Earth Science Solid Earth General Science l How


















































- Slides: 56

Earth Science Solid Earth

General Science l How can Wind Erosion be prevented? (two ways) l Planting of cover crops keeps soil from being carried away by the wind. l A row of trees planted at the edge of a field blocks and reduces the force of wind (a windbreak)

Calculations l Why would you weigh less at the equator than at the North Pole? l The earth is not a perfect sphere. It is slightly flattened at the poles. The earth’s rotation increases this effect further. At the North Pole you are closer to the earth’s center; therefore the gravitational attraction between you and the earth is greater.

Calculations l GRAVITY l The universal force that attracts all objects to each other. Gravity holds our planet in orbit around the sun and the moon in orbit around the earth. All objects on the surface of the earth are attracted to the center of the earth.

Calculations l List the three PHASES of MATTER from least to most dense. l Gas l Liquid l Solid

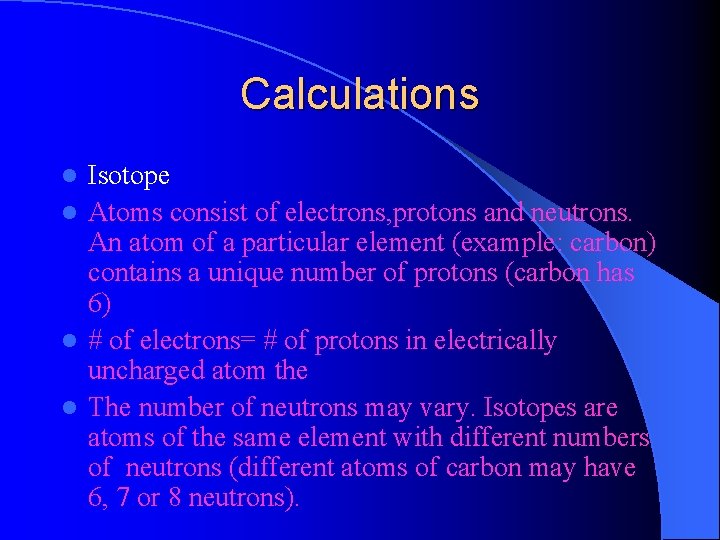
Calculations Isotope l Atoms consist of electrons, protons and neutrons. An atom of a particular element (example: carbon) contains a unique number of protons (carbon has 6) l # of electrons= # of protons in electrically uncharged atom the l The number of neutrons may vary. Isotopes are atoms of the same element with different numbers of neutrons (different atoms of carbon may have 6, 7 or 8 neutrons). l

Calculations l Density l What is the equation? l The amount of material in a given space (or volume). Density =Mass/Volume

Calculations l 1. 2. 3. 4. 5. List the five Phase Changes. Melting: solid to liquid Freezing (or Fusion): liquid to solid Boiling ( or evaporation): liquid to gas Condensation: gas to liquid Sublimation: solid to gas

Calculations Absolute Zero 1. Value in Kelvin 2. Value in Celsius The lowest possible temperature at which all atomic motion ceases. 1. 0 Kelvin 2. -273 Celsius l

Calculations l 1. 2. 3. Water: Heat of fusion Heat of vaporization Density 80 calories/gram 540 calories/gram 1 gram/milliliter ( at 4 C)

Calculations l Absolute Temperature Scale l Measures temperature in degrees Kelvin. * Starts at 0 * Freezing point of water = 273 * Boiling point of water = 373

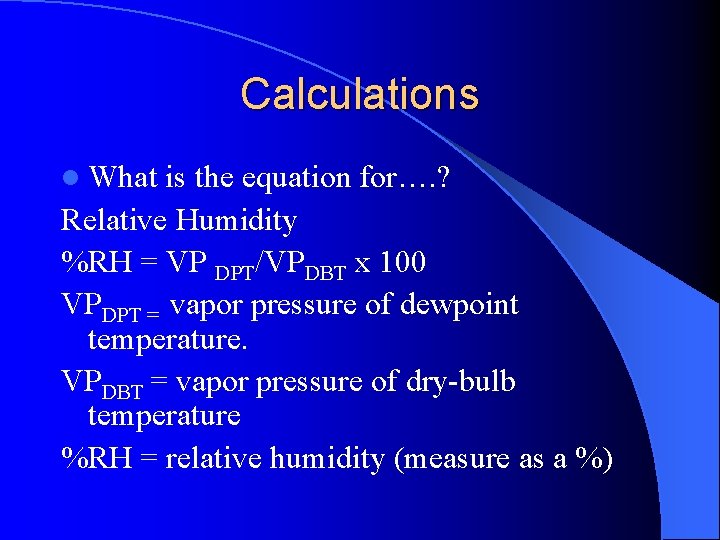
Calculations l What is the equation for…. ? Relative Humidity %RH = VP DPT/VPDBT x 100 VPDPT = vapor pressure of dewpoint temperature. VPDBT = vapor pressure of dry-bulb temperature %RH = relative humidity (measure as a %)

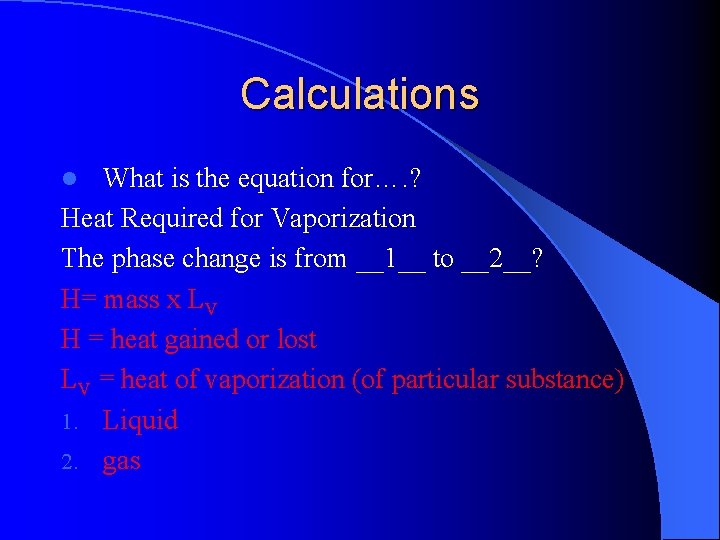
Calculations What is the equation for…. ? Heat Required for Vaporization The phase change is from __1__ to __2__? H= mass x LV H = heat gained or lost LV = heat of vaporization (of particular substance) 1. Liquid 2. gas l

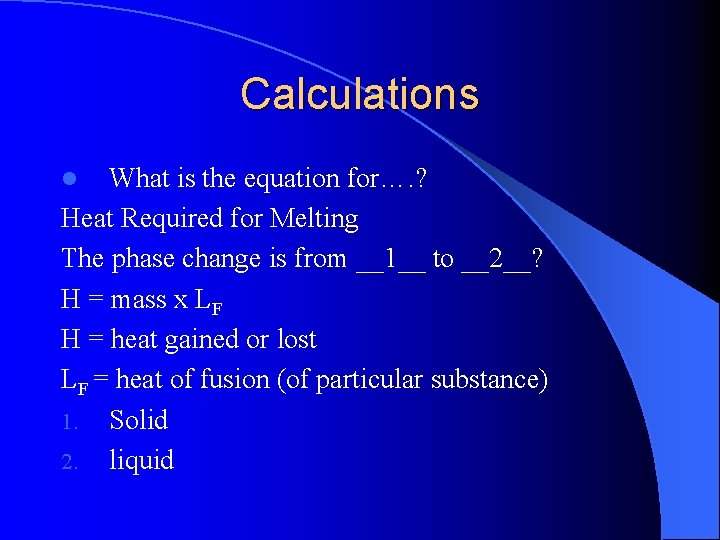
Calculations What is the equation for…. ? Heat Required for Melting The phase change is from __1__ to __2__? H = mass x LF H = heat gained or lost LF = heat of fusion (of particular substance) 1. Solid 2. liquid l

Maps 1. Conic Projection l A method of transferring the features of the earth from a sphere to a flat surface where lines of longitude are shown to converge as they do on the globe. l 2. Topographic Map l A map showing the physical features of the surface of the earth (mountains, valleys…) useful for hikers – shows the shape of the land. l

Atomic Number What are the atomic numbers of…? 1. Carbon 2. Oxygen 3. Hydrogen l

Continuing…. The number of protons in the nucleus of an atom of an element. Each element has a unique number. 1. Carbon: 6 2. Oxygen: 8 3. Hydrogen: 1 l

Maps l Prime Meridian l Located in Greenwich, England. It has a value of 0 degrees of longitude. All other meridians are measured in degrees of longitude east or west of the prime meridian.

Save our planet Denudation What is it caused by? Why is it dangerous?

Continuing…. . The loss of the layer on the earth’s surface which contains living things. (trees, soil, insects etc. ) l Erosion causes it naturally but human activities, glaciation and volcanic eruptions accelerate it unnaturally. l We upset the natural balance of nature; losing soil to grow food and tress to produce oxygen. The food chain and water cycles are interrupted.

Save our planet l Describe Four methods to Prevent Soil Erosion. l Contour plowing l Terracing l Strip-cropping l Damming of gullies

The Oceans How is the ocean heated? 2. Where is it warmer? 3. Where is it cooler? 1. By the sun. 2. It is warmer near the Equator and from the surface to a few hundred feet deep. 3. It is cooler farther from the Equator and closer to the poles of the Earth; and at depths below a few hundred feet. 1.

The Oceans Sandbar 2. Offshore Bar 1. Deposits of sand are moved by waves and currents moving parallel to the shore. 2. A sandbar not connected to the mainland. 1.

The Oceans Swells 2. Breakers 1. Waves traveling away from their source appear as rounded peaks which rise and fall with regular rhythm. 2. When a swell reaches shallow water the bottom of the wave drags along the bottom and slows. The top moves forward and eventually falls. Before the wave reaches shallow water it’s motion is circular. Waves washing up on the beach are breakers. 1.

The Oceans Drift 2. Tide 1. A shallow, wide mass of ocean water that moves more slowly than a current. 2. The rise and fall of the ocean as a whole in regular rhythm. 1.

The Oceans Polar Current 2. Polar Creep 1. A substance current originating in the polar regions. Polar water is denser because it is cold and often has high salinity. 2. The water from polar currents moves very slowly towards the equator, taking years to reach it. Hence the term “polar creep”. 1.

Weathering Mechanical Weathering: The breakdown of rock by physical means: frost, temperature changes, plant growth, condensation, animal and human activities.

Weathering Chemical Weathering: The breakdown of rock by chemical reaction with various substances: acids, water, oxygen, carbon dioxide.

Weathering Humus: Decayed plant and animal remains; a major part of soil.

Running Water Flood Plain Materials carried by the river are deposited in the valley after a flood. Eventually a large, flat area is formed. The flood plain can become a swamp if there is nowhere for flood water to drain.

Running Water Meander How are they formed? “S-shaped” curves found in a mature river. They are formed when the river flows around an obstruction rather than eroding it.

Running Water 1. 2. Alluvial Fan Levee 1. A deposit of rock formed when a river flows from high in the mountains into flatter land. Usually forms in arid areas where there is erratic rainfall and no rivers exist to carry away the sediment. 2. A low wall formed on the banks of a river; created by deposits of material collected every time overflows its bank and the flow decreases.

Winds and Glacier Continental Glacier cover large areas of land made of huge ice sheets Example: Greenland, Antarctica; northern-North America during the Pleistocene era.

Wind and Glaciers 1. 2. Horn Cirque 1. Sharp-edged, pyramid-shaped mountain peak presents in areas where there are many alpine glaciers. 2. A circular scoop high in a mountain area formed by erosion by frost; usually where alpine glaciers begin. Cirques on several sides of the mountain create the horns.

Wind & Glaciers Glacier- Ice Age How many ice ages were there? When was the most recent ice age? Glacier: a mass of slowly moving ice Ice Age: a period of time during which glaciers existed. There is evidence for four ice ages; the most recent ice age: about 10, 00 years ago.

Groundwater Water Cycle 1. Rain falls to earth; runs to the oceans and lakes 2. Water evaporates 3. Rains falls to earth (cycle continues)

The living crust Three main types of EARTHQUAKE WAVES. P-waves: primary waves S-waves: secondary waves L-waves: long (or surface) waves

The Living Crust Tsunami: Powerful and possibly destructive ocean waves produced by an earthquake.

The Living Crust Lava: Magma which has come to the surface of the earth.

The Living Crust Three types of…… Faults 1. Normal fault 2. Reverse fault 3. Thrust fault

The Living Crust What causes Earthquakes? Internal pressure causes the earth’s crust to fracture. The vibrations in the crust from this are felt as an earthquake.

The living Crust Earthquake 2. Fault 1. The crust of the earth is alive and moving all the time. The crust is under stress. When the strength of the rock is less than the stress, the crust breaks. This sudden and strong movement is called an earthquake. 2. A break in the earth’s crust where it has cracked from internal pressure. Most strong earthquakes occur along faults. There are three kinds. 1.

The Living Crust Shock Waves 1. Where do they travel? 2. What determines their speed? Vibrations which travel through the earth; produced by earthquakes. 1. Waves travel through the crust and also through the core and mantle. 2. speed depends on the rock material, density, and temperature the wave is traveling through.

Rocks Coal Organic Sedimentary Rock Remains of dead plants accumulate in swamps and are compacted along with other rocks to form coal; primarily made of carbon.

Rocks Three types of rock in the earth’s crust 1. Igneous 2. Sedimentary 3. metamorphic

Rocks How are Oil and Natural Gas formed? The remains of the plants and tiny animals over millions of years settled in sea and swamp bottoms. Heat and pressure of burial under rock layers changed the carbon in them to oil and gas.

Rocks Five common minerals in rocks: Quartz Feldspar Mica Calcite hornblende

Rocks Magma 2. Lava 1. Molten rock inside the earth, including any dissolved gases and crystals. 2. Molten rock (originally magma) that has come out to the surface of the earth; often from a volcano. Similar to magma except most of the gaseous component has escaped. 1.

Minerals Inner & Outer Core Combined thickness of two zones is approximately 2100 miles Both consist of iron and nickel Outer core-molten Inner core-solid, about 7400 F, high pressure (100 million pounds/in)

Minerals Crust Thinnest zone of earth’s lithosphereoutermost solid shell or layer of earth. 3 -40 kilometers thick; composed largely of silicate minerals The continents are the crust and are about 40 km thick. The ocean crust is the thinner part of the crust (about 3 -8 km thick).

Minerals Mantle Plastic deformable layer under the crust in the earth’s lithosphere Approximately 1800 miles thick Made of dense rock Contains iron.

Minerals Element 2. Compound 1. A substance consisting of only one type of atom. There are more than 100 elements which occur in nature. 2. A substance consisting of two or more elements chemically combined. 1.

Minerals How are minerals identified? By their chemical and physical properties. Example: * Diamonds are very hard * Water is liquid at room temperature and boils at 100 Celsius.

Minerals Physical Properties 2. Chemical Properties 1. Properties that can be seen, felt etc. 2. Properties that describe how a substance reacts with other substances. 1.

Minerals Cleavage 2. Fracture 1. Because of the weaknesses in atomic structure, some minerals break along predictable planes of weakness. These are cleavage planes. 2. Some minerals have no cleavage zones. They spilt with rough irregular surfaces. 1.

Minerals Radioactivity 2. Double Refraction 1. A physical property of a few minerals. The nucleus of element’s atoms decays to another element. 2. The ability of a crystal to split a beam of light in two. You will see two images when you look through the mineral. 1.