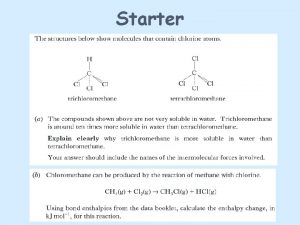
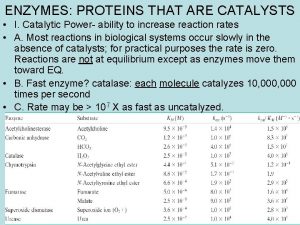
Enzymes Organic Catalysts Enzymes are Proteins Proteins contain



- Slides: 28

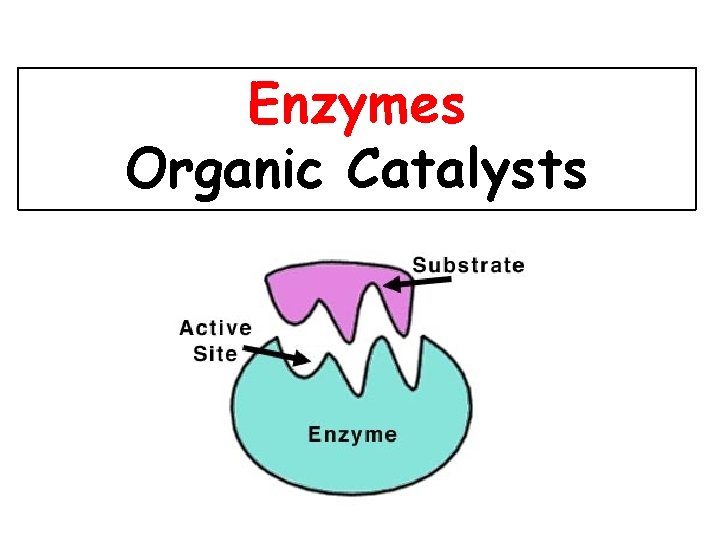
Enzymes Organic Catalysts

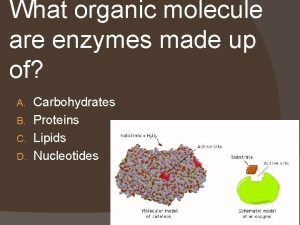
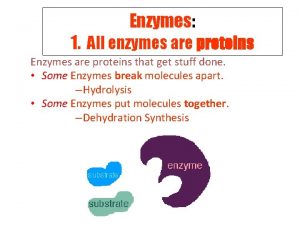
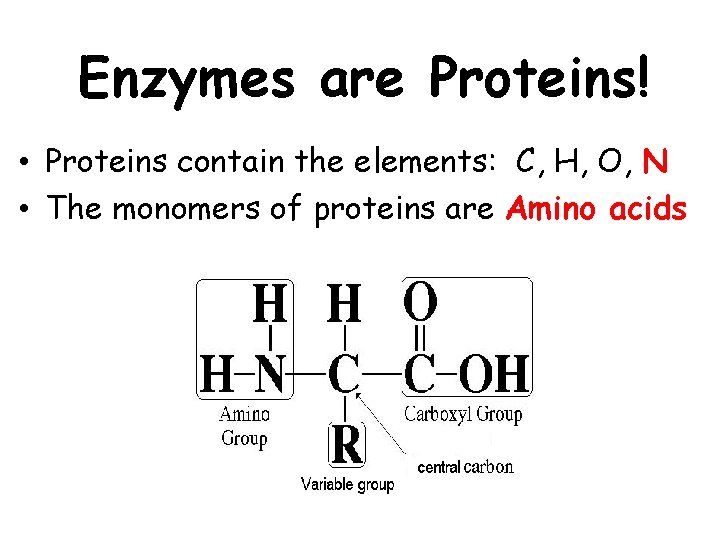
Enzymes are Proteins! • Proteins contain the elements: C, H, O, N • The monomers of proteins are Amino acids

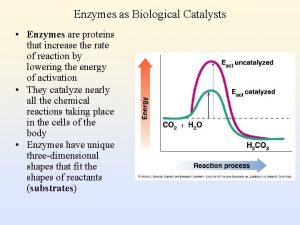
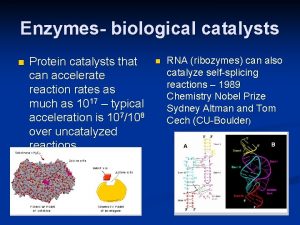
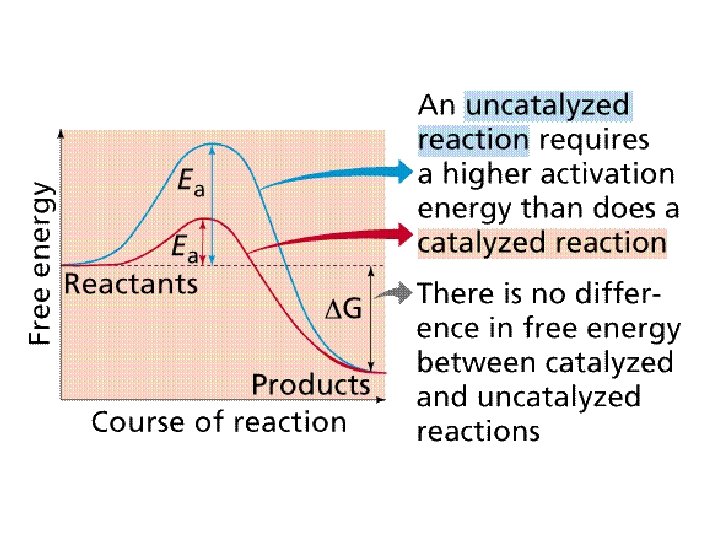
Characteristics of Enzymes • Facilitate or catalyze chemical reactions in cells – Speed them up – By lowering the activation energy (the energy needed to start the reaction) – W/O enzymes, chemical reactions would occur too slowly at the temperatures found in organisms to sustain life processes


A Catalyst Analogy* • You are at the American Museum of Natural History (because you are a science nerd and like to look at Dinosaur Bones • You need to get to Babo (because you are a big fan of Iron Chef) • What are your transportation options? *Analogy provided thanks to Ms. Walker

A Catalyst Analogy* • You can walk… • If you walk like a • You can take the C New Yorker – about 1 from 81 st to West block a minute – you 4 th St would walk over 80 • Once the train blocks – so an hour arrives – about 15 -17 and 20 minutes. • Longer if you walk • Less time if you can like a Livingston High transfer to the A School student on their way to class *Analogy provided thanks to Ms. Walker

The train is the “enzyme” that catalyzes your transportation *Analogy provided thanks to Ms. Walker

Characteristics of Enzymes • 3 D shape (tertiary or quaternary) confers specificity to the substrate upon which it binds to facilitate the reaction • Many enzymes are named after the substrate on which they act and end in –ase • Sucrase (enzyme) acts upon sucrose (substrate)

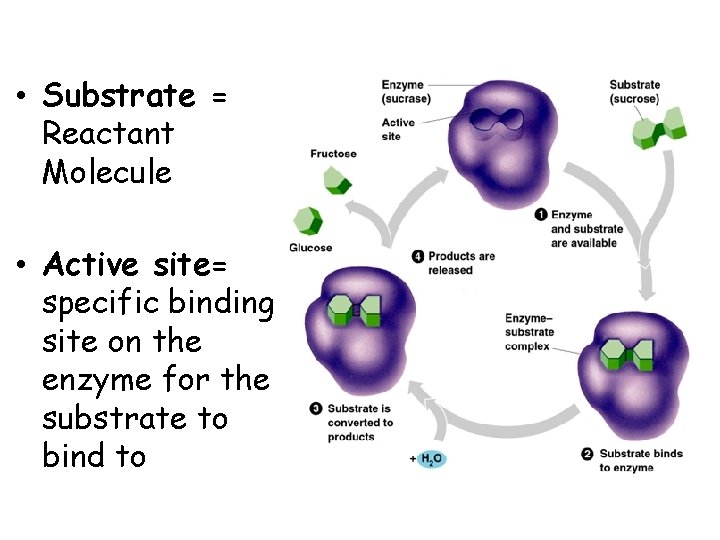
• Substrate = Reactant Molecule • Active site= specific binding site on the enzyme for the substrate to bind to

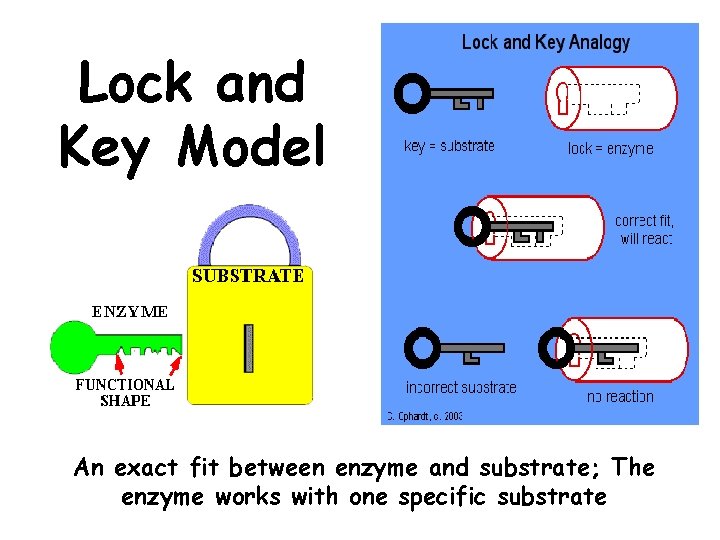
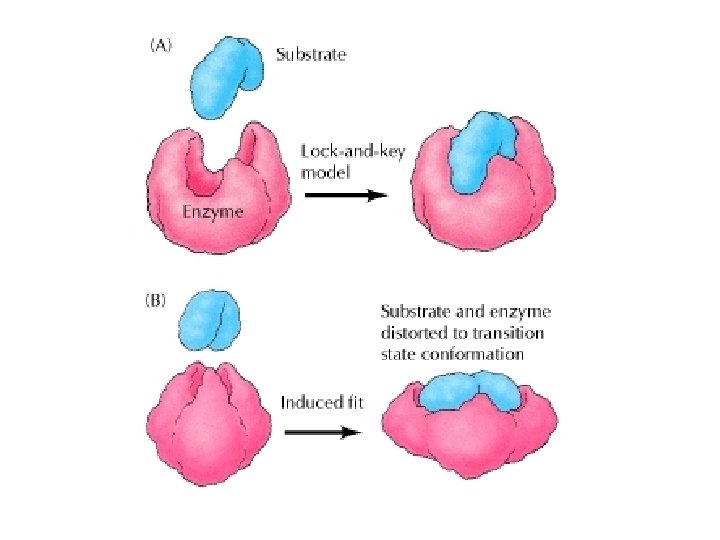
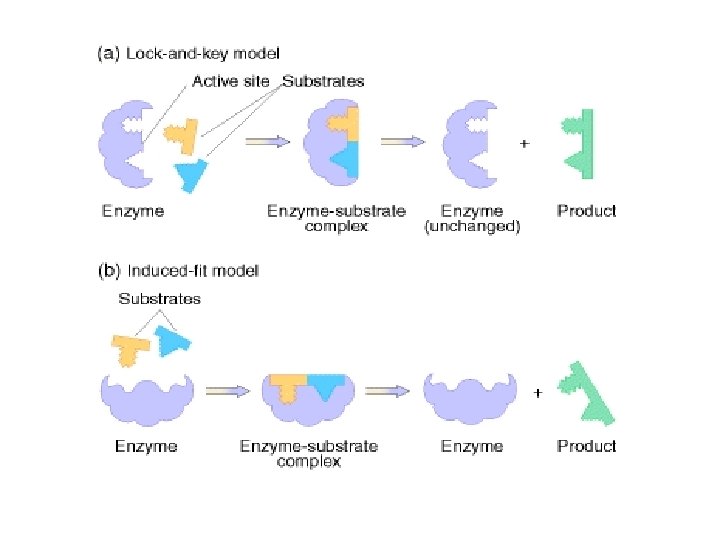
Lock and Key Model An exact fit between enzyme and substrate; The enzyme works with one specific substrate

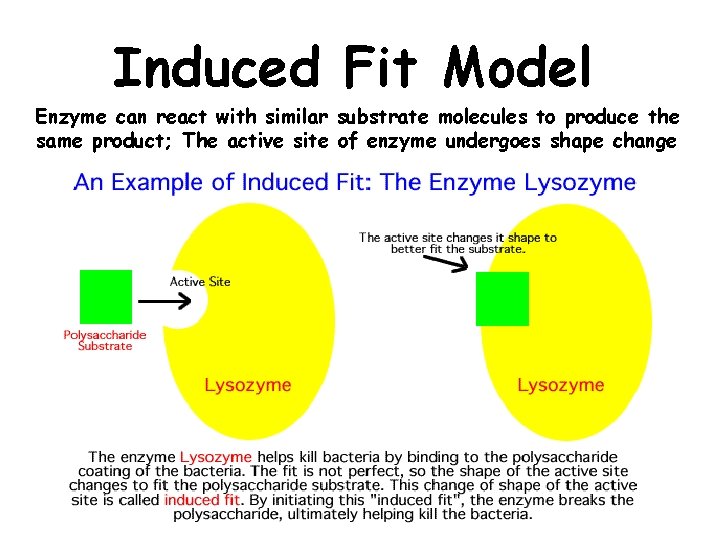
Induced Fit Model Enzyme can react with similar substrate molecules to produce the same product; The active site of enzyme undergoes shape change



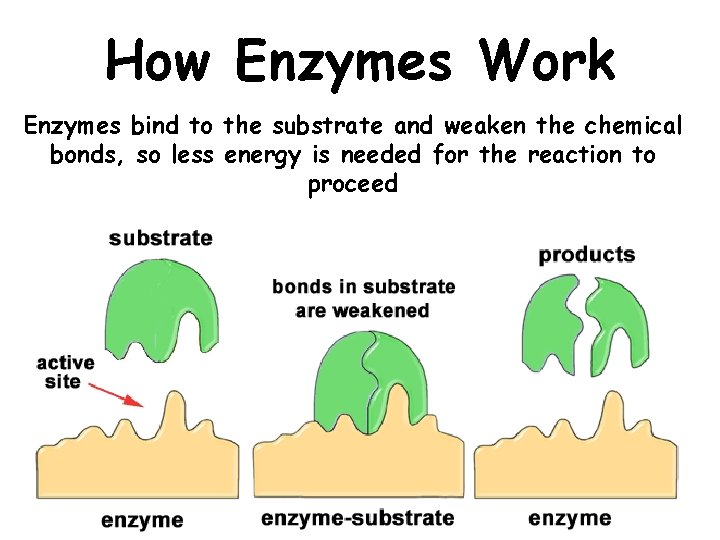
How Enzymes Work Enzymes bind to the substrate and weaken the chemical bonds, so less energy is needed for the reaction to proceed

Factors that affect Enzymes • • Enzymes are affected by their environment There are optimal conditions for every enzyme Optimal conditions = optimal performance Deviations= Denaturization = Loss of Function – Substrate & Enzyme Concentration – Higher temperatures – Changes in salt concentrations & p. H

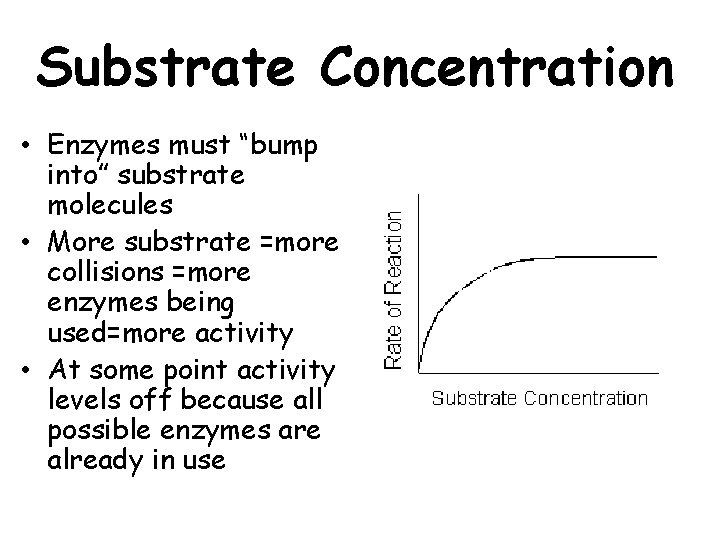
Substrate Concentration • Enzymes must “bump into” substrate molecules • More substrate =more collisions =more enzymes being used=more activity • At some point activity levels off because all possible enzymes are already in use

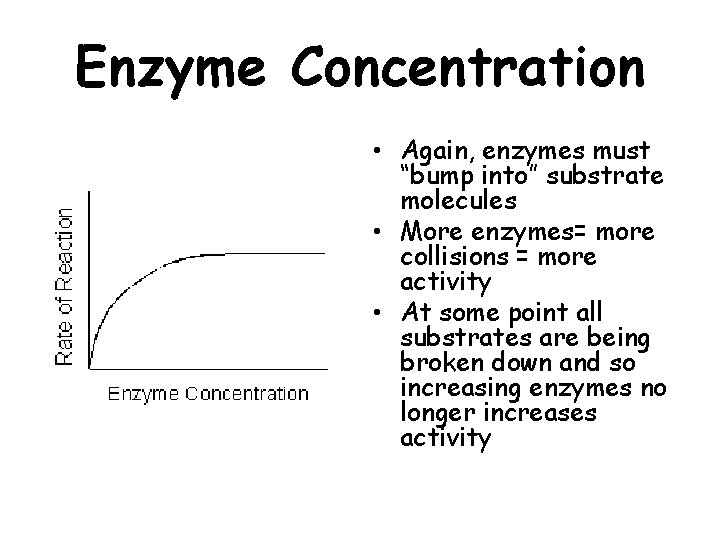
Enzyme Concentration • Again, enzymes must “bump into” substrate molecules • More enzymes= more collisions = more activity • At some point all substrates are being broken down and so increasing enzymes no longer increases activity

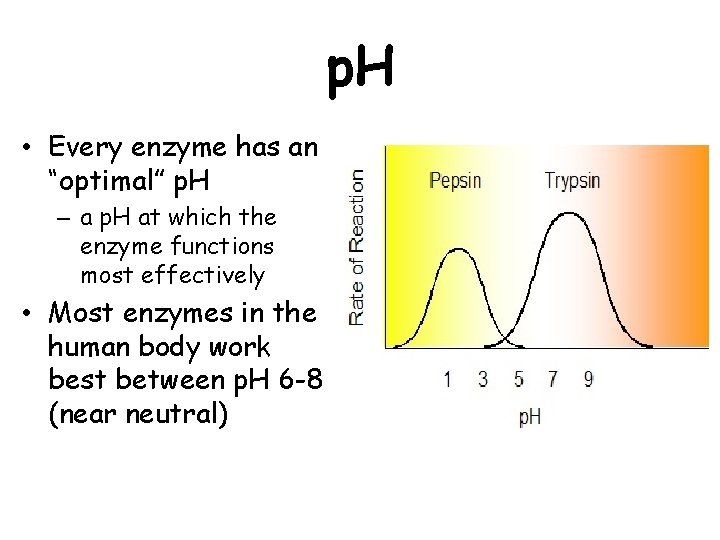
p. H • Every enzyme has an “optimal” p. H – a p. H at which the enzyme functions most effectively • Most enzymes in the human body work best between p. H 6 -8 (near neutral)

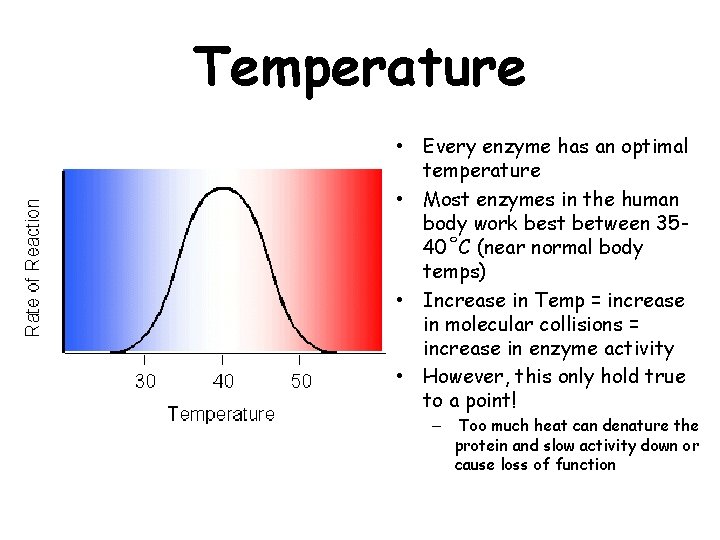
Temperature • Every enzyme has an optimal temperature • Most enzymes in the human body work best between 3540˚C (near normal body temps) • Increase in Temp = increase in molecular collisions = increase in enzyme activity • However, this only hold true to a point! – Too much heat can denature the protein and slow activity down or cause loss of function

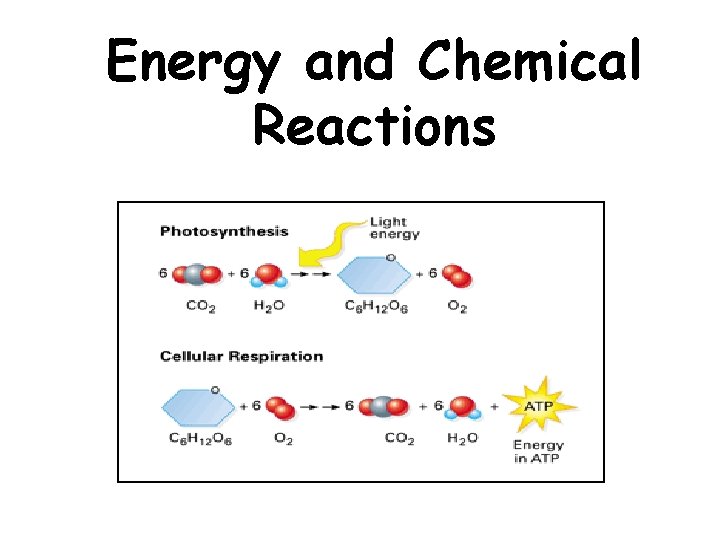
Energy and Chemical Reactions

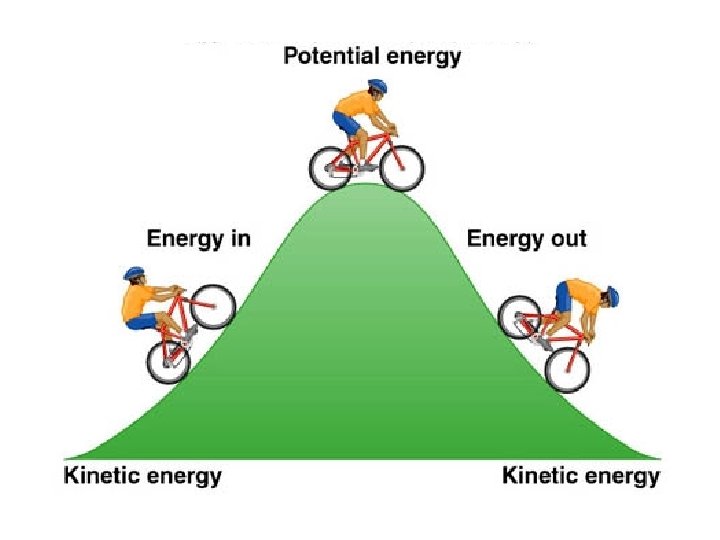
What is Energy? • Energy – The ability to do work • Potential – Stored Energy – Chemical Energy = potential energy of molecules • Kinetic – Energy of doing work (motion) – Heat • Energy associated with the movement of molecules


Life Depends on Energy Conversion Thermodynamics =The study of energy conversions • Two laws govern energy conversion: – First Law of Thermodynamics • The law of energy conservation • Energy can be transferred and transformed, but not created or destroyed – Second Law of Thermodynamics • Energy conversions reduce order (increase disorder) • Amount of disorder = Entropy

Chemical Reactions • Chemical reactions are involved when energy conversions occur – Atoms are rearranged – Law of Conservation of Matter – Matter is neither created nor destroyed • The sum of all chemical reactions in an organism – Metabolism • Chemical reactions either store or release energy

Endothermic and Exothermic Reactions Chemical reactions either store or release energy • Endergonic Reactions = Endothermic Reactions – Store energy – Yield products high in potential energy – Photosynthesis • Exergonic Reactions = Exothermic Reactions – Release energy – Potential energy is high in the reactants – Cellular Respiration

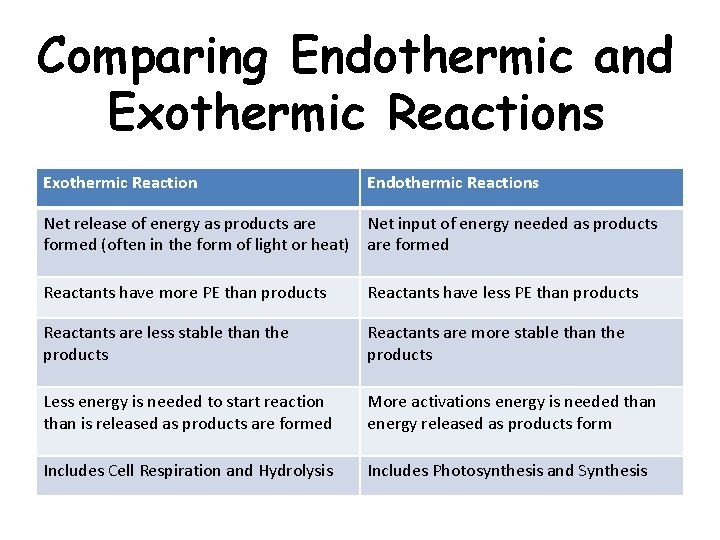
Comparing Endothermic and Exothermic Reactions Exothermic Reaction Endothermic Reactions Net release of energy as products are Net input of energy needed as products formed (often in the form of light or heat) are formed Reactants have more PE than products Reactants have less PE than products Reactants are less stable than the products Reactants are more stable than the products Less energy is needed to start reaction than is released as products are formed More activations energy is needed than energy released as products form Includes Cell Respiration and Hydrolysis Includes Photosynthesis and Synthesis

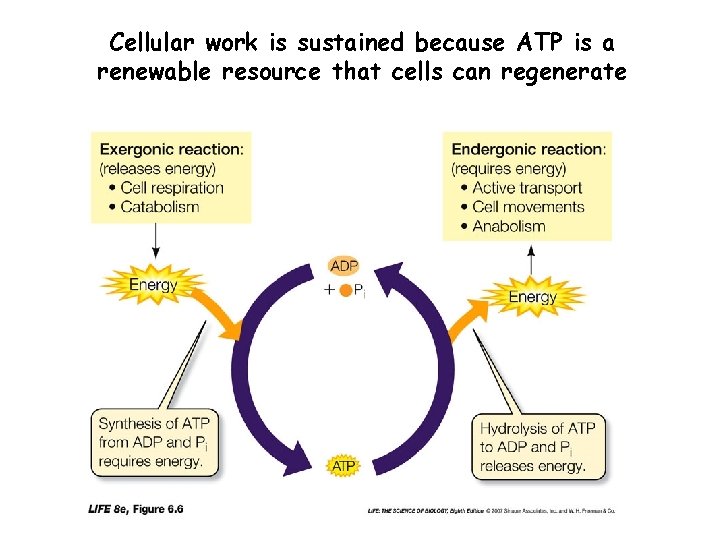
Coupled Reactions • Many reactions use the energy released from exothermic reactions to drive or fuel endothermic reactions • These are called “Coupled Reactions” – ATP Regeneration is the main example in cells • The usable energy released by most exothermic reactions is stored in ATP • The energy used in most endothermic reactions comes from ATP

Cellular work is sustained because ATP is a renewable resource that cells can regenerate
 Mikael ferm
Mikael ferm Relational escalation catalysts
Relational escalation catalysts Proteins contain what elements
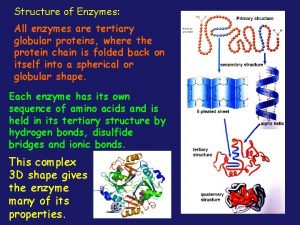
Proteins contain what elements All enzymes are globular proteins
All enzymes are globular proteins Not all enzymes are proteins
Not all enzymes are proteins Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Organic compounds must contain:
Organic compounds must contain: A carbohydrate is an organic compound because it contain
A carbohydrate is an organic compound because it contain Weathering and soil erosion
Weathering and soil erosion All organic compounds contain carbon and ________.
All organic compounds contain carbon and ________. All organic compounds must contain the element
All organic compounds must contain the element Enzymes are composed of what organic molecule
Enzymes are composed of what organic molecule Membrane synthesis
Membrane synthesis Function of muscle tissue
Function of muscle tissue Protein domains and motifs
Protein domains and motifs Characteristics of proteins
Characteristics of proteins Ace-2 expression
Ace-2 expression Tertiary protein structure
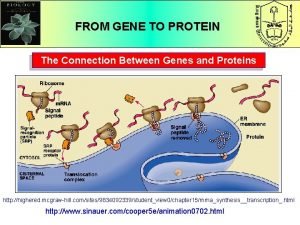
Tertiary protein structure What is the connection between genes and proteins
What is the connection between genes and proteins What is a negative acute phase protein
What is a negative acute phase protein Food sources of nucleic acids
Food sources of nucleic acids Which organelle prepares proteins for specific jobs
Which organelle prepares proteins for specific jobs How many amino acids are there
How many amino acids are there Primary secondary tertiary quaternary structure of proteins
Primary secondary tertiary quaternary structure of proteins Nuclear pores function
Nuclear pores function Carrier vs channel proteins
Carrier vs channel proteins Salting in salting out
Salting in salting out Does exocytosis require energy
Does exocytosis require energy The smallest living unit of all living things is
The smallest living unit of all living things is