ENZYMES PROTEINS THAT ARE CATALYSTS I Catalytic Power

![Metabolic Logic of Regulatory Effects • a. When [CTP] is high, ATCase activity to Metabolic Logic of Regulatory Effects • a. When [CTP] is high, ATCase activity to](https://slidetodoc.com/presentation_image/419df40707226a9669e54a352ec07bb0/image-16.jpg)
![Kinetics of ATCase • Kinetics of E: (rate(v) vs. [asp]) • The curve (Fig Kinetics of ATCase • Kinetics of E: (rate(v) vs. [asp]) • The curve (Fig](https://slidetodoc.com/presentation_image/419df40707226a9669e54a352ec07bb0/image-17.jpg)



- Slides: 41

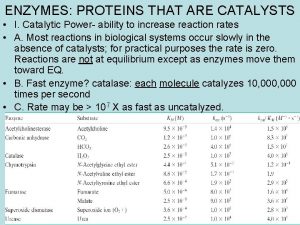
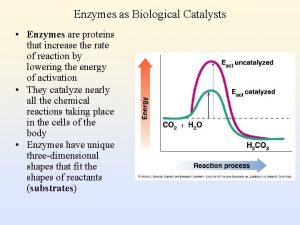
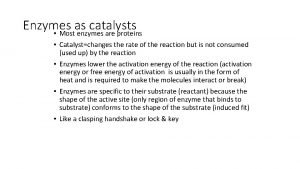
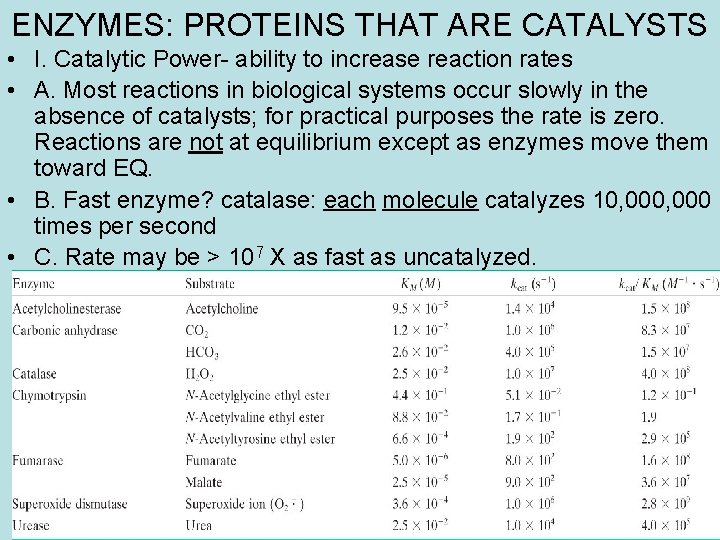
ENZYMES: PROTEINS THAT ARE CATALYSTS • I. Catalytic Power- ability to increase reaction rates • A. Most reactions in biological systems occur slowly in the absence of catalysts; for practical purposes the rate is zero. Reactions are not at equilibrium except as enzymes move them toward EQ. • B. Fast enzyme? catalase: each molecule catalyzes 10, 000 times per second • C. Rate may be > 107 X as fast as uncatalyzed.

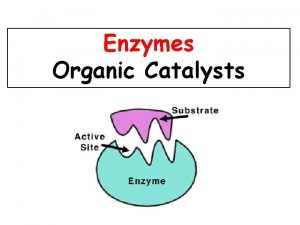
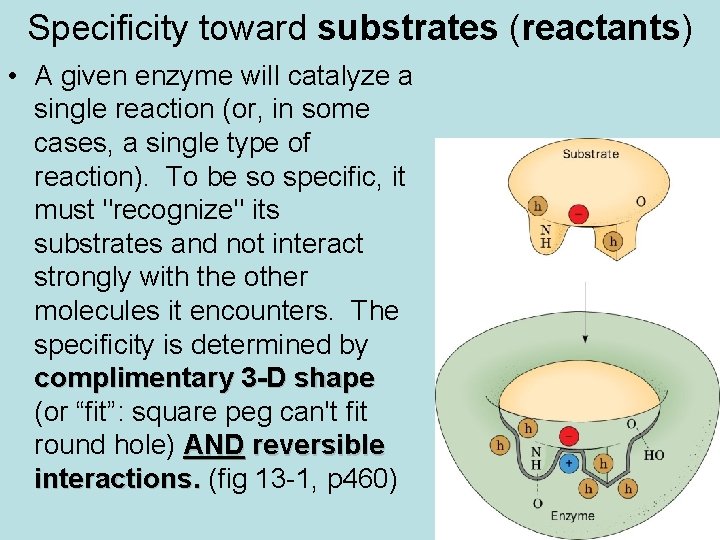
Specificity toward substrates (reactants) • A given enzyme will catalyze a single reaction (or, in some cases, a single type of reaction). To be so specific, it must "recognize" its substrates and not interact strongly with the other molecules it encounters. The specificity is determined by complimentary 3 -D shape (or “fit”: square peg can't fit round hole) AND reversible interactions. (fig 13 -1, p 460)

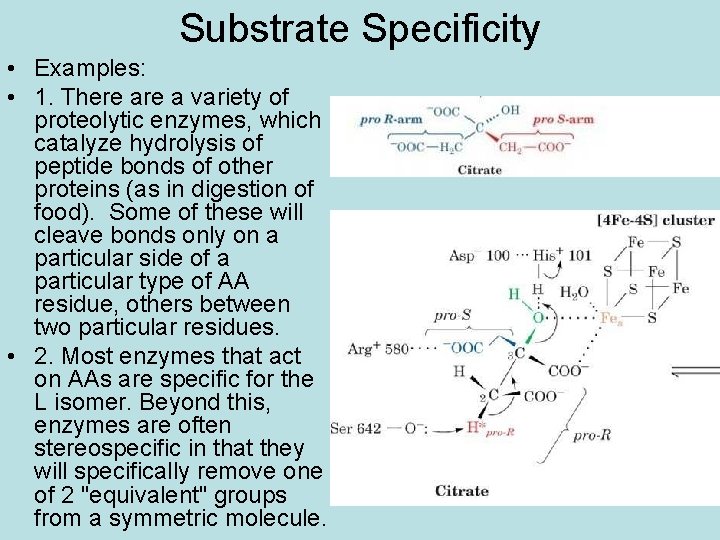
Substrate Specificity • Examples: • 1. There a variety of proteolytic enzymes, which catalyze hydrolysis of peptide bonds of other proteins (as in digestion of food). Some of these will cleave bonds only on a particular side of a particular type of AA residue, others between two particular residues. • 2. Most enzymes that act on AAs are specific for the L isomer. Beyond this, enzymes are often stereospecific in that they will specifically remove one of 2 "equivalent" groups from a symmetric molecule.

Regulation: Control of Enzyme Activity • If all molecules of a given enzyme are inactivated, reaction rate approaches zero. If all are activated (or not deactivated), reaction proceeds toward EQ at high rate. We shall see that usually control of one reaction in a pathway results in control of all of them. • Types: • 1. Allosteric: Allosteric • a. as with Hb: small indicator molecules • b. Regulatory proteins are themselves regulated and then act to control other proteins. • 2. Covalent modification: modification often a hydroxyl containing residue (ser, thr, tyr) will be phosphorylated at its –OH group. This results in stabilization of one form of the protein, as in CO 2 effect on Hb. • 3. Proteolytic activation: the enzyme is synthesized in inactive form, becomes active after a segment of its polypeptide chain is removed. (common for food digesting proteases)

• Enzymes interconvert forms of energy. • Living things are "chemical engines". • Foods are energy fuels; the energy released when they are metabolized is used as mechanical (muscle contraction), osmotic (and nutrient transport), chemical (biosynthesis), etc. energy.

Energy: Thermodynamics • A. Second Law: For all spontaneous processes, the entropy of the universe increases. In biosynthetic processes the entropy decreases, but this is offset by a larger increase in the entropy of surroundings. (ΔS (universe) = ΔS (system) + ΔS (surroundings) ) • B. The above statement of the Second Law is difficult to use practically: • 1. It can be converted mathematically to the following result: a reaction occurs spontaneously only if ΔG < 0. It is at equilibrium if ΔG = 0. 0 (ΔG is the "free energy change") (spontaneously means left to right. We say the reaction is not spontaneous if ΔG > 0, but what this means is that the reaction proceeds from right to left as written. )

Energy: Thermodynamics • 2. We will often be concerned with readily reversible reactions. The reaction proceeds at a significant rate from right to left and from left to right: • at equilibrium the rates of the reactions in each direction are equal and ΔG = 0. • If ΔG < 0 the rate of the reaction to the right is greater than that to the left and we say the (net) “reaction goes to the right” (until EQ is reached). • If ΔG > 0 we say the (net) “reaction goes to the left”. • Note: Spontaneously means "without proportional input of energy". It does not mean "occurs immediately" or "occurs rapidly". There is no relationship between ΔG and rate. • 3. ΔG is independent of path. The ΔG for glucose oxidation is the same whether the glucose is burned in air (a reaction in which oxygen molecules collide with glucose molecules) or occurs in a living system without flame or any direct contact between oxygen and glucose.

Thermodynamics and Equilibrium • • • For the reaction: a. A + b. B ---> c. C + d. D; ΔG = ΔGo' + 2. 303 RT log Q; where Q = [C]c[D]d/[A]a[B]b at the existing conditions and ΔGo' = -2. 303 RT log Keq. The superscripts on ΔGo' indicate standard conditions, namely [A] = [B] = [C] = [D] = 1 M (if A, B, C, and/or D are aqueous substances) (and if A, B, C, or D is a gas, PA =PB = PC = PD = 1 atm for a gas), and the prime indicates a special standard condition for biochemistry: p. H = 7. The value of ΔGo' indicates the spontaneous direction of the reaction under standard conditions as for ΔG above. Note that to calculate ΔGo' we must first calculate Keq from the EQ concentrations of A, B, C, and D. If the system is at EQ: ΔG = 0 and Q = Keq. (Note: R= 8. 31 J/mol. K = 8. 31 X 10 -3 k. J/mol. K and 2. 303 RT = 5. 7 k. J at 298 K. )

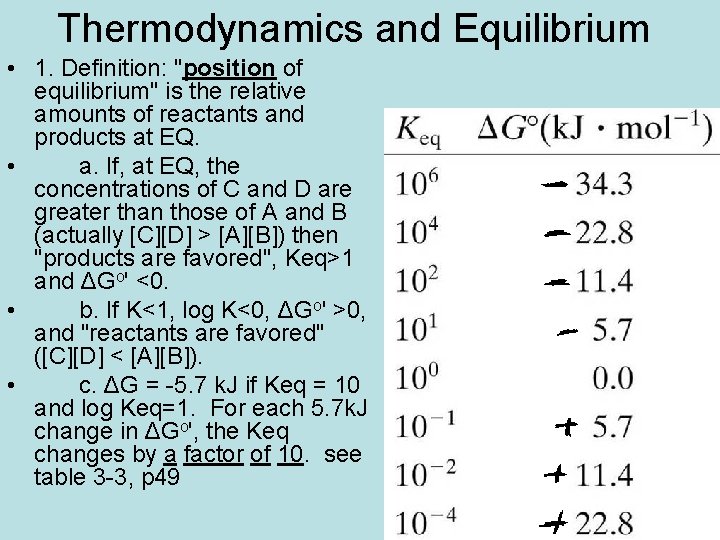
Thermodynamics and Equilibrium • 1. Definition: "position of equilibrium" is the relative amounts of reactants and products at EQ. • a. If, at EQ, the concentrations of C and D are greater than those of A and B (actually [C][D] > [A][B]) then "products are favored", Keq>1 and ΔGo' <0. • b. If K<1, log K<0, ΔGo' >0, and "reactants are favored" ([C][D] < [A][B]). • c. ΔG = -5. 7 k. J if Keq = 10 and log Keq=1. For each 5. 7 k. J change in ΔGo', the Keq changes by a factor of 10. see table 3 -3, p 49

Thermodynamics and Equilibrium • 2. Note that the spontaneity of a reaction depends on the 2 factors in the ΔG expression: i. ΔGo' indicates the characteristics of the substances in the reaction regarding which are more stable and favored at equilibrium. • ii. Q indicates existing concentrations. • If Q=10 and Keq=3 then there are more products (and/or less reactants) than at EQ and the reaction will go left even though products are favored.

Kinetics: effects of enzymes on reaction rates • 1. Enzymes have no effect on ΔGo', ΔG, Keq, or the position of EQ. • 2. Enzymes cannot make a reaction go in a certain direction, they only make it go faster in the direction (net) specified by ΔG. • 3. Enzymes actually accelerate the rate in both directions equally (by an equal factor). • 4. They increase the rate at which the reaction goes toward EQ. Most biological reactions are not at EQ in a living cell.

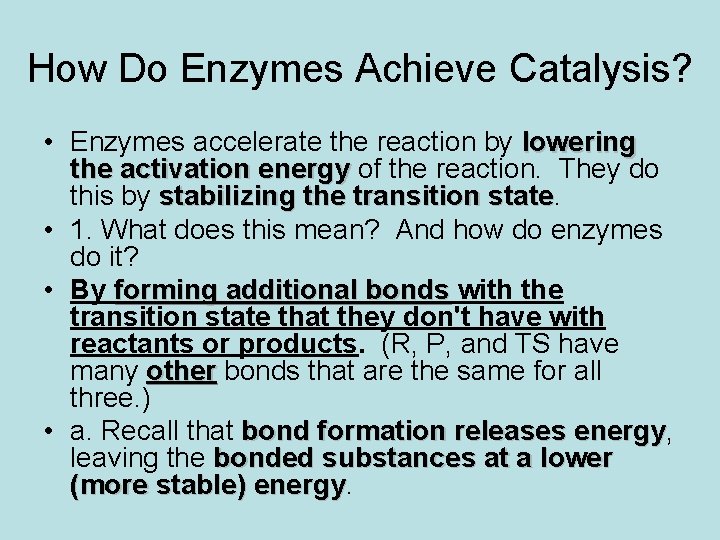
How Do Enzymes Achieve Catalysis? • Enzymes accelerate the reaction by lowering the activation energy of the reaction. They do this by stabilizing the transition state • 1. What does this mean? And how do enzymes do it? • By forming additional bonds with the transition state that they don't have with reactants or products. (R, P, and TS have many other bonds that are the same for all three. ) • a. Recall that bond formation releases energy, energy leaving the bonded substances at a lower (more stable) energy

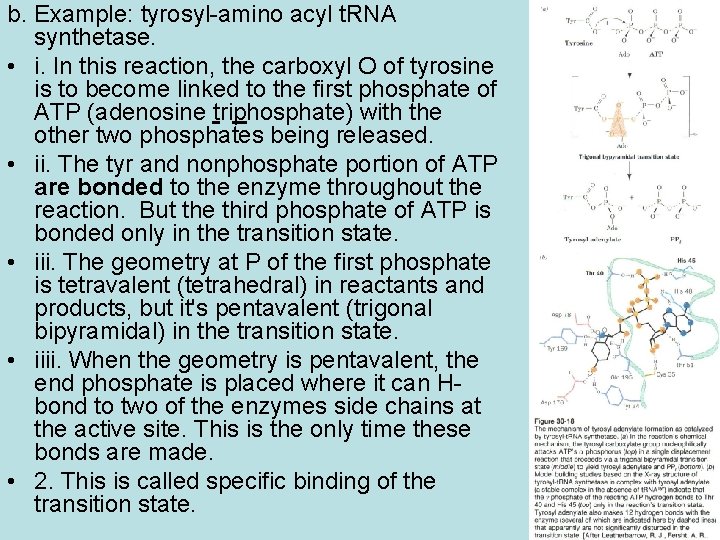
b. Example: tyrosyl-amino acyl t. RNA synthetase. • i. In this reaction, the carboxyl O of tyrosine is to become linked to the first phosphate of ATP (adenosine triphosphate) with the other two phosphates being released. • ii. The tyr and nonphosphate portion of ATP are bonded to the enzyme throughout the reaction. But the third phosphate of ATP is bonded only in the transition state. • iii. The geometry at P of the first phosphate is tetravalent (tetrahedral) in reactants and products, but it's pentavalent (trigonal bipyramidal) in the transition state. • iiii. When the geometry is pentavalent, the end phosphate is placed where it can Hbond to two of the enzymes side chains at the active site. This is the only time these bonds are made. • 2. This is called specific binding of the transition state.

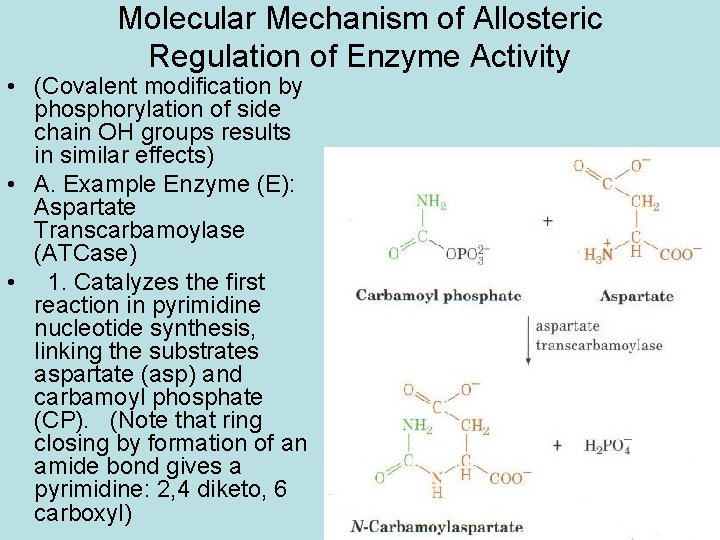
Molecular Mechanism of Allosteric Regulation of Enzyme Activity • (Covalent modification by phosphorylation of side chain OH groups results in similar effects) • A. Example Enzyme (E): Aspartate Transcarbamoylase (ATCase) • 1. Catalyzes the first reaction in pyrimidine nucleotide synthesis, linking the substrates aspartate (asp) and carbamoyl phosphate (CP). (Note that ring closing by formation of an amide bond gives a pyrimidine: 2, 4 diketo, 6 carboxyl)

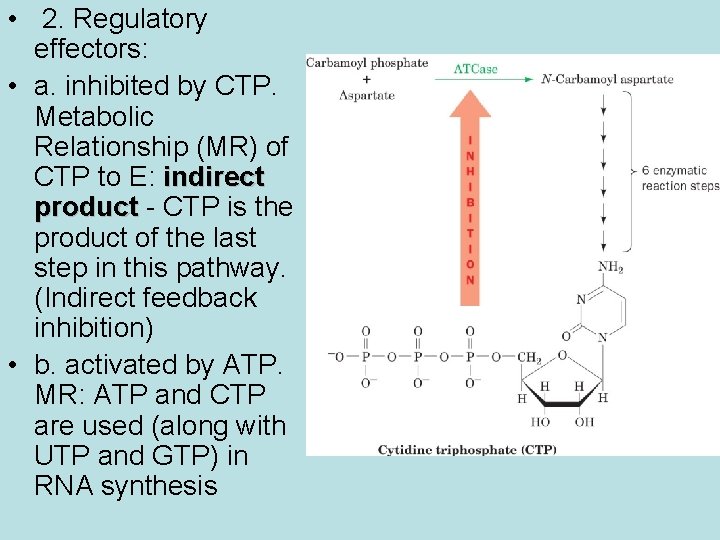
• 2. Regulatory effectors: • a. inhibited by CTP. Metabolic Relationship (MR) of CTP to E: indirect product - CTP is the product of the last step in this pathway. (Indirect feedback inhibition) • b. activated by ATP. MR: ATP and CTP are used (along with UTP and GTP) in RNA synthesis
![Metabolic Logic of Regulatory Effects a When CTP is high ATCase activity to Metabolic Logic of Regulatory Effects • a. When [CTP] is high, ATCase activity to](https://slidetodoc.com/presentation_image/419df40707226a9669e54a352ec07bb0/image-16.jpg)
Metabolic Logic of Regulatory Effects • a. When [CTP] is high, ATCase activity to produce more is not needed. Inhibition by CTP conserves substrates for other uses. • b. ATP and CTP are used in RNA synthesis (along with GTP and UTP). When [ATP] is high, RNA synthesis is well supplied ONLY IF [CTP] is also high. Activation of E by ATP coordinates nucleotide synthesis/supply.
![Kinetics of ATCase Kinetics of E ratev vs asp The curve Fig Kinetics of ATCase • Kinetics of E: (rate(v) vs. [asp]) • The curve (Fig](https://slidetodoc.com/presentation_image/419df40707226a9669e54a352ec07bb0/image-17.jpg)
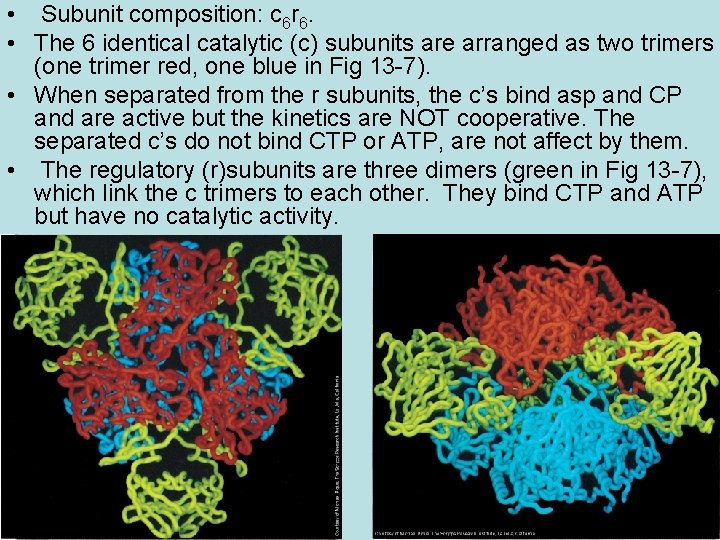
Kinetics of ATCase • Kinetics of E: (rate(v) vs. [asp]) • The curve (Fig 13 -5) indicates positive cooperativity, as with Hb. The affinity for asp is low at low [asp], and is higher at higher [asp], where several molecules of asp are bound.

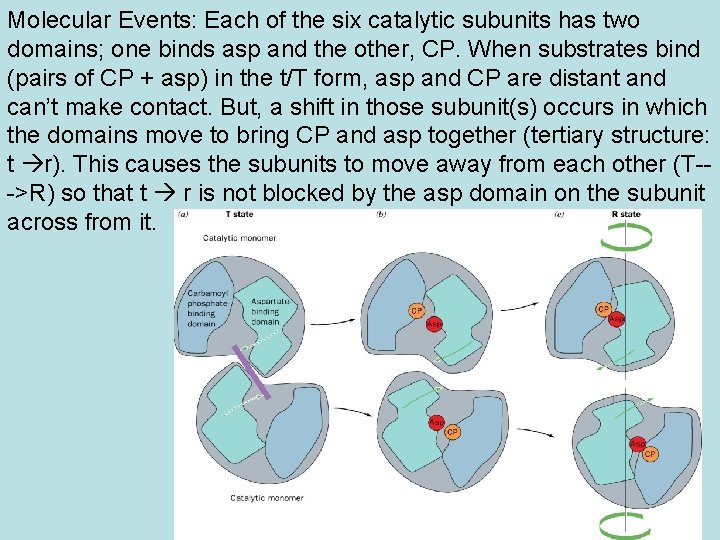
• Subunit composition: c 6 r 6. • The 6 identical catalytic (c) subunits are arranged as two trimers (one trimer red, one blue in Fig 13 -7). • When separated from the r subunits, the c’s bind asp and CP and are active but the kinetics are NOT cooperative. The separated c’s do not bind CTP or ATP, are not affect by them. • The regulatory (r)subunits are three dimers (green in Fig 13 -7), which link the c trimers to each other. They bind CTP and ATP but have no catalytic activity.

Molecular Events: Each of the six catalytic subunits has two domains; one binds asp and the other, CP. When substrates bind (pairs of CP + asp) in the t/T form, asp and CP are distant and can’t make contact. But, a shift in those subunit(s) occurs in which the domains move to bring CP and asp together (tertiary structure: t r). This causes the subunits to move away from each other (T-->R) so that t r is not blocked by the asp domain on the subunit across from it.

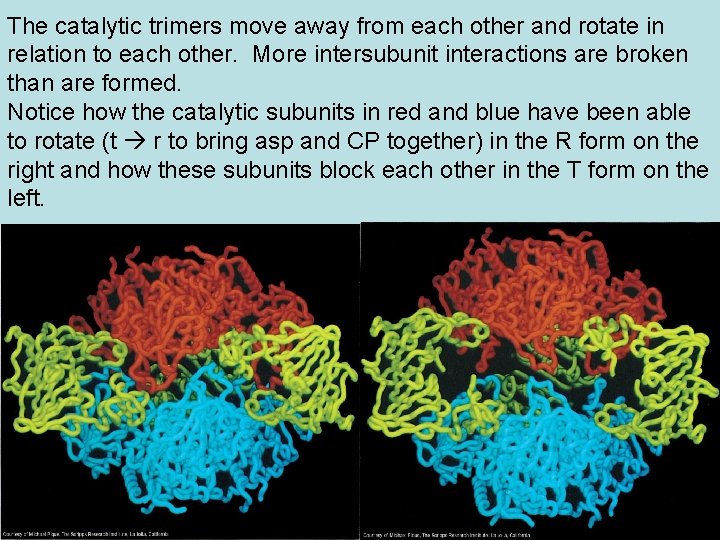
The catalytic trimers move away from each other and rotate in relation to each other. More intersubunit interactions are broken than are formed. Notice how the catalytic subunits in red and blue have been able to rotate (t r to bring asp and CP together) in the R form on the right and how these subunits block each other in the T form on the left.

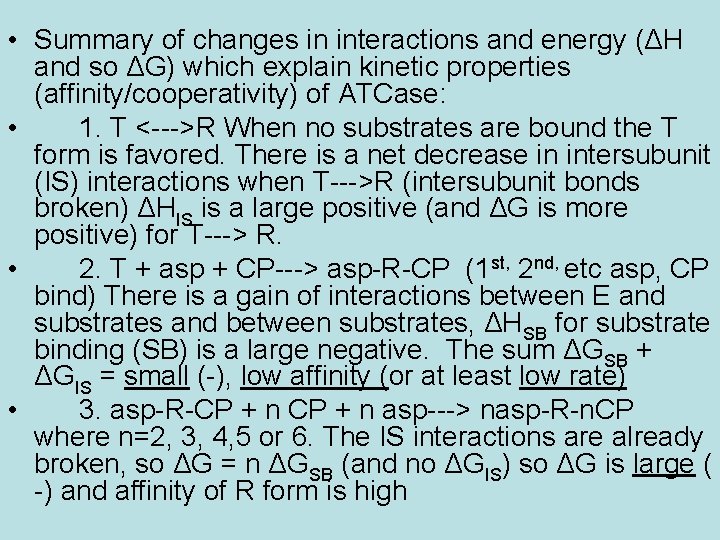
• Summary of changes in interactions and energy (ΔH and so ΔG) which explain kinetic properties (affinity/cooperativity) of ATCase: • 1. T <--->R When no substrates are bound the T form is favored. There is a net decrease in intersubunit (IS) interactions when T--->R (intersubunit bonds broken) ΔHIS is a large positive (and ΔG is more positive) for T---> R. • 2. T + asp + CP---> asp-R-CP (1 st, 2 nd, etc asp, CP bind) There is a gain of interactions between E and substrates and between substrates, ΔHSB for substrate binding (SB) is a large negative. The sum ΔGSB + ΔGIS = small (-), low affinity (or at least low rate) • 3. asp-R-CP + n asp---> nasp-R-n. CP where n=2, 3, 4, 5 or 6. The IS interactions are already broken, so ΔG = n ΔGSB (and no ΔGIS) so ΔG is large ( -) and affinity of R form is high

• 4. Activation by ATP, which binds strongly only to R form, keeps more E in R form. • R-n. ATP ---> T + n. ATP breaks interactions between ATPs and E, so ΔGATP is +. ΔGIS is - ; sum ΔGATP + ΔGIS favors T form less than in 1 above, so more (or most? ) of E is in R form • T + CP + asp + ATP (cp, asp) – R- ATP (Binding of ATP along with substrates) will have a ΔGATP that is – to go along with the – value for ΔGSB, and this will make the overall ΔG more negative and the affinity higher than in 2 above. • 5. Inhibition by CTP (analagous to CO 2 effect on Hb) keeps more E in T form, makes T---> R harder and makes R---> T more favorable; CTP only binds strongly to T. In T-CTP ---> R + CTP, these are additional interactions that must be broken in T to R. ΔGCTP + ΔGIS for T---> R is more + so T form is even more favored than in 1, and affinity is even lower than in 2.

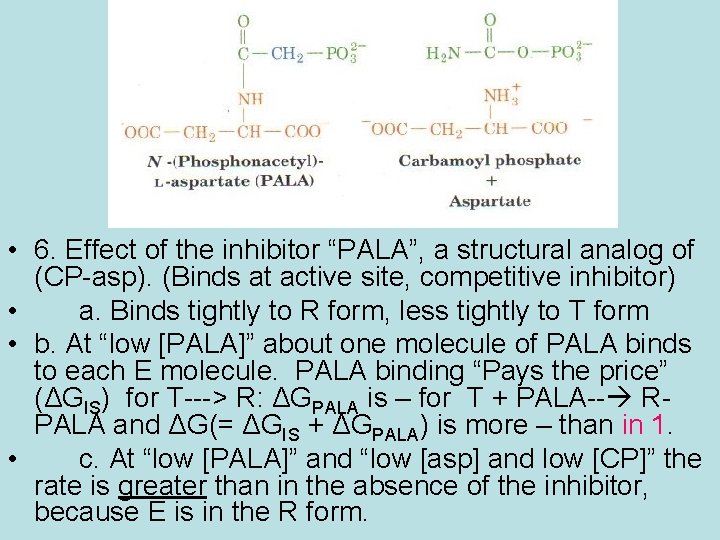
• 6. Effect of the inhibitor “PALA”, a structural analog of (CP-asp). (Binds at active site, competitive inhibitor) • a. Binds tightly to R form, less tightly to T form • b. At “low [PALA]” about one molecule of PALA binds to each E molecule. PALA binding “Pays the price” (ΔGIS) for T---> R: ΔGPALA is – for T + PALA-- RPALA and ΔG(= ΔGIS + ΔGPALA) is more – than in 1. • c. At “low [PALA]” and “low [asp] and low [CP]” the rate is greater than in the absence of the inhibitor, because E is in the R form.

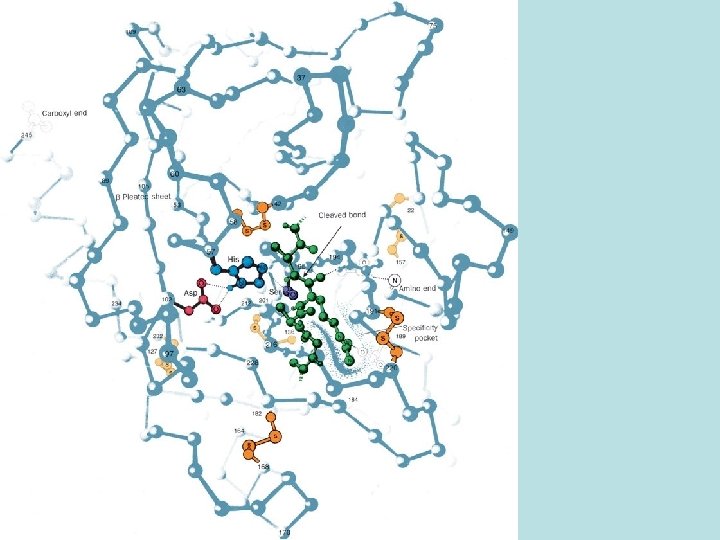
Chymotrypsin • Reason to study chymotrypsin: as an example of the mechanisms and strategies enzymes employ to achieve catalysis. • Chymotrypsin catalyzes hydrolysis of specific peptide bonds: those preceeded by large hydrophobic R groups on the substrate. (This is the R group of the residue containing the carbonyl group of the peptide bond that is hydrolyzed) • How does E achieve this substrate specificity? Hydrophobic/nonpolar/HC side chains on the E “line” a pocket across from the active site that the hydrophobic side chain on the substrate fits into and interacts with. • Effects of these interactions: • 1. puts the susceptible peptide bond in extended (β) conformation so that • 2. the peptide N is positioned close to H of ser OH on E and the peptide C is positioned close to O of ser OH on E. These are “proximity and orientation effects”.


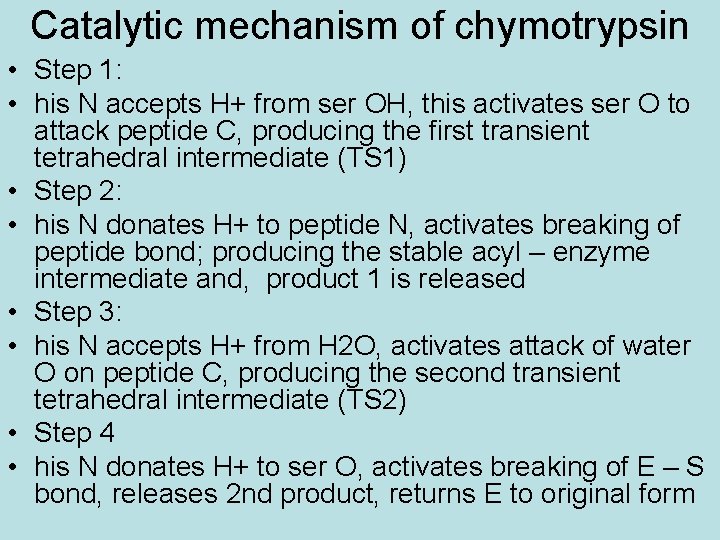
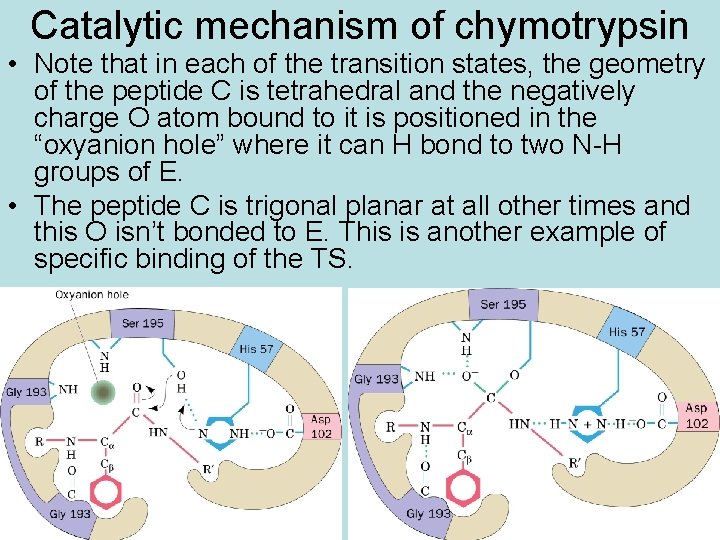
Catalytic mechanism of chymotrypsin • Step 1: • his N accepts H+ from ser OH, this activates ser O to attack peptide C, producing the first transient tetrahedral intermediate (TS 1) • Step 2: • his N donates H+ to peptide N, activates breaking of peptide bond; producing the stable acyl – enzyme intermediate and, product 1 is released • Step 3: • his N accepts H+ from H 2 O, activates attack of water O on peptide C, producing the second transient tetrahedral intermediate (TS 2) • Step 4 • his N donates H+ to ser O, activates breaking of E – S bond, releases 2 nd product, returns E to original form

Catalytic mechanism of chymotrypsin • Note that in each of the transition states, the geometry of the peptide C is tetrahedral and the negatively charge O atom bound to it is positioned in the “oxyanion hole” where it can H bond to two N-H groups of E. • The peptide C is trigonal planar at all other times and this O isn’t bonded to E. This is another example of specific binding of the TS.

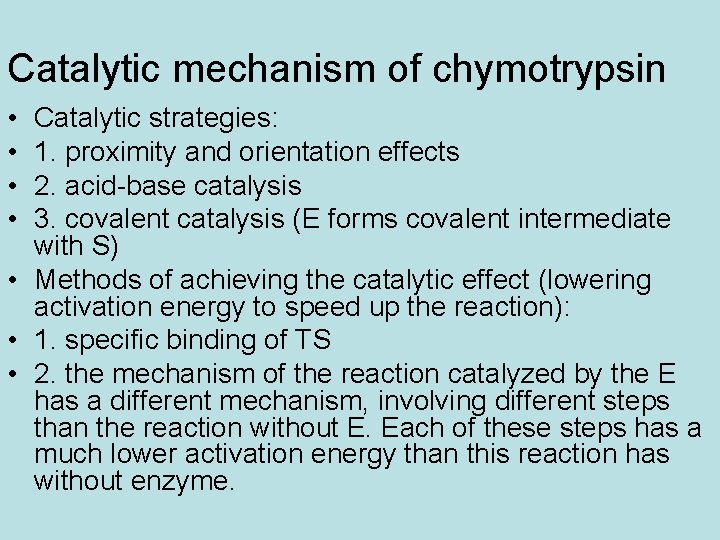
Catalytic mechanism of chymotrypsin • • Catalytic strategies: 1. proximity and orientation effects 2. acid-base catalysis 3. covalent catalysis (E forms covalent intermediate with S) • Methods of achieving the catalytic effect (lowering activation energy to speed up the reaction): • 1. specific binding of TS • 2. the mechanism of the reaction catalyzed by the E has a different mechanism, involving different steps than the reaction without E. Each of these steps has a much lower activation energy than this reaction has without enzyme.

Chymotrypsin employs many of the catalytic strategies that are available to enzymes: 1. proximity and orientation effects 2. acid-base catalysis 3. covalent catalysis (forms covalent intermediate with S) But these are NOT the answers to the quiz: • Of the 2 methods by which it achieves the catalytic effect (increase in reaction rate), one is the same as that of tyrosyl-amino acyl t. RNA synthetase. Describe the two methods and give the details for chymotrypsin. • Compare this slide to slide 28.

• Serine proteases: a group of related enzymes that use the same catalytic mechanism as chymotrypsin • Trypsin catalyses the same reaction on peptide bonds preceeded by lys and arg side chains: long + charged groups. It has an asp side chain at the bottom of its specificity pocket that can form an ionic bond with the substrate side chain.

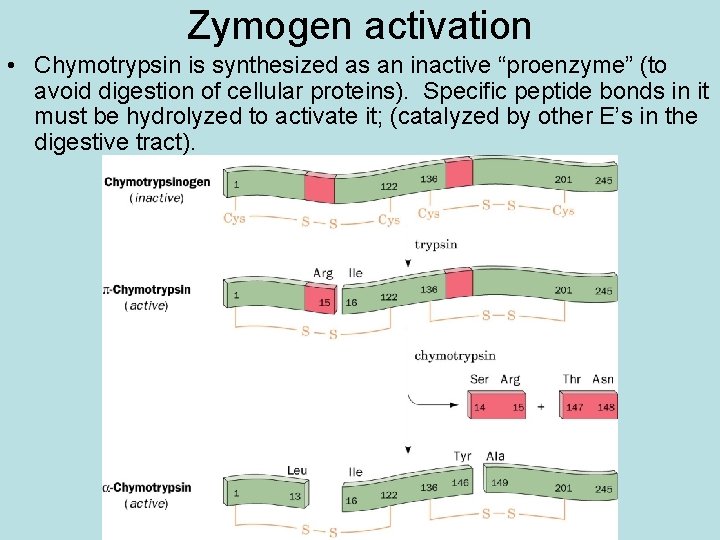
Zymogen activation • Chymotrypsin is synthesized as an inactive “proenzyme” (to avoid digestion of cellular proteins). Specific peptide bonds in it must be hydrolyzed to activate it; (catalyzed by other E’s in the digestive tract).

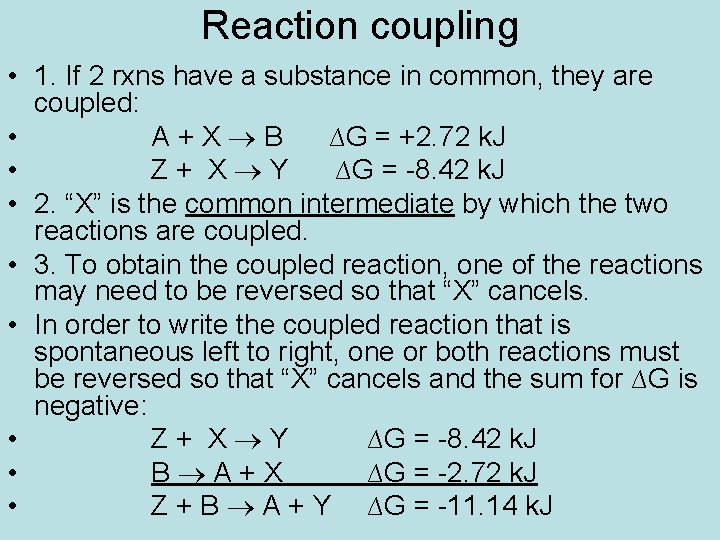
Reaction coupling • 1. If 2 rxns have a substance in common, they are coupled: • A+X B ∆G = +2. 72 k. J • Z+ X Y ∆G = -8. 42 k. J • 2. “X” is the common intermediate by which the two reactions are coupled. • 3. To obtain the coupled reaction, one of the reactions may need to be reversed so that “X” cancels. • In order to write the coupled reaction that is spontaneous left to right, one or both reactions must be reversed so that “X” cancels and the sum for ∆G is negative: • Z+ X Y ∆G = -8. 42 k. J • B A+X ∆G = -2. 72 k. J • Z + B A + Y ∆G = -11. 14 k. J

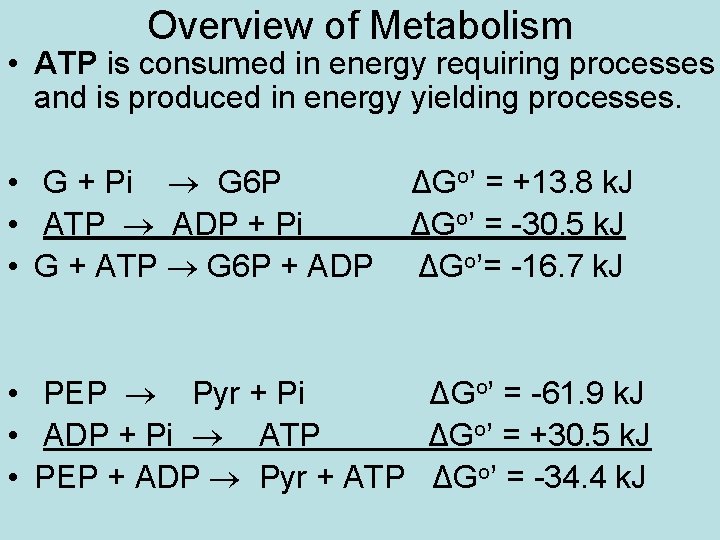
Overview of Metabolism • ATP is consumed in energy requiring processes and is produced in energy yielding processes. • G + Pi G 6 P • ATP ADP + Pi • G + ATP G 6 P + ADP ΔGo’ = +13. 8 k. J ΔGo’ = -30. 5 k. J ΔGo’= -16. 7 k. J • PEP Pyr + Pi ΔGo’ = -61. 9 k. J • ADP + Pi ATP ΔGo’ = +30. 5 k. J • PEP + ADP Pyr + ATP ΔGo’ = -34. 4 k. J


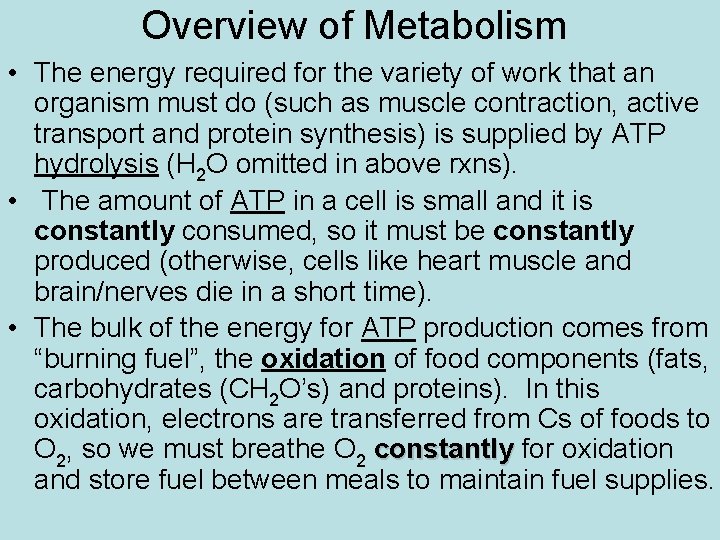
Overview of Metabolism • The energy required for the variety of work that an organism must do (such as muscle contraction, active transport and protein synthesis) is supplied by ATP hydrolysis (H 2 O omitted in above rxns). • The amount of ATP in a cell is small and it is constantly consumed, so it must be constantly produced (otherwise, cells like heart muscle and brain/nerves die in a short time). • The bulk of the energy for ATP production comes from “burning fuel”, the oxidation of food components (fats, carbohydrates (CH 2 O’s) and proteins). In this oxidation, electrons are transferred from Cs of foods to O 2, so we must breathe O 2 constantly for oxidation and store fuel between meals to maintain fuel supplies.

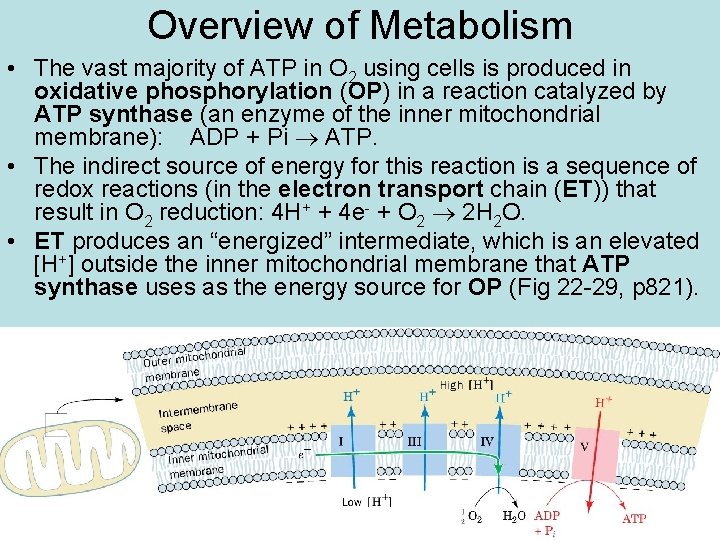
Overview of Metabolism • The vast majority of ATP in O 2 using cells is produced in oxidative phosphorylation (OP) in a reaction catalyzed by ATP synthase (an enzyme of the inner mitochondrial membrane): ADP + Pi ATP. • The indirect source of energy for this reaction is a sequence of redox reactions (in the electron transport chain (ET)) that result in O 2 reduction: 4 H+ + 4 e- + O 2 2 H 2 O. • ET produces an “energized” intermediate, which is an elevated [H+] outside the inner mitochondrial membrane that ATP synthase uses as the energy source for OP (Fig 22 -29, p 821).

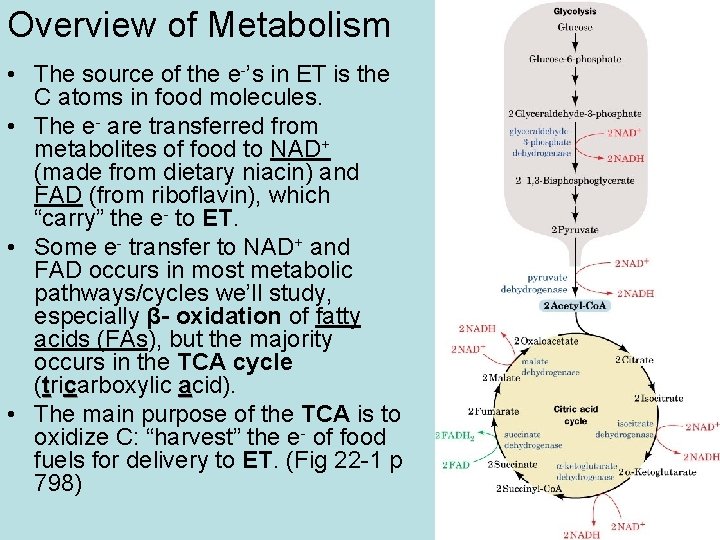
Overview of Metabolism • The source of the e-’s in ET is the C atoms in food molecules. • The e- are transferred from metabolites of food to NAD+ (made from dietary niacin) and FAD (from riboflavin), which “carry” the e- to ET. • Some e- transfer to NAD+ and FAD occurs in most metabolic pathways/cycles we’ll study, especially β- oxidation of fatty acids (FAs), but the majority occurs in the TCA cycle (tricarboxylic acid). • The main purpose of the TCA is to oxidize C: “harvest” the e- of food fuels for delivery to ET. (Fig 22 -1 p 798)

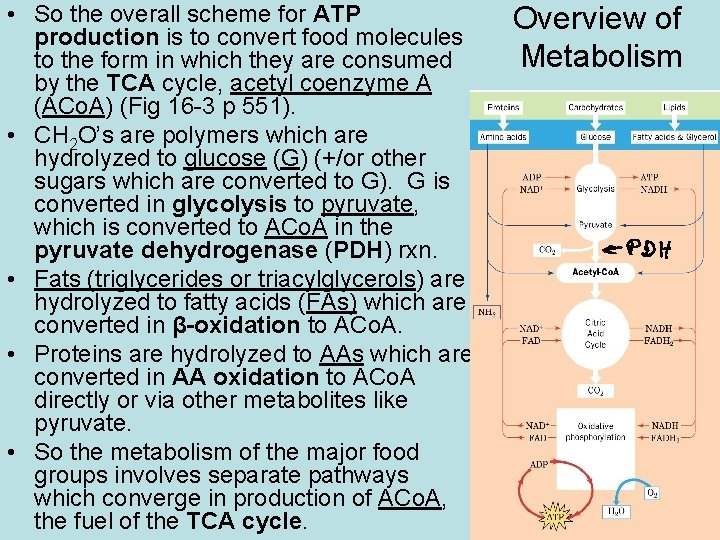
• So the overall scheme for ATP production is to convert food molecules to the form in which they are consumed by the TCA cycle, acetyl coenzyme A (ACo. A) (Fig 16 -3 p 551). • CH 2 O’s are polymers which are hydrolyzed to glucose (G) (+/or other sugars which are converted to G). G is converted in glycolysis to pyruvate, which is converted to ACo. A in the pyruvate dehydrogenase (PDH) rxn. • Fats (triglycerides or triacylglycerols) are hydrolyzed to fatty acids (FAs) which are converted in β-oxidation to ACo. A. • Proteins are hydrolyzed to AAs which are converted in AA oxidation to ACo. A directly or via other metabolites like pyruvate. • So the metabolism of the major food groups involves separate pathways which converge in production of ACo. A, the fuel of the TCA cycle. Overview of Metabolism

Overview of Metabolism • Aside from ATP production, our other main concern will be storage of fuel after a meal and its release as needed. • AAs are stored as muscle protein and are released in short term (> 8 hr) CH 2 O starvation. • FAs are stored as fat (which is the major stored fuel by far) and released continuously since they are the main fuel for most cells most of the time. • G is stored as the polymer glycogen and released in muscle cells to do muscle work and in liver for export to the blood to supply the brain its preferred fuel. • G is also produced from AAs in gluconeogenesis for export to blood in times of CH 2 O starvation. • Since the capacity to synthesize and store glycogen is limited, much of the G in a high CH 2 O meal is converted to fatty acids via glycolysis, PDH and FA synthesis.

Overview of Metabolism • The pathways and cycles must work together. • For brain to function the nerve cells must get ATP from the sequential action of glycolysis, then PDH, then TCA, then ET and OP. • Glycogen breakdown and gluconeogenesis work in concert to increase blood G starting a few hours after a meal. • Glycolysis does not convert G pyr at a high rate when gluconeogenesis is converting pyr G at a high rate. • Also, a given pathway may have many functions: glycolysis is not only involved in ATP production and feeding FA synthesis, it also feeds AA synthesis and other biosynthetic pathways and the production of TCA intermediates (as distinct from ACo. A).

Overview of Metabolism • So, how does a pathway “know” when to “stop” and when to “go”? • We will focus on 2 mechanisms: • allosteric regulation of specific enzymes by effectors that indicate the need (or lack thereof) in that cell for their activity; • and control of activity by reversible phosphorylation in response to the hormones insulin, adrenaline and glucagon. • These hormones signal the cells, especially liver cells as to the needs of the organism. • CO 2 regulation of Hb is a molecular model for both of these mechanisms.
 Ace-2 expression
Ace-2 expression Antigentest åre
Antigentest åre Relational escalation catalysts
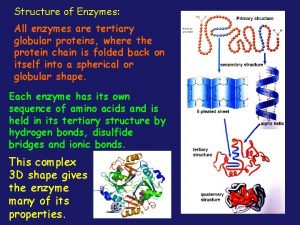
Relational escalation catalysts All enzymes are globular proteins
All enzymes are globular proteins Not all enzymes are proteins
Not all enzymes are proteins Tôn thất thuyết là ai
Tôn thất thuyết là ai Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Walmart thất bại ở nhật
Walmart thất bại ở nhật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block nhĩ thất độ 2 mobitz 2
Block nhĩ thất độ 2 mobitz 2 Tìm vết của đường thẳng
Tìm vết của đường thẳng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Thơ thất ngôn tứ tuyệt đường luật
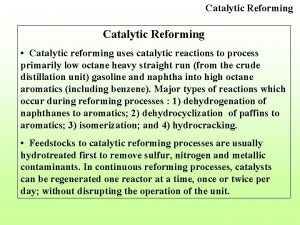
Thơ thất ngôn tứ tuyệt đường luật Catalytic cracking of octane
Catalytic cracking of octane Catalytic heater oil and gas
Catalytic heater oil and gas Site:slidetodoc.com
Site:slidetodoc.com Catalytic reforming of hexane
Catalytic reforming of hexane Catalytic cracking
Catalytic cracking Ethanol
Ethanol Chantal stieber
Chantal stieber Catalytic converter ingredients
Catalytic converter ingredients Catalytic reforming bottleneck
Catalytic reforming bottleneck Naphthenes in crude oil
Naphthenes in crude oil Catalytic converter reaction mechanism
Catalytic converter reaction mechanism Catalytic functions
Catalytic functions You can air
You can air Catalytic converter
Catalytic converter Emery oil prices
Emery oil prices Triangle of power
Triangle of power 4 macromolecules
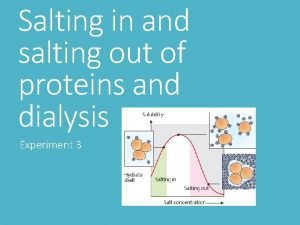
4 macromolecules Salting out proteins
Salting out proteins Translation refers to:
Translation refers to: Amphoteric proteins
Amphoteric proteins Where are proteins found
Where are proteins found Section 8-1 carbohydrates fats and proteins answer key
Section 8-1 carbohydrates fats and proteins answer key What is protein
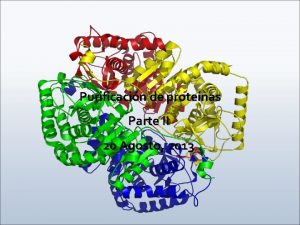
What is protein Higher order structure of proteins
Higher order structure of proteins Nuclear pores function
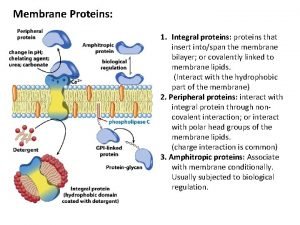
Nuclear pores function Integral proteins
Integral proteins Dna denaturation definition
Dna denaturation definition