube rack. �Use razor to cut three pieces of potato into approximately equal size cubes that can fit into a 25 m. L test tube. �Determine mass of 1 st potato cube, record initial mass, place in tube “A. ” �Determine mass of 2 nd cube of potato, record initial mass, place in tube “B. ” �Determine mass of 3 rd cube of potato, record initial mass, place in tube “C. ”

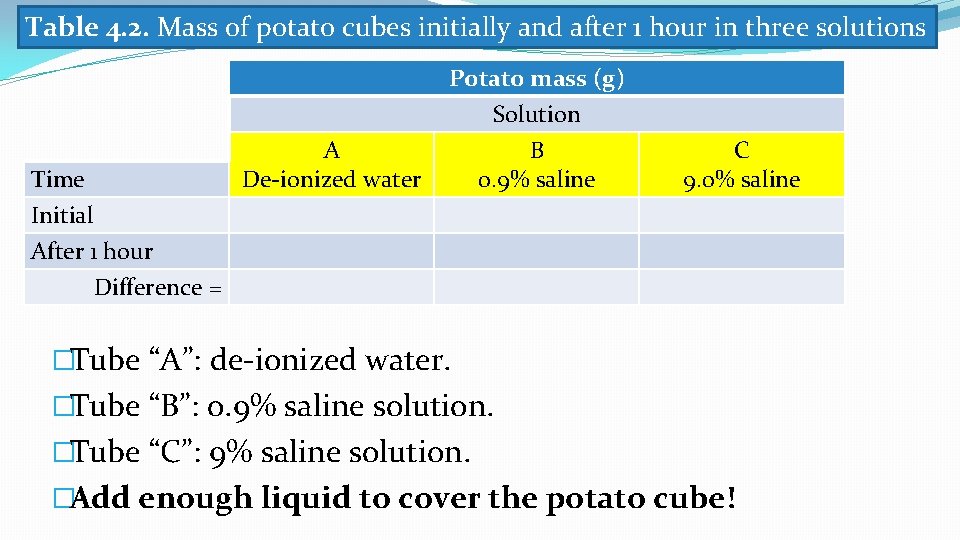
Table 4. 2. Mass of potato cubes initially and after 1 hour in three solutions Time Initial After 1 hour A De-ionized water Potato mass (g) Solution B 0. 9% saline C 9. 0% saline Difference = �Tube “A”: de-ionized water. �Tube “B”: 0. 9% saline solution. �Tube “C”: 9% saline solution. �Add enough liquid to cover the potato cube!

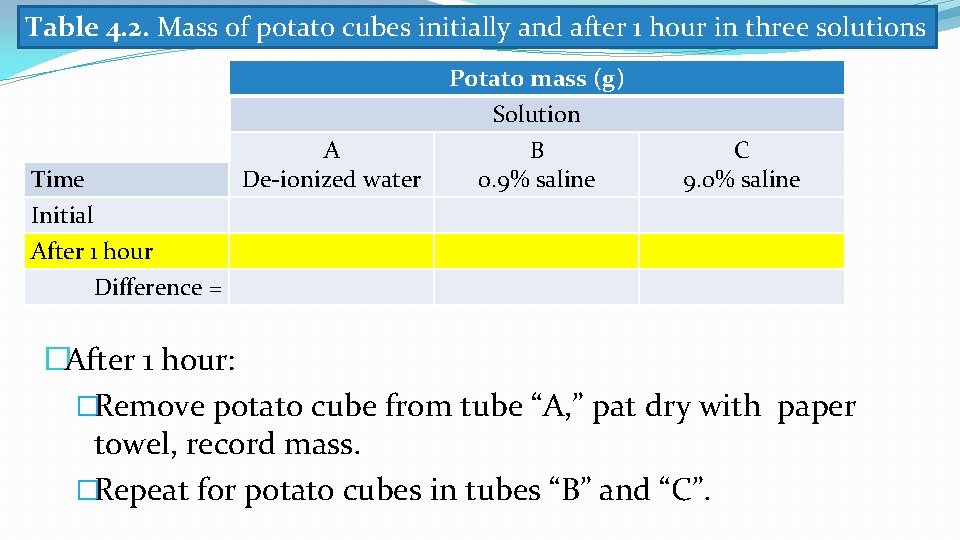
Table 4. 2. Mass of potato cubes initially and after 1 hour in three solutions Time Initial After 1 hour A De-ionized water Potato mass (g) Solution B 0. 9% saline C 9. 0% saline Difference = �After 1 hour: �Remove potato cube from tube “A, ” pat dry with paper towel, record mass. �Repeat for potato cubes in tubes “B” and “C”.

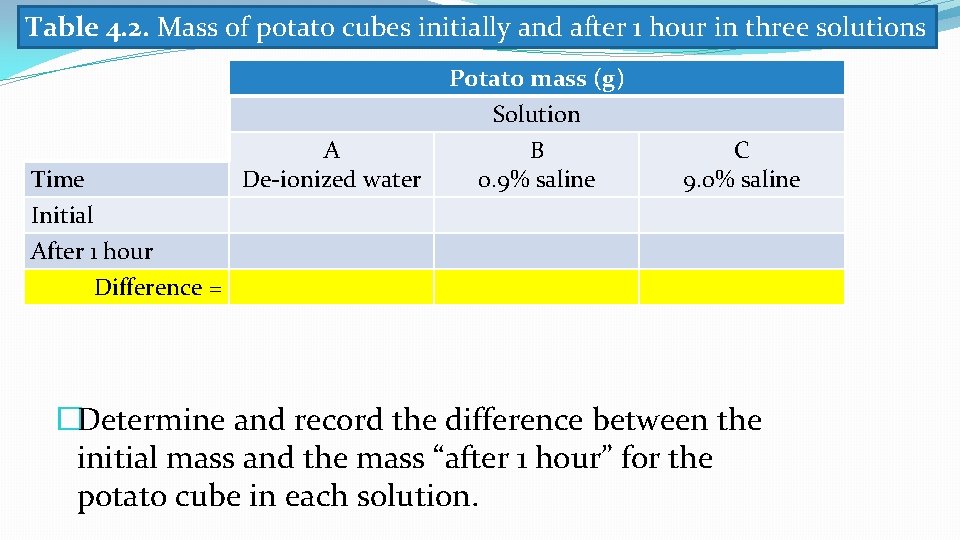
Table 4. 2. Mass of potato cubes initially and after 1 hour in three solutions Time Initial After 1 hour A De-ionized water Potato mass (g) Solution B 0. 9% saline C 9. 0% saline Difference = �Determine and record the difference between the initial mass and the mass “after 1 hour” for the potato cube in each solution.

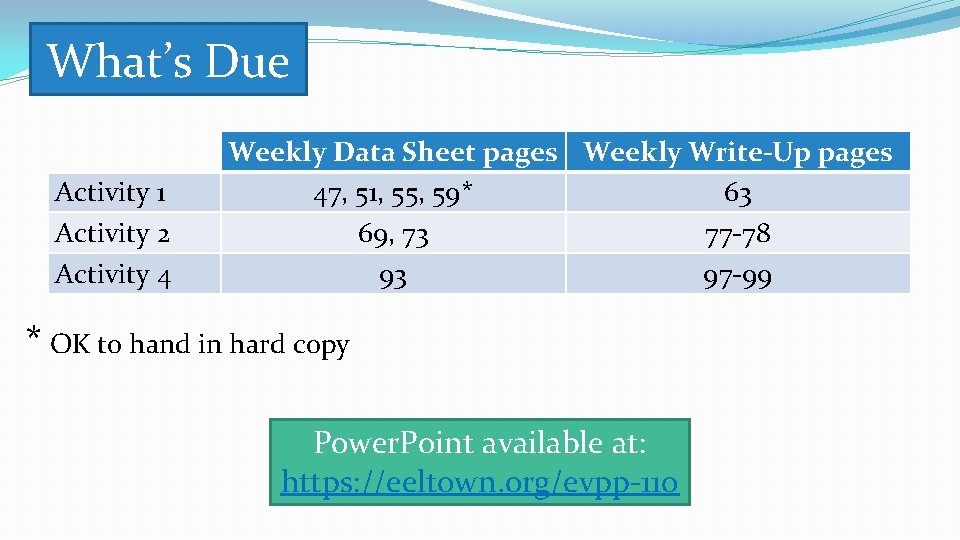
What’s Due Activity 1 Activity 2 Activity 4 Weekly Data Sheet pages Weekly Write-Up pages 47, 51, 55, 59* 63 69, 73 77 -78 93 97 -99 * OK to hand in hard copy Power. Point available at: https: //eeltown. org/evpp-110