Empirical and Molecular Formulas Composition of Ca CO




- Slides: 12

Empirical and Molecular Formulas

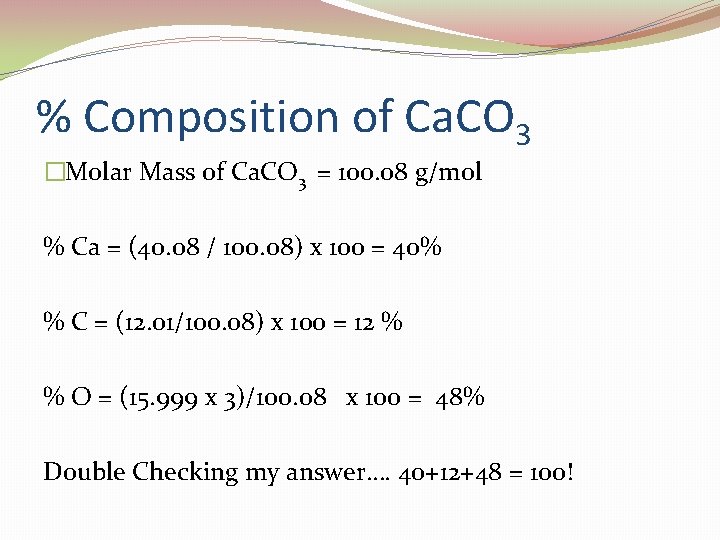
% Composition of Ca. CO 3 �Molar Mass of Ca. CO 3 = 100. 08 g/mol % Ca = (40. 08 / 100. 08) x 100 = 40% % C = (12. 01/100. 08) x 100 = 12 % % O = (15. 999 x 3)/100. 08 x 100 = 48% Double Checking my answer…. 40+12+48 = 100!

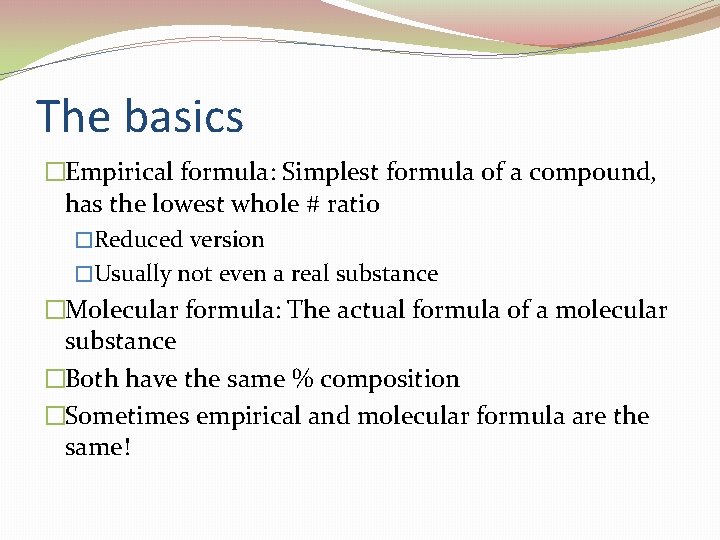
The basics �Empirical formula: Simplest formula of a compound, has the lowest whole # ratio �Reduced version �Usually not even a real substance �Molecular formula: The actual formula of a molecular substance �Both have the same % composition �Sometimes empirical and molecular formula are the same!

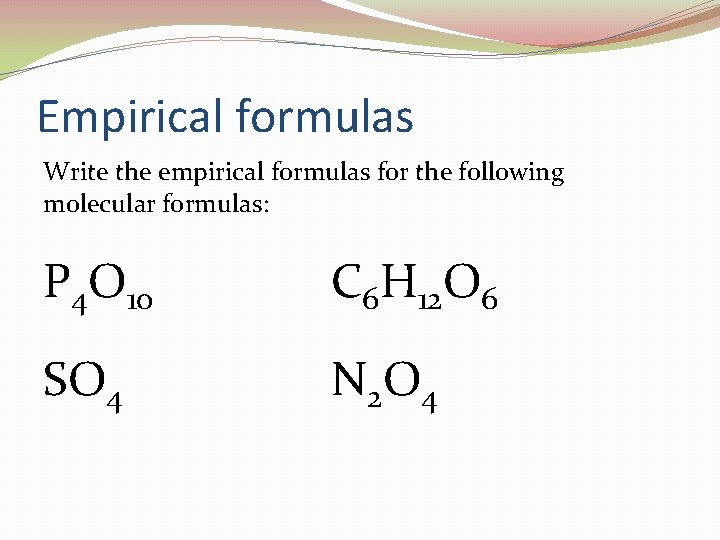
Empirical formulas Write the empirical formulas for the following molecular formulas: P 4 O 10 C 6 H 12 O 6 SO 4 N 2 O 4

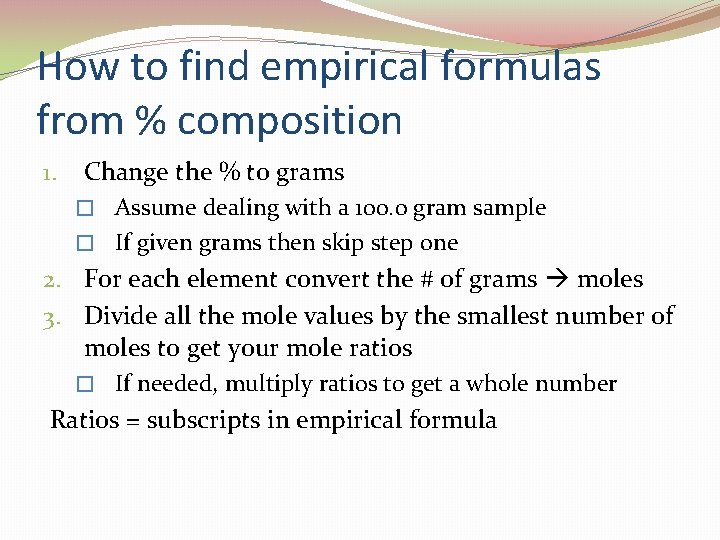
How to find empirical formulas from % composition 1. Change the % to grams � Assume dealing with a 100. 0 gram sample � If given grams then skip step one 2. For each element convert the # of grams moles 3. Divide all the mole values by the smallest number of moles to get your mole ratios � If needed, multiply ratios to get a whole number Ratios = subscripts in empirical formula

Empirical Formula Ex. 1 A compound contains 27. 29% Carbon and 72. 71% Oxygen, what is the empirical formula?

Empirical Formula Ex. 2 A compound contains 25% Potassium, 35% Manganese, and 40% Oxygen, what is the empirical formula?

Empirical Formula Ex. 3 Find the empirical formula of the compound that contains 27. 36 % Na, 1. 20 % H, 14. 30 % C, and 57. 14 % O Na. HCO 3

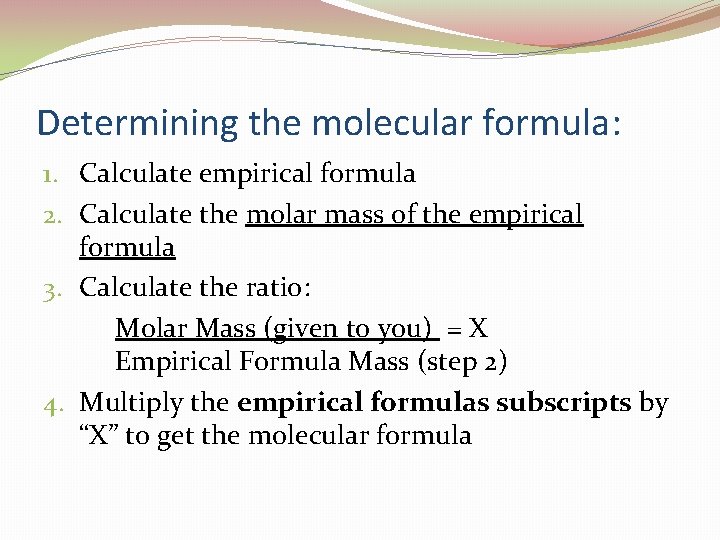
Determining the molecular formula: 1. Calculate empirical formula 2. Calculate the molar mass of the empirical formula 3. Calculate the ratio: Molar Mass (given to you) = X Empirical Formula Mass (step 2) 4. Multiply the empirical formulas subscripts by “X” to get the molecular formula

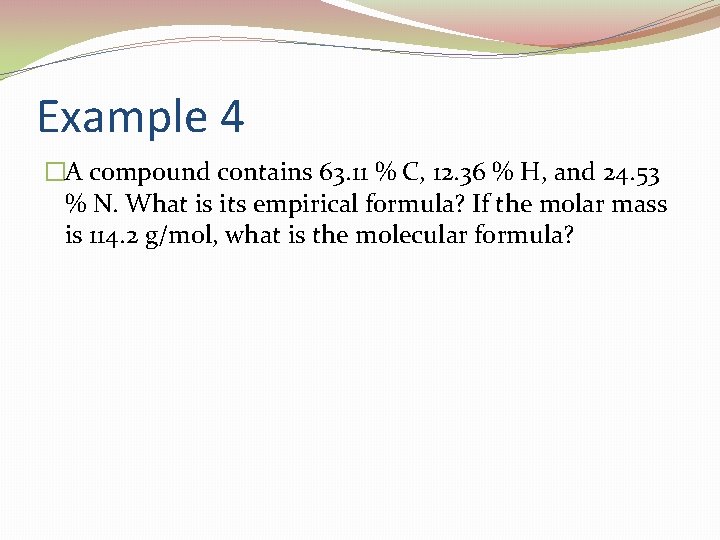
Example 4 �A compound contains 63. 11 % C, 12. 36 % H, and 24. 53 % N. What is its empirical formula? If the molar mass is 114. 2 g/mol, what is the molecular formula?

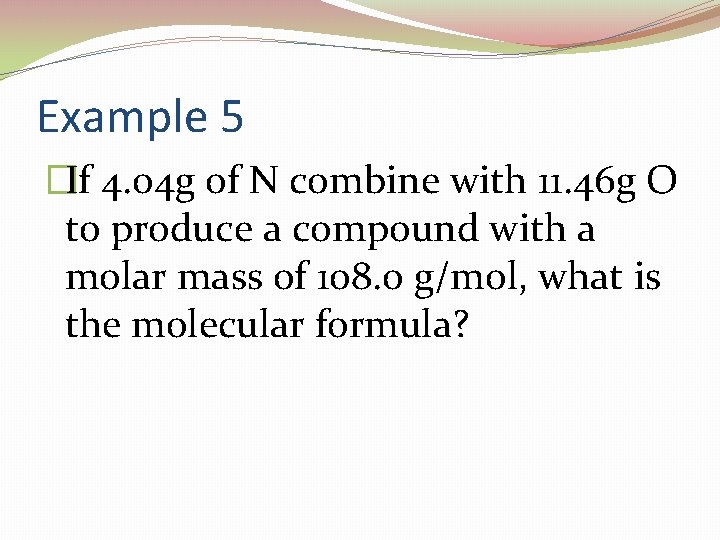
Example 5 �If 4. 04 g of N combine with 11. 46 g O to produce a compound with a molar mass of 108. 0 g/mol, what is the molecular formula?

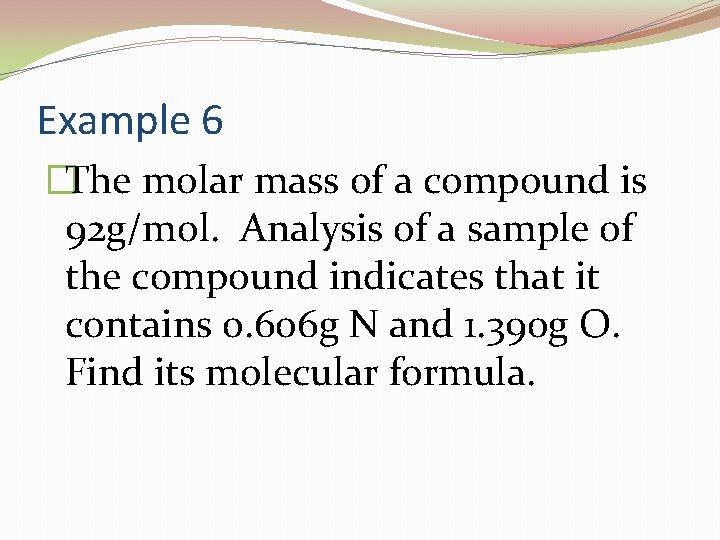
Example 6 �The molar mass of a compound is 92 g/mol. Analysis of a sample of the compound indicates that it contains 0. 606 g N and 1. 390 g O. Find its molecular formula.
 Empirical and molecular formulas worksheet
Empirical and molecular formulas worksheet Molar mass of ibuprofen
Molar mass of ibuprofen Empirical formula from percent composition
Empirical formula from percent composition Molecular formula and empirical formula
Molecular formula and empirical formula Molecular formula from empirical formula
Molecular formula from empirical formula How to find empirical formula
How to find empirical formula Empirical formula pogil
Empirical formula pogil Molar mass quiz
Molar mass quiz Empirical formula of c5h12
Empirical formula of c5h12 Empirical formula
Empirical formula Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc How to find molecular formula
How to find molecular formula Empirical formula vs molecular formula
Empirical formula vs molecular formula