Ensuring Plastic Additives used in Sensitive Applications are







- Slides: 26

Ensuring Plastic Additives used in Sensitive Applications are Fit for Use 2019 SPE International Polyolefins Conference David Horst, BASF Corporation Dr. Tobias Eltze, BASF SE February 27, 2019 Houston, TX 1 2019 International Polyolefins Conference

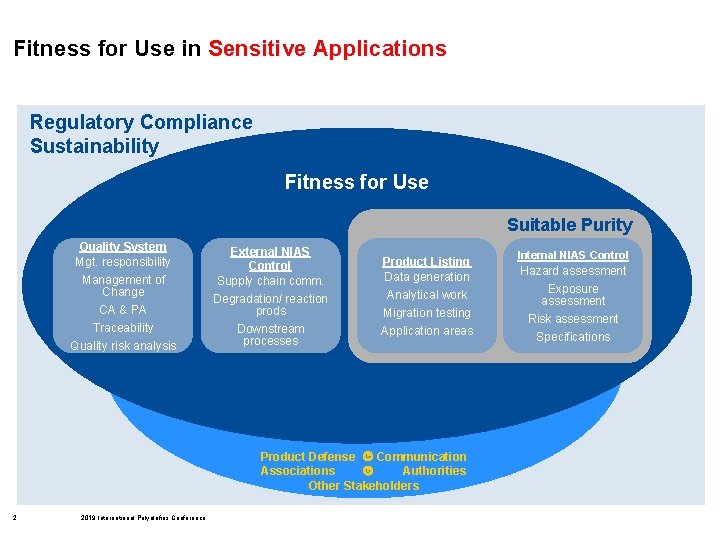
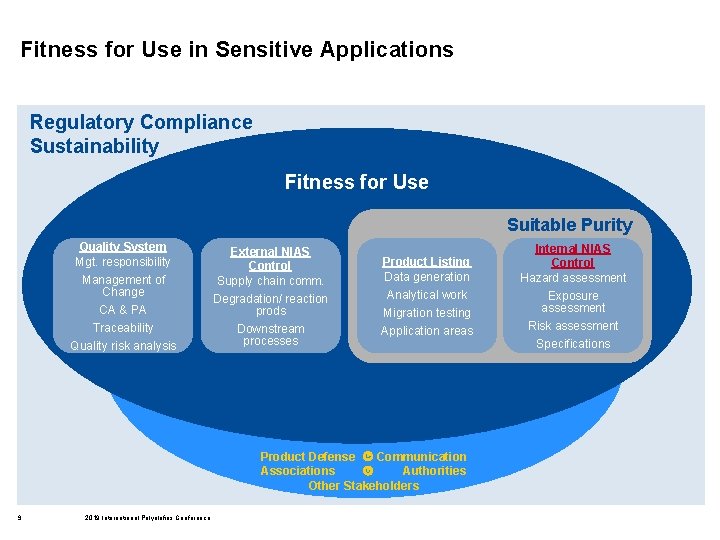
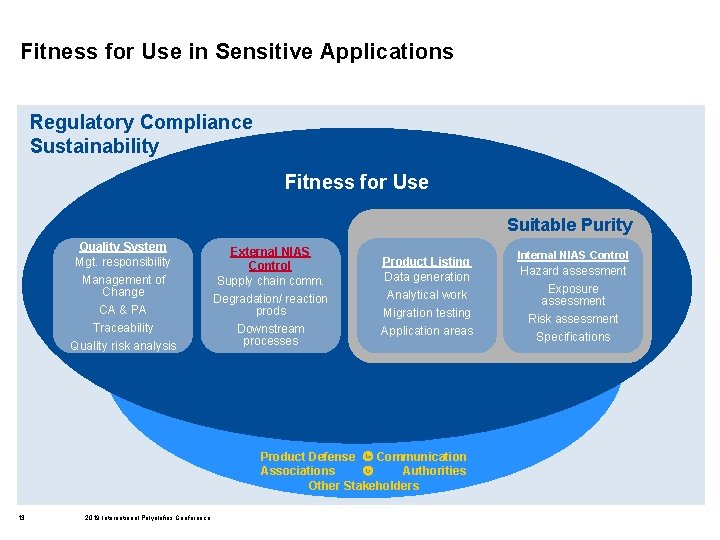
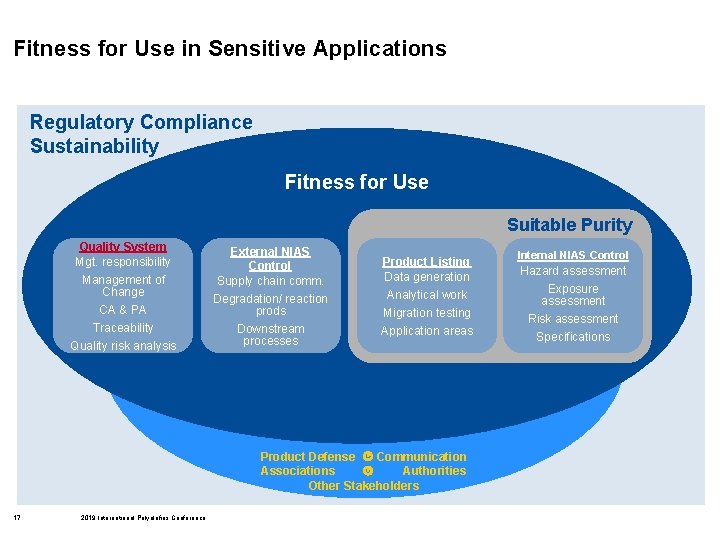
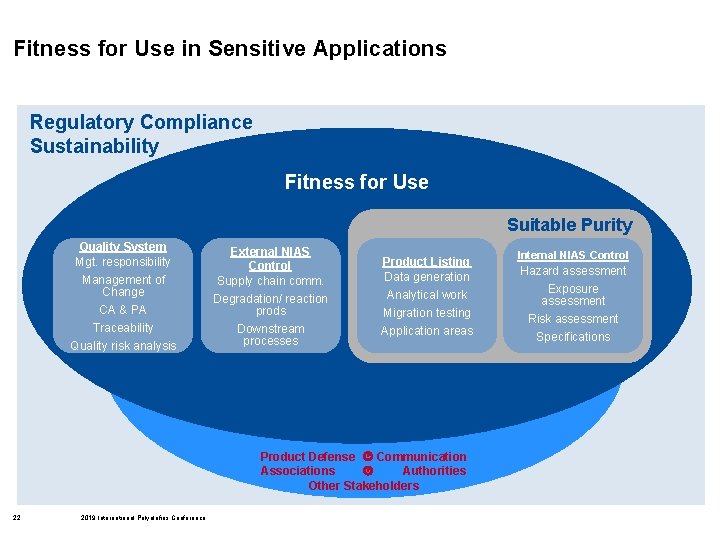
Fitness for Use in Sensitive Applications Regulatory Compliance Sustainability Fitness for Use Suitable Purity Quality System Mgt. responsibility Management of Change CA & PA Traceability Quality risk analysis External NIAS Control Supply chain comm. Degradation/ reaction prods Downstream processes Product Listing Data generation Analytical work Migration testing Application areas Product Defense Communication Associations Authorities Other Stakeholders 2 2019 International Polyolefins Conference Internal NIAS Control Hazard assessment Exposure assessment Risk assessment Specifications

Sensitive Applications for Plastic Additive Products § § § § § 2019 International Polyolefins Conference Antioxidants Process stabilizers UV absorbers Light stabilizers Clarifying agents Nucleating agents Antistatic agents Slip agents Acid neutralizers Visbreakers

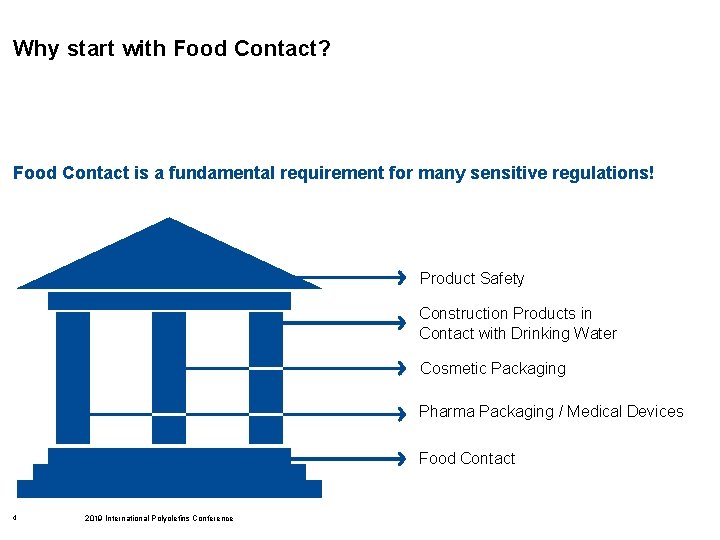
Why start with Food Contact? Food Contact is a fundamental requirement for many sensitive regulations! Product Safety Construction Products in Contact with Drinking Water Cosmetic Packaging Pharma Packaging / Medical Devices Food Contact 4 2019 International Polyolefins Conference

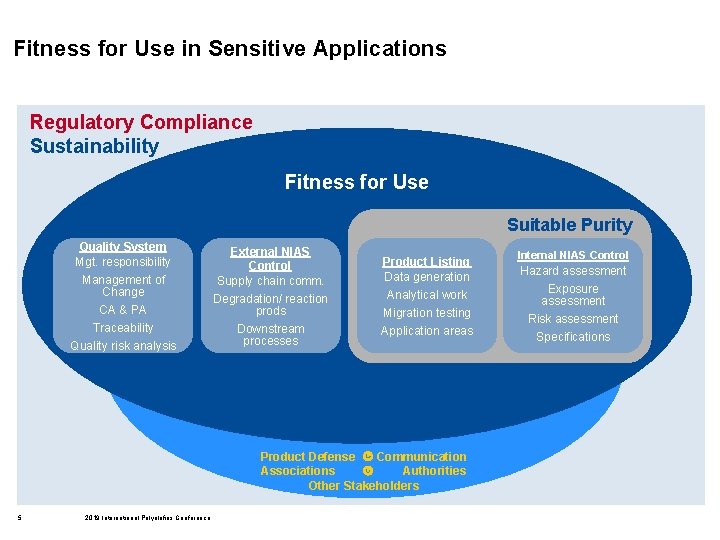
Fitness for Use in Sensitive Applications Regulatory Compliance Sustainability Fitness for Use Suitable Purity Quality System Mgt. responsibility Management of Change CA & PA Traceability Quality risk analysis External NIAS Control Supply chain comm. Degradation/ reaction prods Downstream processes Product Listing Data generation Analytical work Migration testing Application areas Product Defense Communication Associations Authorities Other Stakeholders 5 2019 International Polyolefins Conference Internal NIAS Control Hazard assessment Exposure assessment Risk assessment Specifications

Food Regulation Compliance with food contact regulations involves 5 aspects: 1. Listing of the product on the positive list – Declaration of Compliance 2. Byproducts and residuals are controlled within safe levels 3. Degradation products from normal use must be assessed 4. Adequate control of product purity (major requirement for shared facilities) 5. Specific quality management system components 2019 International Polyolefins Conference

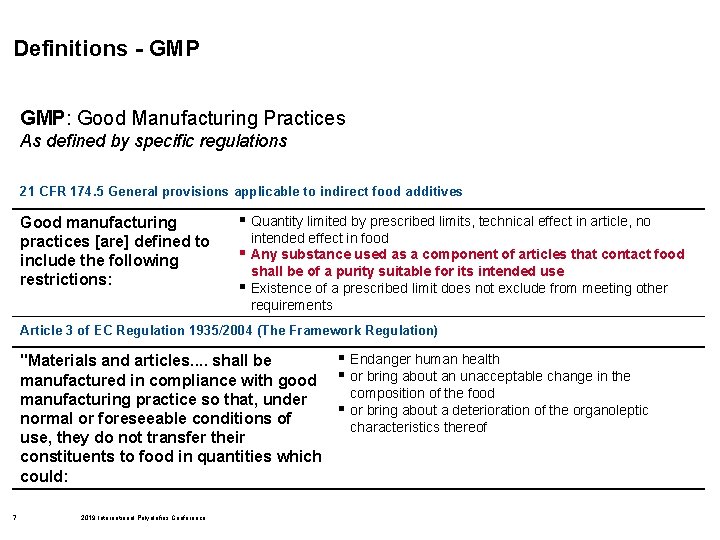
Definitions - GMP: Good Manufacturing Practices As defined by specific regulations 21 CFR 174. 5 General provisions applicable to indirect food additives Good manufacturing practices [are] defined to include the following restrictions: § Quantity limited by prescribed limits, technical effect in article, no § § intended effect in food Any substance used as a component of articles that contact food shall be of a purity suitable for its intended use Existence of a prescribed limit does not exclude from meeting other requirements Article 3 of EC Regulation 1935/2004 (The Framework Regulation) § Endanger human health "Materials and articles. . shall be manufactured in compliance with good § or bring about an unacceptable change in the composition of the food manufacturing practice so that, under § or bring about a deterioration of the organoleptic normal or foreseeable conditions of characteristics thereof use, they do not transfer their constituents to food in quantities which could: 7 2019 International Polyolefins Conference

Changes from New EU Regulations EC 2023/2007; EC 10/2011 § More explicit requirements for the quality system u Management responsibility u Training u Evaluation of starting materials u Documentation § More explicit requirements for Quality Control u Monitoring of GMP attainment u Corrective actions for failures § NIAS are defined to include impurities, reaction intermediates from the production process and reaction or degradation products § Risk assessment in accordance with internationally recognized scientific principles 8 2019 International Polyolefins Conference

Fitness for Use in Sensitive Applications Regulatory Compliance Sustainability Fitness for Use Suitable Purity Quality System Mgt. responsibility Management of Change CA & PA Traceability Quality risk analysis External NIAS Control Supply chain comm. Degradation/ reaction prods Downstream processes Product Listing Data generation Analytical work Migration testing Application areas Product Defense Communication Associations Authorities Other Stakeholders 9 2019 International Polyolefins Conference Internal NIAS Control Hazard assessment Exposure assessment Risk assessment Specifications

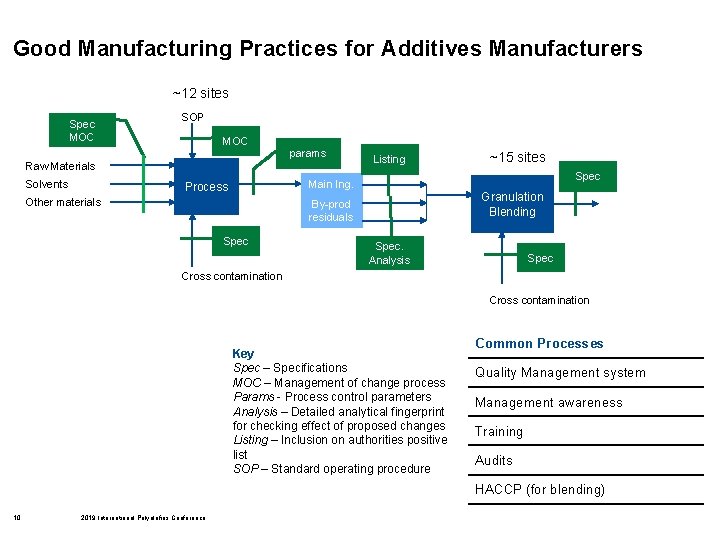
Good Manufacturing Practices for Additives Manufacturers ~12 sites Spec MOC SOP MOC params Raw Materials Solvents Listing ~15 sites Spec Main Ing. Process Other materials Granulation Blending By-prod residuals Spec. Analysis Spec Cross contamination Key Spec – Specifications MOC – Management of change process Params - Process control parameters Analysis – Detailed analytical fingerprint for checking effect of proposed changes Listing – Inclusion on authorities positive list SOP – Standard operating procedure Common Processes Quality Management system Management awareness Training Audits HACCP (for blending) 10 2019 International Polyolefins Conference

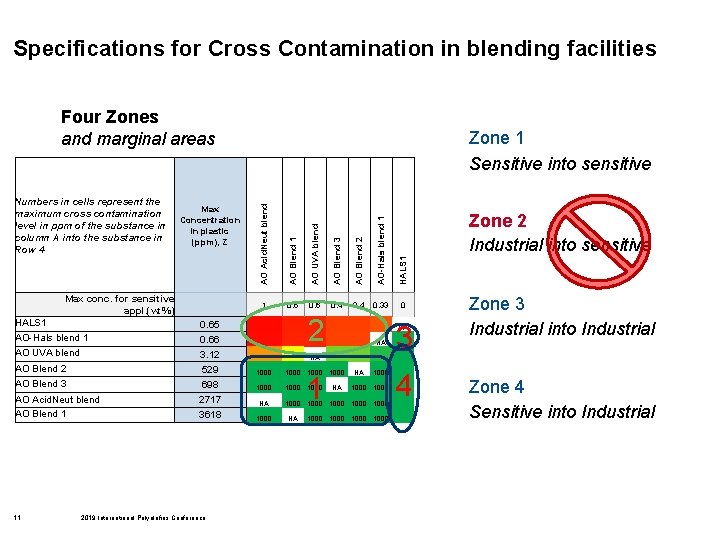
Specifications for Cross Contamination in blending facilities Four Zones and marginal areas Max Concentration in plastic (ppm), Z AO Acid. Neut blend AO Blend 1 AO UVA blend AO Blend 3 AO Blend 2 AO-Hals blend 1 HALS 1 Numbers in cells represent the maximum cross contamination level in ppm of the substance in column A into the substance in Row 4 Zone 1 Sensitive into sensitive 1 0. 6 0. 4 0. 33 0 Max conc. for sensitive appl. (wt%) 2 HALS 1 0. 65 AO-Hals blend 1 0. 66 AO UVA blend 3. 12 AO Blend 2 529 1000 AO Blend 3 698 1000 AO Acid. Neut blend AO Blend 1 2717 NA 3618 1000 11 2019 International Polyolefins Conference NA NA NA 1000 1000 NA 3 NA NA 1 Zone 2 Industrial into sensitive 1000 4 Zone 3 Industrial into Industrial Zone 4 Sensitive into Industrial

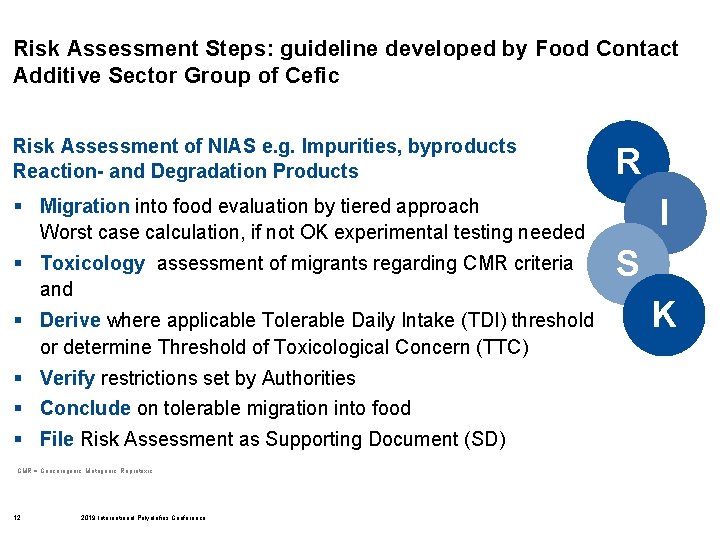
Risk Assessment Steps: guideline developed by Food Contact Additive Sector Group of Cefic Risk Assessment of NIAS e. g. Impurities, byproducts Reaction- and Degradation Products R I § Migration into food evaluation by tiered approach Worst case calculation, if not OK experimental testing needed § Toxicology assessment of migrants regarding CMR criteria and § Derive where applicable Tolerable Daily Intake (TDI) threshold or determine Threshold of Toxicological Concern (TTC) § Verify restrictions set by Authorities § Conclude on tolerable migration into food § File Risk Assessment as Supporting Document (SD) CMR = Cancerogenic, Mutagenic, Reprotoxic 12 2019 International Polyolefins Conference S K

Fitness for Use in Sensitive Applications Regulatory Compliance Sustainability Fitness for Use Suitable Purity Quality System Mgt. responsibility Management of Change CA & PA Traceability Quality risk analysis External NIAS Control Supply chain comm. Degradation/ reaction prods Downstream processes Product Listing Data generation Analytical work Migration testing Application areas Product Defense Communication Associations Authorities Other Stakeholders 13 2019 International Polyolefins Conference Internal NIAS Control Hazard assessment Exposure assessment Risk assessment Specifications

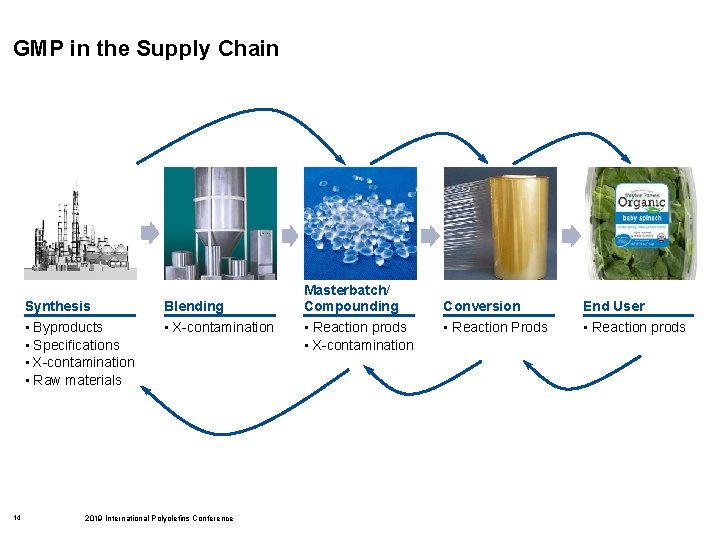
GMP in the Supply Chain 14 Synthesis Blending • Byproducts • Specifications • X-contamination • Raw materials • X-contamination 2019 International Polyolefins Conference Masterbatch/ Compounding • Reaction prods • X-contamination Conversion End User • Reaction Prods • Reaction prods

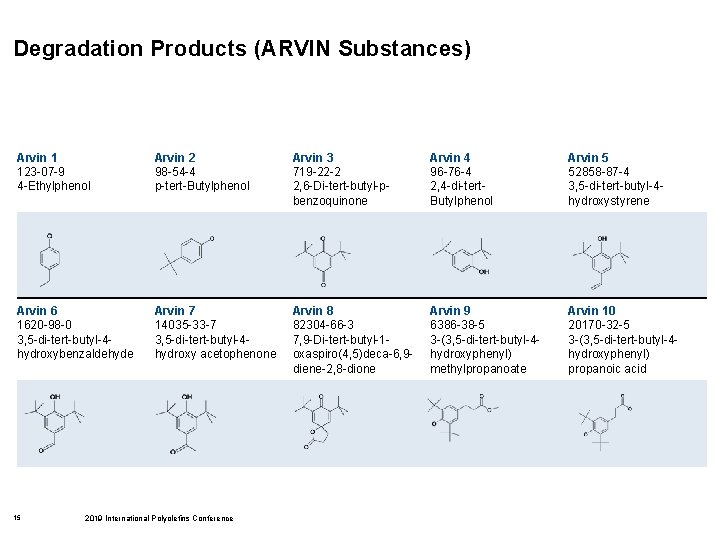
Degradation Products (ARVIN Substances) Arvin 1 123 -07 -9 4 -Ethylphenol Arvin 2 98 -54 -4 p-tert-Butylphenol Arvin 3 719 -22 -2 2, 6 -Di-tert-butyl-pbenzoquinone Arvin 4 96 -76 -4 2, 4 -di-tert. Butylphenol Arvin 5 52858 -87 -4 3, 5 -di-tert-butyl-4 hydroxystyrene Arvin 6 1620 -98 -0 3, 5 -di-tert-butyl-4 hydroxybenzaldehyde Arvin 7 14035 -33 -7 3, 5 -di-tert-butyl-4 hydroxy acetophenone Arvin 8 82304 -66 -3 7, 9 -Di-tert-butyl-1 oxaspiro(4, 5)deca-6, 9 diene-2, 8 -dione Arvin 9 6386 -38 -5 3 -(3, 5 -di-tert-butyl-4 hydroxyphenyl) methylpropanoate Arvin 10 20170 -32 -5 3 -(3, 5 -di-tert-butyl-4 hydroxyphenyl) propanoic acid 15 2019 International Polyolefins Conference

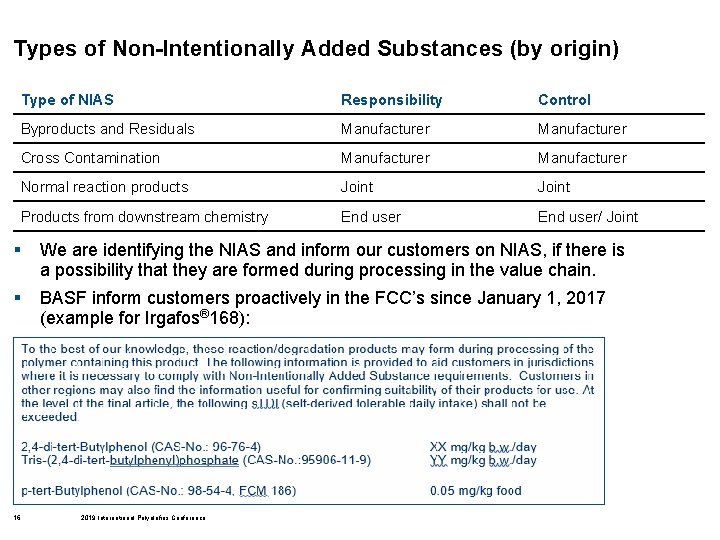
Types of Non-Intentionally Added Substances (by origin) Type of NIAS Responsibility Control Byproducts and Residuals Manufacturer Cross Contamination Manufacturer Normal reaction products Joint Products from downstream chemistry End user/ Joint § We are identifying the NIAS and inform our customers on NIAS, if there is a possibility that they are formed during processing in the value chain. § BASF inform customers proactively in the FCC’s since January 1, 2017 (example for Irgafos® 168): 16 2019 International Polyolefins Conference

Fitness for Use in Sensitive Applications Regulatory Compliance Sustainability Fitness for Use Suitable Purity Quality System Mgt. responsibility Management of Change CA & PA Traceability Quality risk analysis External NIAS Control Supply chain comm. Degradation/ reaction prods Downstream processes Product Listing Data generation Analytical work Migration testing Application areas Product Defense Communication Associations Authorities Other Stakeholders 17 2019 International Polyolefins Conference Internal NIAS Control Hazard assessment Exposure assessment Risk assessment Specifications

Challenges People Turnover? Business awareness? Capital projects coming? Regulatory mandates? 18 2019 International Polyolefins Conference Inventory targets? Business management support? Understand? Changes? Market requires?

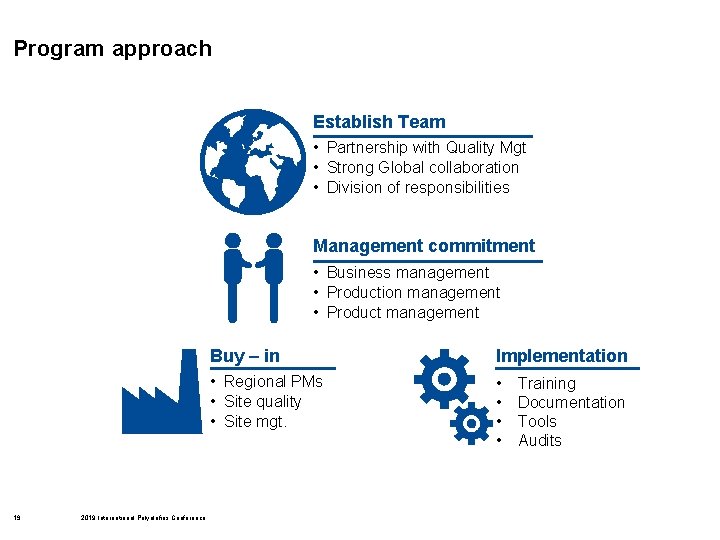
Program approach Establish Team • Partnership with Quality Mgt • Strong Global collaboration • Division of responsibilities Management commitment • Business management • Production management • Product management 19 2019 International Polyolefins Conference Buy – in Implementation • Regional PMs • Site quality • Site mgt. • • Training Documentation Tools Audits

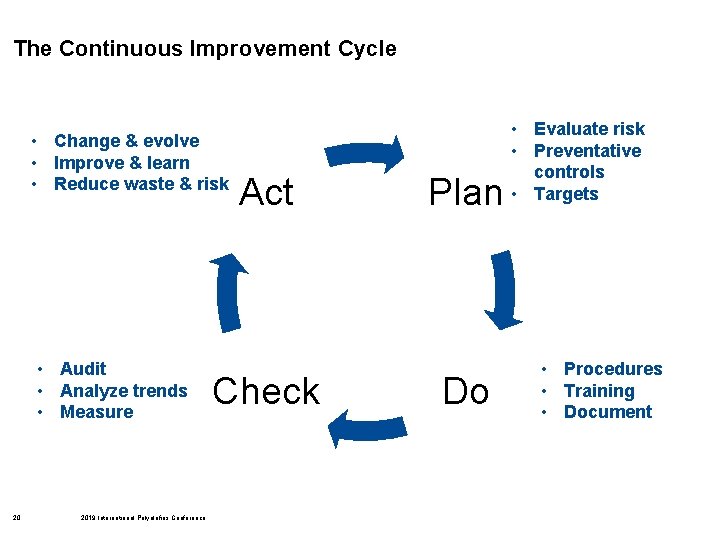
The Continuous Improvement Cycle • Change & evolve • Improve & learn • Reduce waste & risk • Audit • Analyze trends • Measure 20 2019 International Polyolefins Conference Act Check Plan Do • Evaluate risk • Preventative controls • Targets • Procedures • Training • Document

Management of Change Engagement CCC System MOC Process 21 2019 International Polyolefins Conference Training

Fitness for Use in Sensitive Applications Regulatory Compliance Sustainability Fitness for Use Suitable Purity Quality System Mgt. responsibility Management of Change CA & PA Traceability Quality risk analysis External NIAS Control Supply chain comm. Degradation/ reaction prods Downstream processes Product Listing Data generation Analytical work Migration testing Application areas Product Defense Communication Associations Authorities Other Stakeholders 22 2019 International Polyolefins Conference Internal NIAS Control Hazard assessment Exposure assessment Risk assessment Specifications

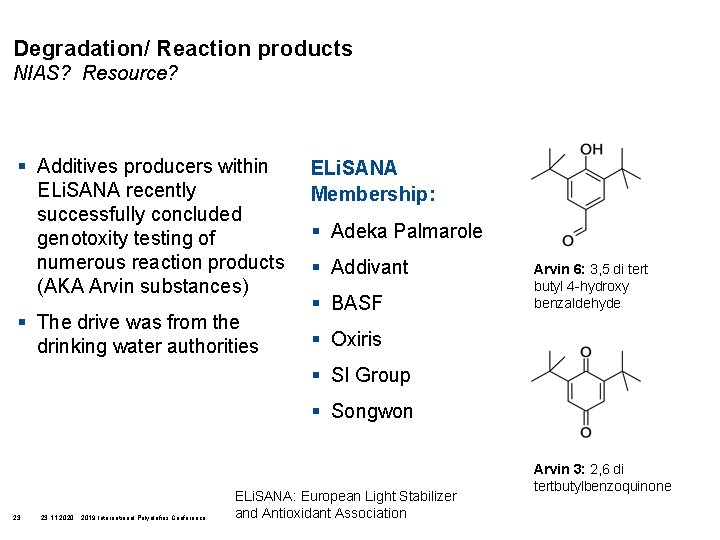
Degradation/ Reaction products NIAS? Resource? § Additives producers within ELi. SANA recently successfully concluded genotoxity testing of numerous reaction products (AKA Arvin substances) § The drive was from the drinking water authorities ELi. SANA Membership: § Adeka Palmarole § Addivant § BASF Arvin 6: 3, 5 di tert butyl 4 -hydroxy benzaldehyde § Oxiris § SI Group § Songwon 23 23. 11. 2020 2019 International Polyolefins Conference ELi. SANA: European Light Stabilizer and Antioxidant Association Arvin 3: 2, 6 di tertbutylbenzoquinone

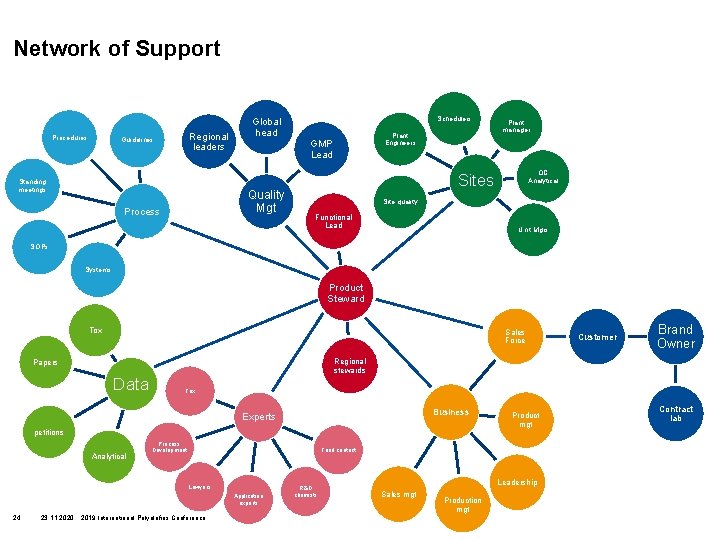
Network of Support Procedures Regional leaders Guidelines Standing meetings Plant Engineers GMP Lead Quality Mgt Process Schedulers Global head Plant manager QC Analytical Sites Site quality Functional Lead Unit Mgrs SOPs Systems Product Steward Tox Sales Force Tox Business Experts petitions Analytical Process Development Product mgt Food contact Lawyers Application experts 24 Brand Owner Regional stewards Papers Data Customer 23. 11. 2020 2019 International Polyolefins Conference R&D chemists Leadership Sales mgt Production mgt Contract lab

Summary § Assuring Fitness for Use is complex § Not just a regulatory activity § Requires u Management commitment/ awareness u Team of experts u Systems u Data § Communication in the supply chain Our Program is in Place and Working 25 23. 11. 2020 2019 International Polyolefins Conference
