Electron Configurations THE QUANTUM MECHANICAL MODEL OF THE



- Slides: 17

Electron Configurations THE QUANTUM MECHANICAL MODEL OF THE ATOM

Quantum Mechanical Model �Developed by Erwin Schrodinger �aka “Electron Cloud” model �Doesn’t define an exact path of electron; estimates probability of finding electron in a certain location �Uses atomic orbitals = a 3 -D region around nucleus that describes the electron’s probable location. Each orbital can hold a maximum of 2 electrons

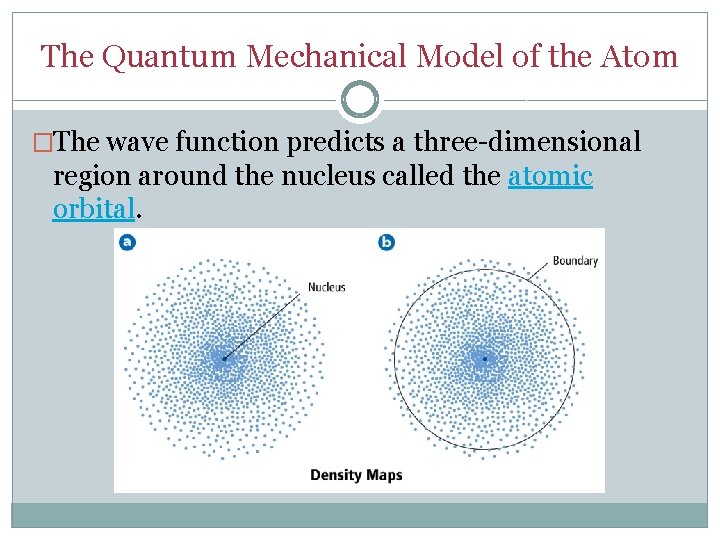
The Quantum Mechanical Model of the Atom �The wave function predicts a three-dimensional region around the nucleus called the atomic orbital.

Atomic Orbitals �Electrons cannot exist between energy levels (just like the rungs of a ladder). �Principal quantum number (n) indicates the relative size and energy of atomic orbitals. n specifies the atom’s major energy levels, called the principal energy levels.

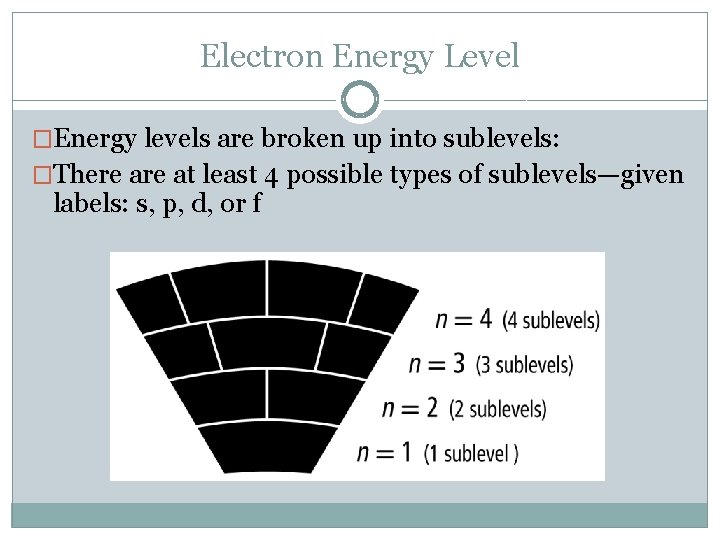
Electron Energy Level �Energy levels are broken up into sublevels: �There at least 4 possible types of sublevels—given labels: s, p, d, or f

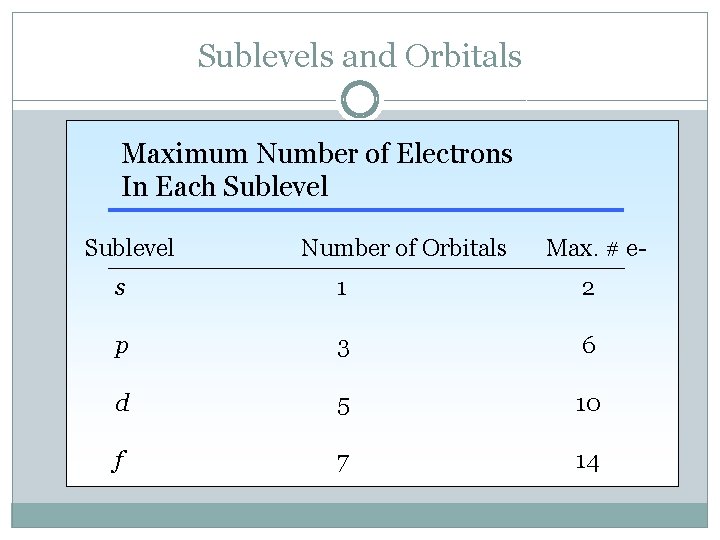
Sublevels and Orbitals Maximum Number of Electrons In Each Sublevel Number of Orbitals Max. # e- s 1 2 p 3 6 d 5 10 f 7 14

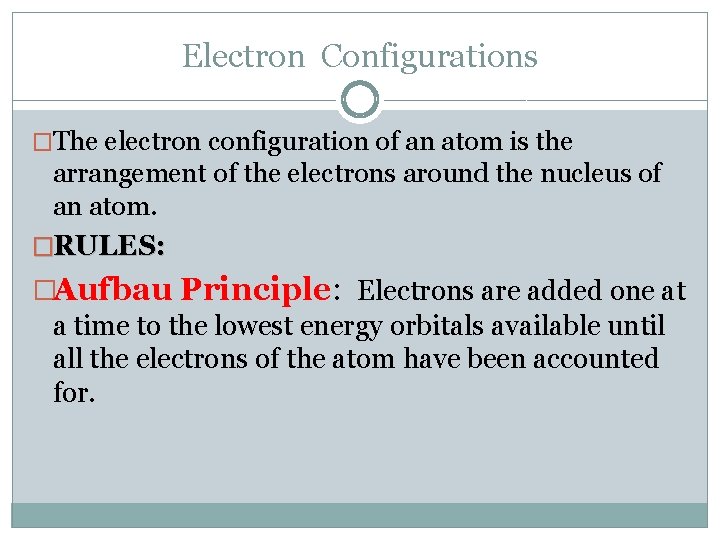
Electron Configurations �The electron configuration of an atom is the arrangement of the electrons around the nucleus of an atom. �RULES: �Aufbau Principle: Electrons are added one at a time to the lowest energy orbitals available until all the electrons of the atom have been accounted for.

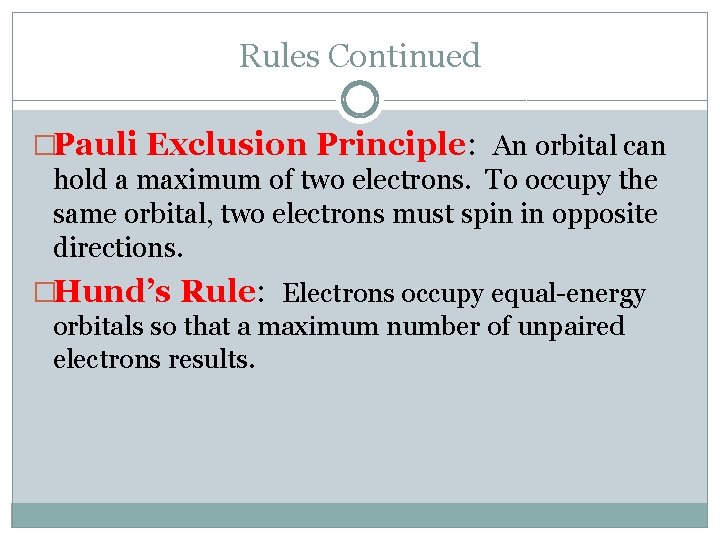
Rules Continued �Pauli Exclusion Principle: An orbital can hold a maximum of two electrons. To occupy the same orbital, two electrons must spin in opposite directions. �Hund’s Rule: Electrons occupy equal-energy orbitals so that a maximum number of unpaired electrons results.

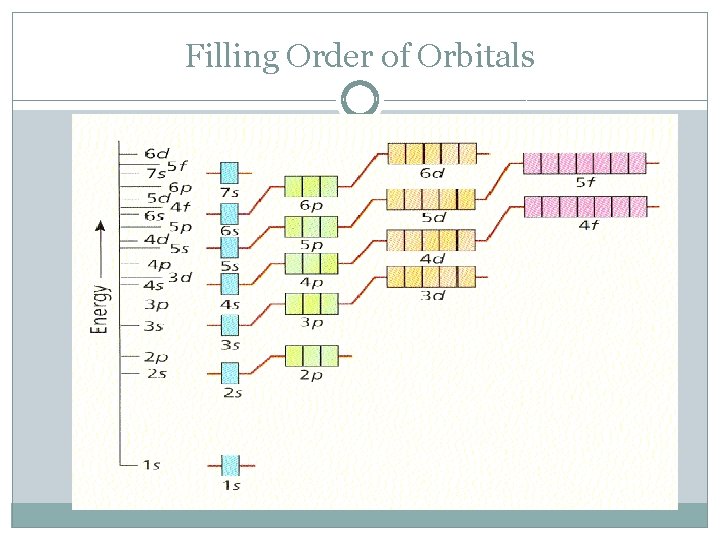
Filling Order of Orbitals

Example of Electron Configurations 1. Hydrogen 2. Lithium 3. Carbon

More Examples �Iron: �Sulfur

Valence Electrons �The electrons occupying the outermost energy levels of an atom �Located in the highest occupied s and p sublevel Maximum Number = 8 �Determined by the location of the element on the periodic table. �Determine the physical and chemical properties of the element

Finding # of Valence Electrons �Group #1 = 1 valence electrons �Group #2 = 2 valence electrons �For Groups #13 -18 Subtract 10 from the group # = # valence electrons Exception Helium only has 2

Noble Gas Stability �Noble gases are usually unreactive �This is because they have max. # valence electrons �For two atoms to join together atoms must gain, lose or share electrons �Elements with max. # of valence electrons do not easily gain or lose electrons

Practice Problems �Determine the number of valence electrons for the following elements: Sodium Chlorine Neon Magnesium Aluminum

Electron (Lewis) Dot Diagrams �Model used to display the valence electrons of an element. �Includes the symbol of the element and the valence electrons represented as dots. �Example: Oxygen

Practice Problems �Draw the electron dot diagram for the following elements. �Calcium �Arsenic