Energy Flow and Geochemical Cycling in an Ecosystem

















- Slides: 54

Energy Flow and Geochemical Cycling in an Ecosystem Ecology

Critical Writing Discuss two manipulator parasites from the talk. Specifically, how did the parasite manipulate its host and why? Artemia (brine shrimp)/tapeworm Cricket/Gordian worm Catapillar/parasitic wasp Snail/trematodes Cockroach/cockroach wasp Toxoplasm/rat Turn in before the bell

Critical Writing from the “Moose is Loose” clicker case In paragraph format, create your own food web consisting of 6 to 9 organisms. Be sure to include producers, and primary and secondary consumers. From here, focus on one particular species in that food web. Create a non-feeding relationship between your species of choice and another organism from your list. Describe what possible disruptions in top-down control of productivity could adversely affect your species? Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Ecology • 1 st coined the word ecology in 1866 • Derived from greek word, oikos meaning “house” • Promoted and popularized Charles Darwin in Germany Recapitulation Theory – “Ontogeny recapitulates Phylogeny” Ernst Haeckel (1834 -1919) Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Energy Flow and Geochemical Cycling in an Ecosystem Energy flow and geochemical cycling Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

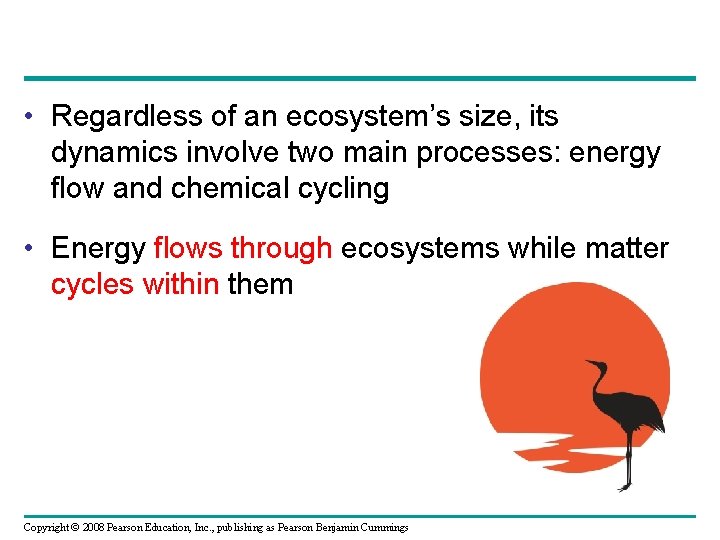
• Regardless of an ecosystem’s size, its dynamics involve two main processes: energy flow and chemical cycling • Energy flows through ecosystems while matter cycles within them Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

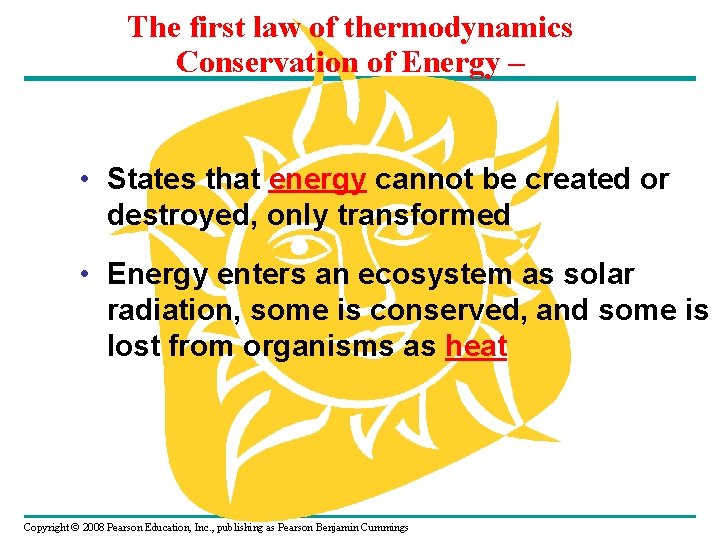
The first law of thermodynamics Conservation of Energy – • States that energy cannot be created or destroyed, only transformed • Energy enters an ecosystem as solar radiation, some is conserved, and some is lost from organisms as heat Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

• The second law of thermodynamics states that every exchange of energy increases the entropy of the universe • In an ecosystem, energy conversions are not completely efficient, and some energy is always lost as heat Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Conservation of Mass • The law of conservation of mass states that matter cannot be created or destroyed • Chemical elements are continually recycled within ecosystems Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

• In a forest ecosystem, most nutrients enter as dust or solutes in rain and are carried away in water • Ecosystems are open systems, absorbing energy and mass and releasing heat and waste products Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

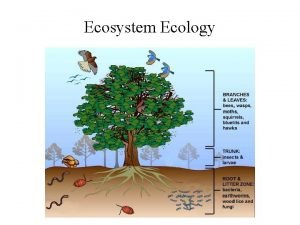
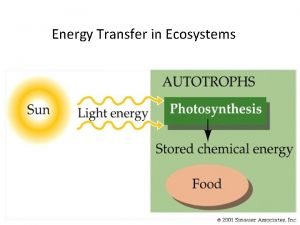
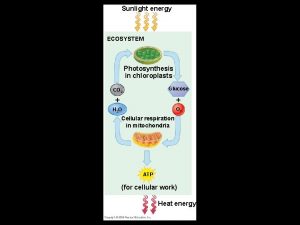
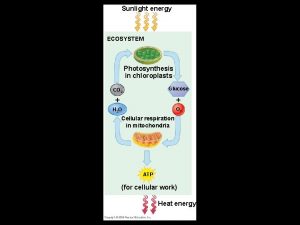
Energy, Mass, and Trophic Levels • Autotrophs build molecules themselves using photosynthesis or chemosynthesis as an energy source. • Heterotrophs depend on the biosynthetic output of other organisms Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

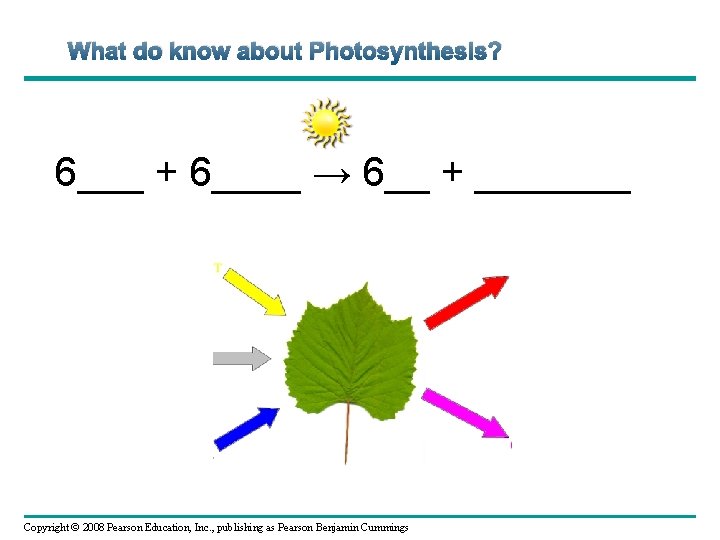
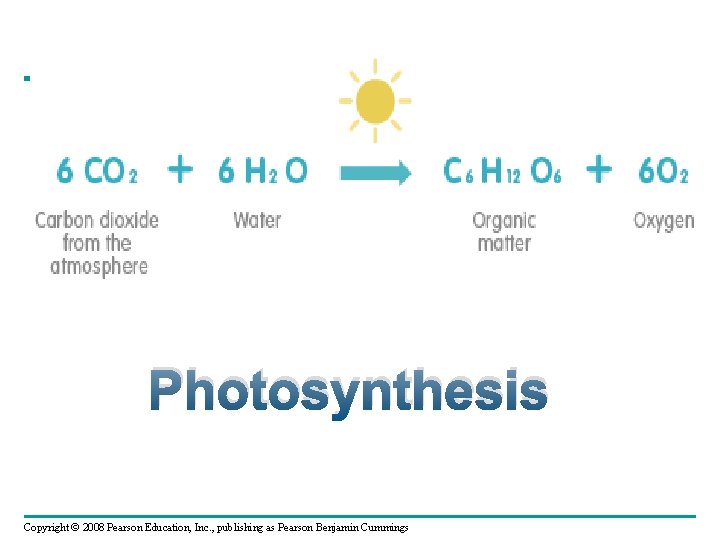
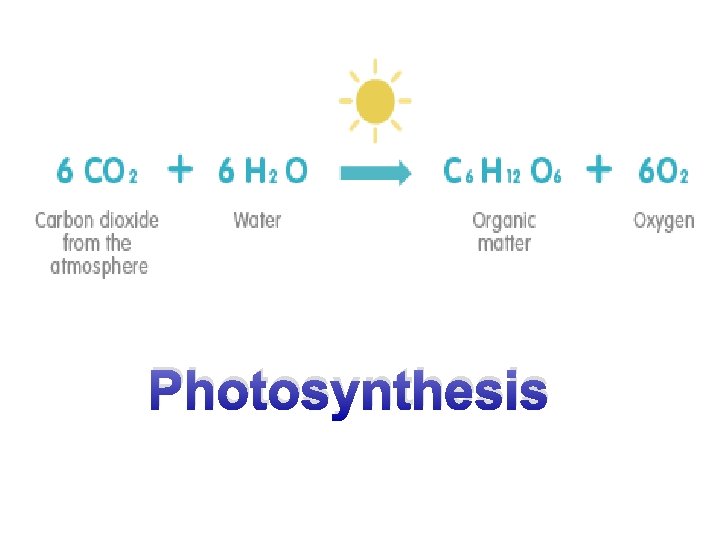
What do know about Photosynthesis? 6___ + 6____ → 6__ + _______ Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

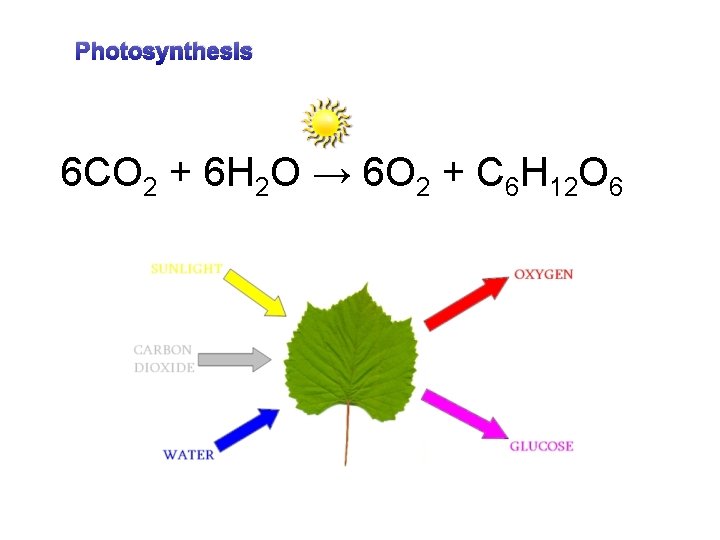
Photosynthesis 6 CO 2 + 6 H 2 O → 6 O 2 + C 6 H 12 O 6

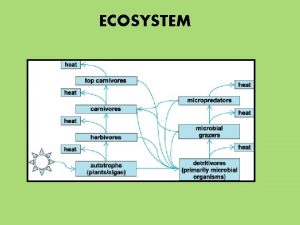
Energy and nutrients pass from primary producers (autotrophs) to primary consumers (herbivores) to secondary consumers (carnivores) to tertiary consumers (carnivores consumers that feed on other carnivores) (carnivores that feed on other carnivores) Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

• Detritivores, or decomposers, are consumers that derive their energy from detritus, nonliving organic matter • Prokaryotes and fungi are important detritivores • Decomposition connects all trophic levels Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

The Global Energy Budget • The amount of solar radiation reaching the Earth’s surface limits photosynthetic output of ecosystems • Only a small fraction of solar energy actually strikes photosynthetic organisms, and even less is of a usable wavelength Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Concept 55. 2: Energy and other limiting factors control primary production in ecosystems • Primary production in an ecosystem is the amount of light energy converted to chemical energy by autotrophs during a given time period Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

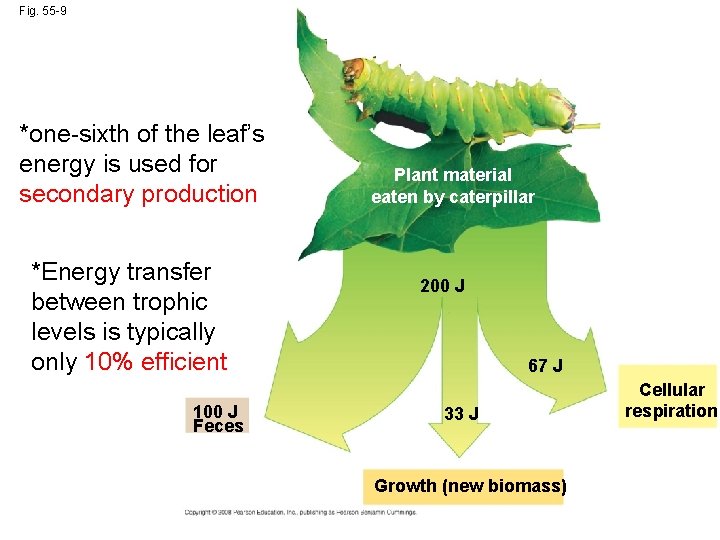
Production Efficiency • When a caterpillar feeds on a leaf, only about one-sixth of the leaf’s energy is used for secondary production • An organism’s production efficiency is the fraction of energy stored in food that is not used for respiration Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Fig. 55 -9 *one-sixth of the leaf’s energy is used for secondary production *Energy transfer between trophic levels is typically only 10% efficient 100 J Feces Plant material eaten by caterpillar 200 J 67 J 33 J Growth (new biomass) Cellular respiration

Concept 55. 3: Energy transfer between trophic levels is typically only 10% efficient • Secondary production of an ecosystem is the amount of chemical energy in food converted to new biomass during a given period of time Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

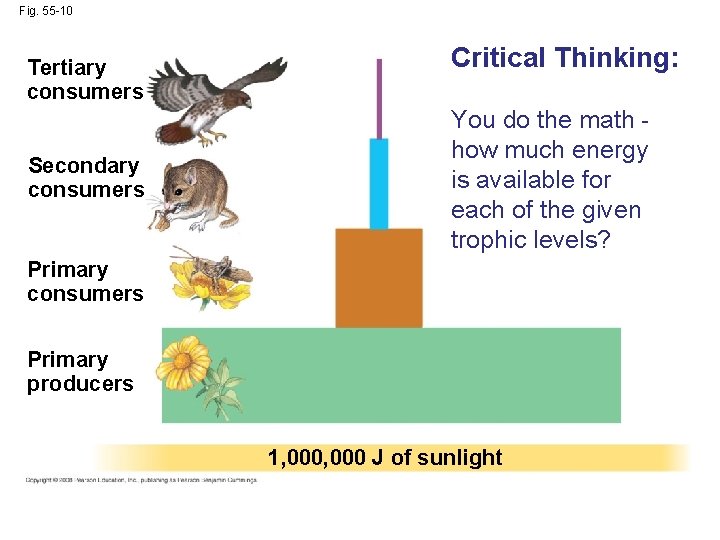
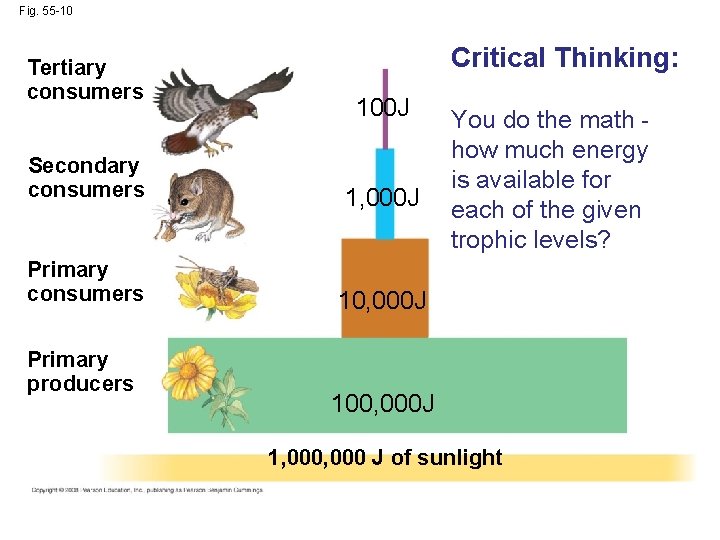
Fig. 55 -10 Tertiary consumers Secondary consumers Critical Thinking: You do the math how much energy is available for each of the given trophic levels? Primary consumers Primary producers 1, 000 J of sunlight

Fig. 55 -10 Tertiary consumers Critical Thinking: 100 J Secondary consumers 1, 000 J Primary consumers 10, 000 J Primary producers You do the math how much energy is available for each of the given trophic levels? 100, 000 J 1, 000 J of sunlight

• Approximately 0. 1% of chemical energy fixed by photosynthesis reaches a tertiary consumer • A pyramid of net production represents the loss of energy with each transfer in a food chain Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings ! ! ! s at c b Bo o G

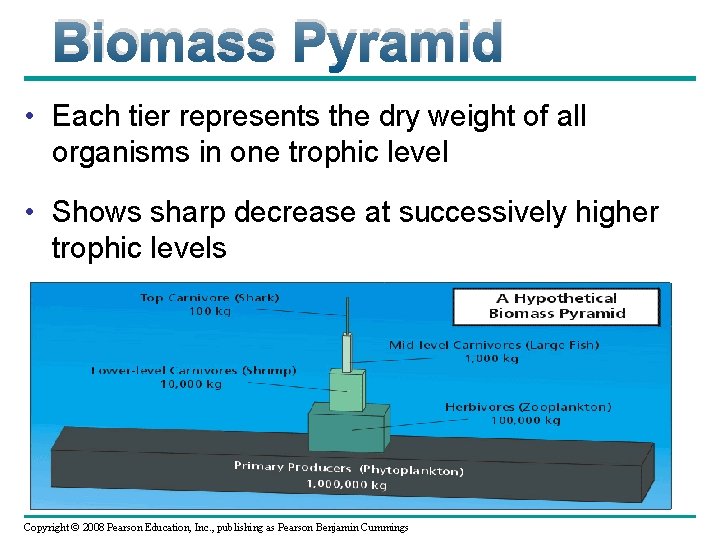
Biomass Pyramid • Each tier represents the dry weight of all organisms in one trophic level • Shows sharp decrease at successively higher trophic levels Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Concept 55. 4: Biological and geochemical processes cycle nutrients between organic and inorganic parts of an ecosystem • Life depends on recycling chemical elements • Nutrient circuits in ecosystems involve biotic and abiotic components and are often called biogeochemical cycles Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Biogeochemical Cycles • Gaseous carbon, oxygen, sulfur, and nitrogen occur in the atmosphere and cycle globally • Less mobile elements such as phosphorus, potassium, and calcium cycle on a more local level Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

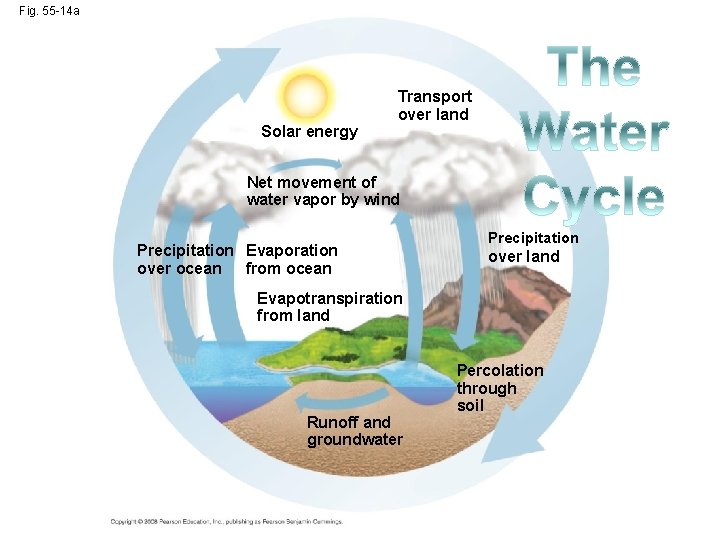
Fig. 55 -14 a Solar energy Transport over land Net movement of water vapor by wind Precipitation Evaporation over ocean from ocean Precipitation over land Evapotranspiration from land Runoff and groundwater Percolation through soil

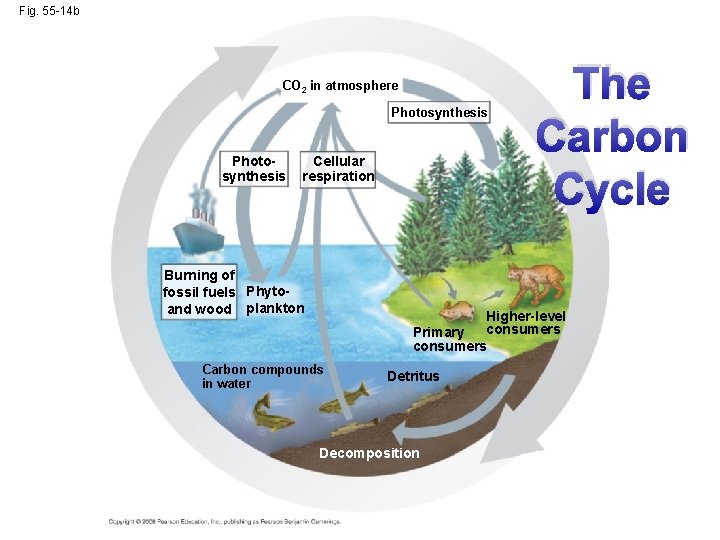
The Carbon Cycle • Carbon-based organic molecules are essential to all organisms • Carbon reservoirs include fossil fuels, soils and sediments, solutes in oceans, plant and animal biomass, and the atmosphere • CO 2 is taken up and released through photosynthesis and respiration; additionally, volcanoes and the burning of fossil fuels contribute CO 2 to the atmosphere Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Photosynthesis Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

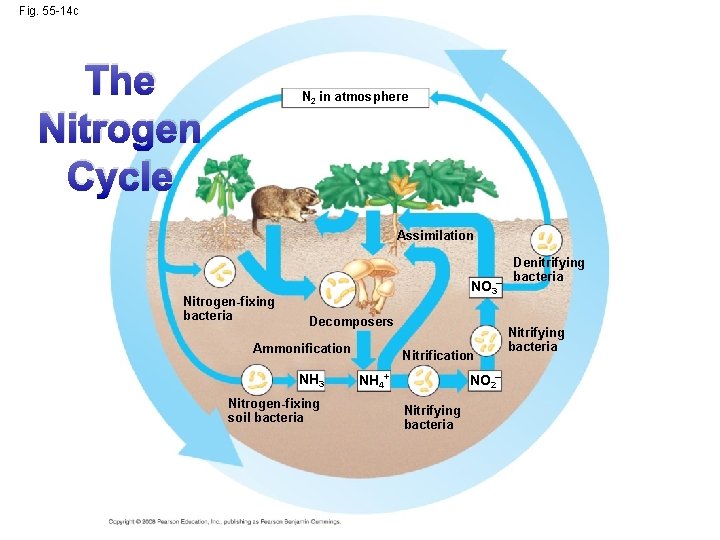
Fig. 55 -14 c The Nitrogen Cycle N 2 in atmosphere Assimilation Nitrogen-fixing bacteria NO 3– Decomposers Ammonification NH 3 Nitrogen-fixing soil bacteria Nitrification NO 2– NH 4+ Nitrifying bacteria Denitrifying bacteria Nitrifying bacteria

The Terrestrial Nitrogen Cycle • The pathway in which nitrogen moves through the ecosystem • Nitrogen is a component of amino acids, proteins, nucleic acids (DNA), ATP • The main reservoir of nitrogen (N 2) is the atmosphere, 78%. N 2 gas must be converted to other forms. Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

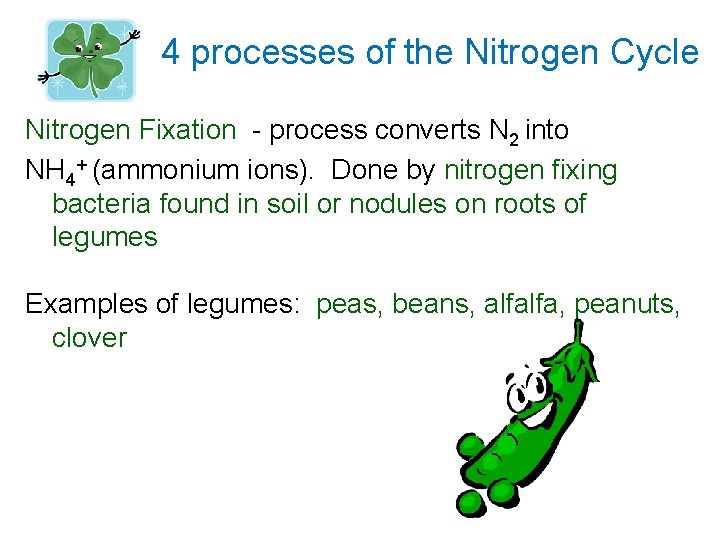
4 processes of the Nitrogen Cycle Nitrogen Fixation - process converts N 2 into NH 4+ (ammonium ions). Done by nitrogen fixing bacteria found in soil or nodules on roots of legumes Examples of legumes: peas, beans, alfalfa, peanuts, clover

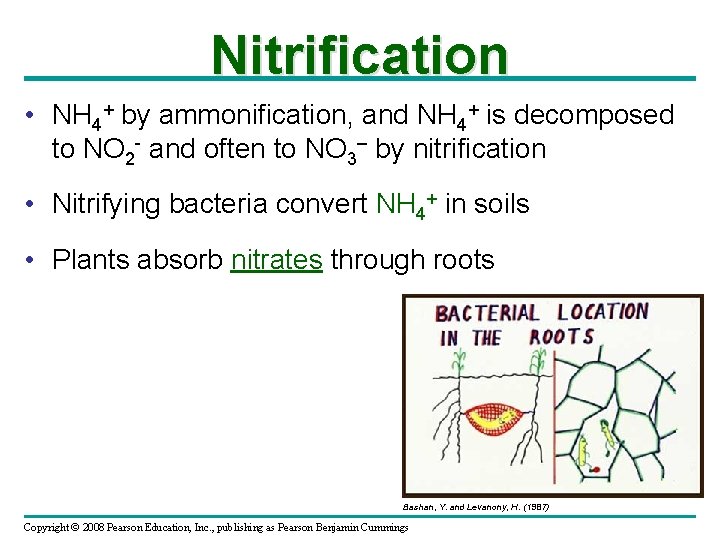
Nitrification • NH 4+ by ammonification, and NH 4+ is decomposed to NO 2 - and often to NO 3– by nitrification • Nitrifying bacteria convert NH 4+ in soils • Plants absorb nitrates through roots Bashan, Y. and Levanony, H. (1987) Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

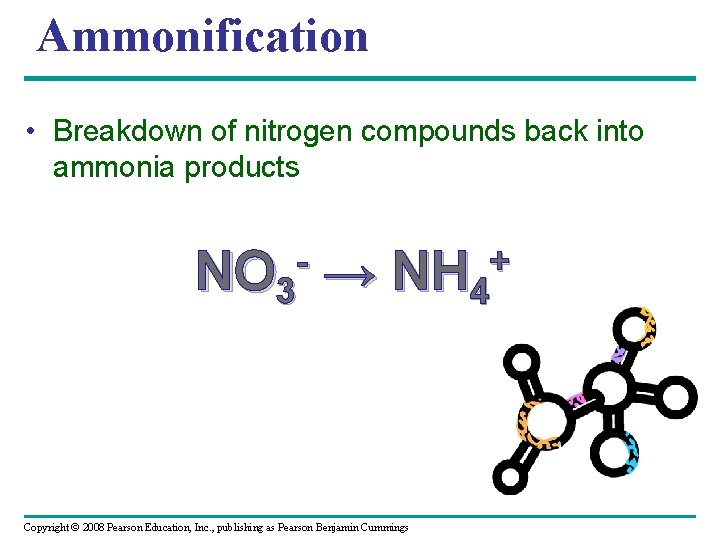
Ammonification • Breakdown of nitrogen compounds back into ammonia products NO 3 → + NH 4 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

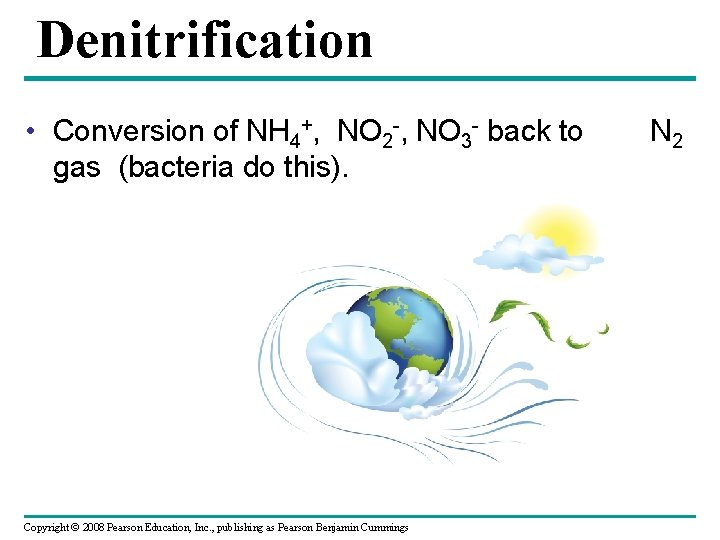
Denitrification • Conversion of NH 4+, NO 2 -, NO 3 - back to gas (bacteria do this). Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings N 2

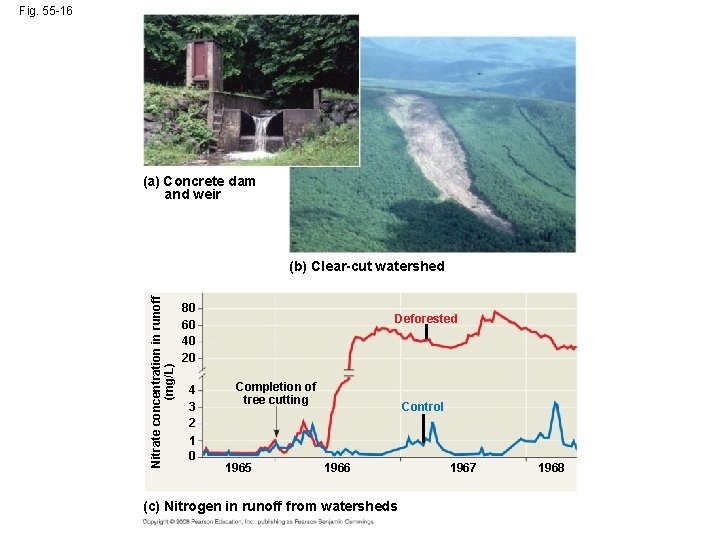
Fig. 55 -16 (a) Concrete dam and weir Nitrate concentration in runoff (mg/L) (b) Clear-cut watershed 80 60 40 20 4 3 2 1 0 Deforested Completion of tree cutting 1965 Control 1966 (c) Nitrogen in runoff from watersheds 1967 1968

Fig. 55 -14 b CO 2 in atmosphere Photosynthesis Cellular respiration Burning of fossil fuels Phytoand wood plankton The Carbon Cycle Higher-level consumers Primary consumers Carbon compounds in water Detritus Decomposition

Photosynthesis



Toxins in the Environment • Humans release many toxic chemicals, including synthetics previously unknown to nature • In some cases, harmful substances persist for long periods in an ecosystem • One reason toxins are harmful is that they become more concentrated in successive trophic levels • Biological magnification concentrates toxins at higher trophic levels, where biomass is lower Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

• PCBs and many pesticides such as DDT are subject to biological magnification in ecosystems • In the 1960 s Rachel Carson brought attention to the biomagnification of DDT in birds in her book Silent Spring Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

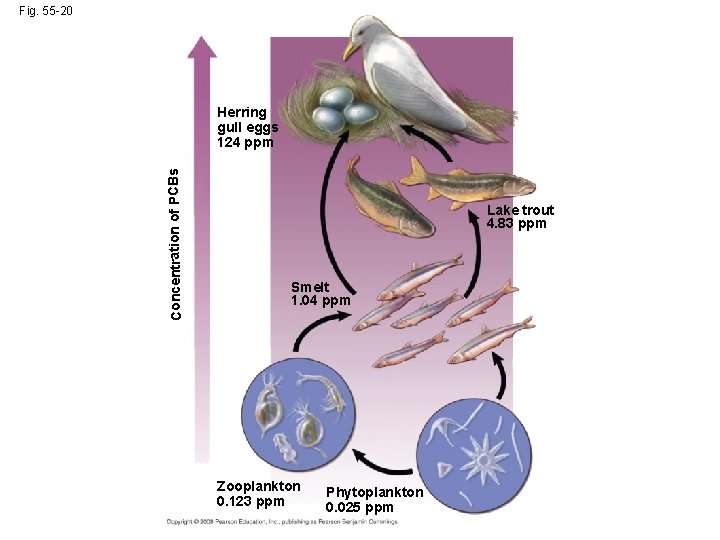
Fig. 55 -20 Concentration of PCBs Herring gull eggs 124 ppm Lake trout 4. 83 ppm Smelt 1. 04 ppm Zooplankton 0. 123 ppm Phytoplankton 0. 025 ppm

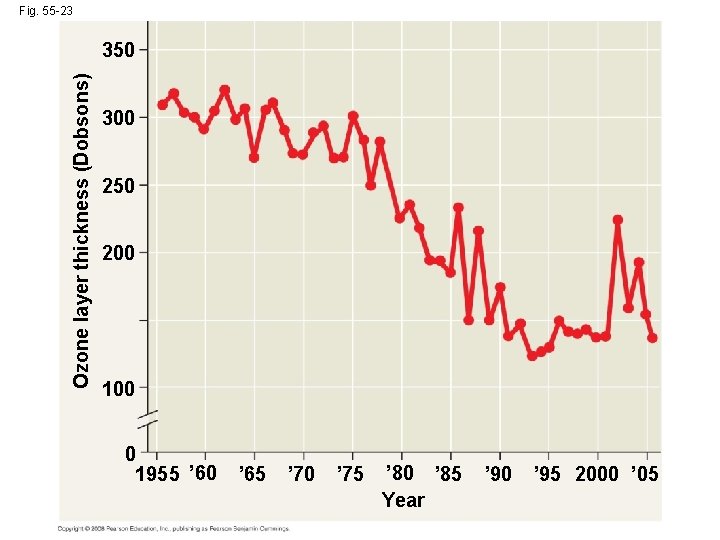
Depletion of Atmospheric Ozone • Life on Earth is protected from damaging effects of UV radiation by a protective layer of ozone molecules in the atmosphere • Satellite studies suggest that the ozone layer has been gradually thinning since 1975 Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Fig. 55 -23 Ozone layer thickness (Dobsons) 350 300 250 200 100 0 1955 ’ 60 ’ 65 ’ 70 ’ 75 ’ 80 ’ 85 Year ’ 90 ’ 95 2000 ’ 05

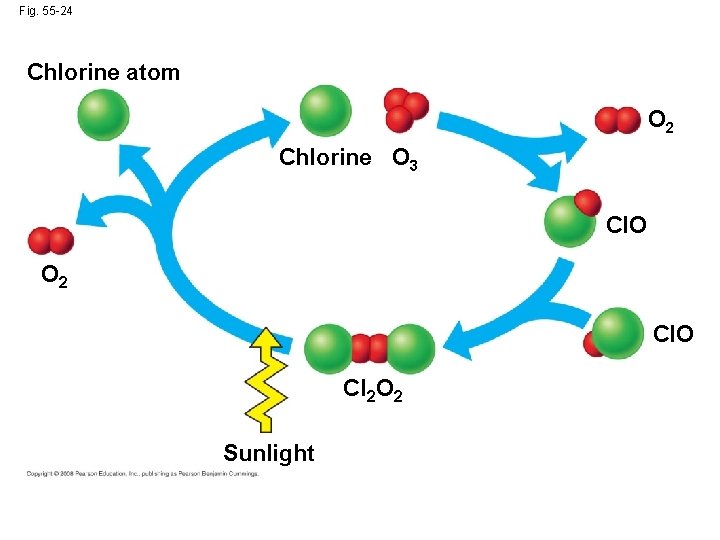
• Destruction of atmospheric ozone probably results from chlorine-releasing pollutants such as CFCs produced by human activity Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Fig. 55 -24 Chlorine atom O 2 Chlorine O 3 Cl. O O 2 Cl. O Cl 2 O 2 Sunlight

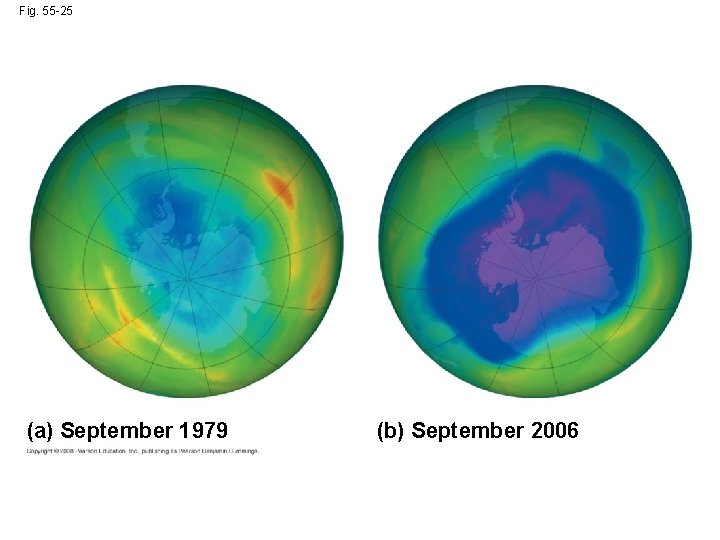
• Scientists first described an “ozone hole” over Antarctica in 1985; it has increased in size as ozone depletion has increased Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Fig. 55 -25 (a) September 1979 (b) September 2006





HOME BACK
 Energy flow and material cycling in ecosystem
Energy flow and material cycling in ecosystem Energy flow and material cycling in ecosystem
Energy flow and material cycling in ecosystem Primary and secondary geochemical dispersion
Primary and secondary geochemical dispersion Practice geochemical cycles answer key
Practice geochemical cycles answer key 4 cycles of matter
4 cycles of matter Chemical cycling in an ecosystem
Chemical cycling in an ecosystem Energy flow in cellular respiration
Energy flow in cellular respiration How does energy flow through the ecosystem
How does energy flow through the ecosystem Chapter 2 section 2 flow of energy in an ecosystem
Chapter 2 section 2 flow of energy in an ecosystem Energy flow in ecosystem
Energy flow in ecosystem Describe the flow of energy in the kelp forest ecosystem
Describe the flow of energy in the kelp forest ecosystem Energy flow through an ecosystem is ----- *
Energy flow through an ecosystem is ----- * Principles of ecology 2 flow of energy in an ecosystem
Principles of ecology 2 flow of energy in an ecosystem Regents biology food chains and energy in ecosystems
Regents biology food chains and energy in ecosystems Energy roles in an ecosystem
Energy roles in an ecosystem How does energy flow through an ecosystem
How does energy flow through an ecosystem Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Oikos meaning
Oikos meaning Carbon and nitrogen cycling in soil:
Carbon and nitrogen cycling in soil: Means using the same pattern of words to show
Means using the same pattern of words to show Mary likes hiking swimming and cycling
Mary likes hiking swimming and cycling Mary likes hiking swimming and cycling
Mary likes hiking swimming and cycling Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Biogeochemical cycling ensures that
Biogeochemical cycling ensures that Cycling swanier
Cycling swanier Section 3 cycling of matter answer key
Section 3 cycling of matter answer key Quantitative research about cycling
Quantitative research about cycling Tpn cycling
Tpn cycling Cycling event sponsorship proposal
Cycling event sponsorship proposal Road cycling 101
Road cycling 101 Buying and disposing consumer behavior
Buying and disposing consumer behavior Fixed orifice tube ac system
Fixed orifice tube ac system Cycling of materials
Cycling of materials Principles of ecology section 3 cycling of matter
Principles of ecology section 3 cycling of matter Matter cycling in ecosystems
Matter cycling in ecosystems Principles of ecology chapter 2 section 1 answer key
Principles of ecology chapter 2 section 1 answer key Principles of ecology chapter 2 section 1 answer key
Principles of ecology chapter 2 section 1 answer key Castleknock cycling club
Castleknock cycling club Cycling of matter definition biology
Cycling of matter definition biology Cycling
Cycling Guide to cycling
Guide to cycling Cycling to work
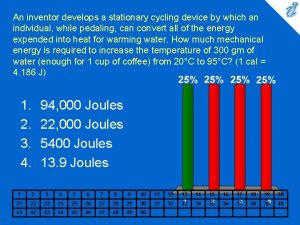
Cycling to work An inventor develops a stationary cycling device
An inventor develops a stationary cycling device Cycling seniors
Cycling seniors In 1896 arbiters of fashion a specific costume for cycling
In 1896 arbiters of fashion a specific costume for cycling What is nitrogen
What is nitrogen Wendy badger cycling
Wendy badger cycling Pan-european master plan for cycling promotion
Pan-european master plan for cycling promotion Cycling
Cycling Structure of ecosystem
Structure of ecosystem Biomass pyramid
Biomass pyramid Abiotic factors
Abiotic factors Detritus food chain
Detritus food chain Ecological pyramids
Ecological pyramids