Dr Vikas A Thakur Asstt Professor in Chemistry

















- Slides: 42

Dr. Vikas A. Thakur Asstt. Professor in Chemistry Mahatma Phule A. S. C. College, Panvel For S. Y. B. Sc. Students

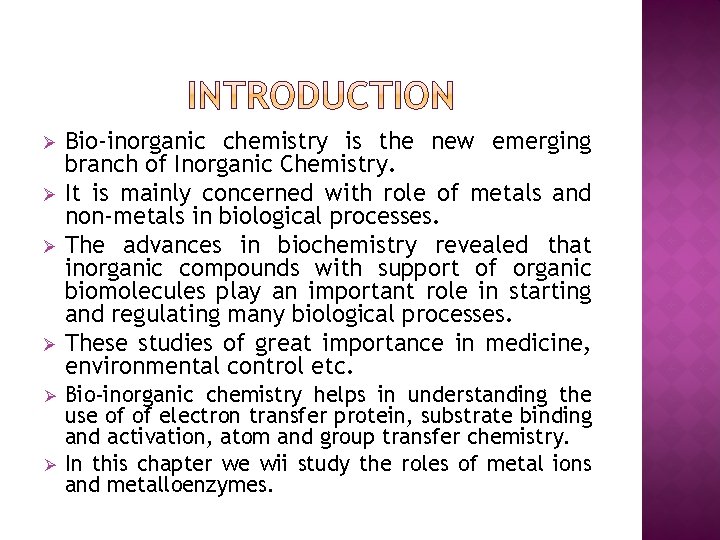
Ø Ø Ø Bio-inorganic chemistry is the new emerging branch of Inorganic Chemistry. It is mainly concerned with role of metals and non-metals in biological processes. The advances in biochemistry revealed that inorganic compounds with support of organic biomolecules play an important role in starting and regulating many biological processes. These studies of great importance in medicine, environmental control etc. Bio-inorganic chemistry helps in understanding the use of of electron transfer protein, substrate binding and activation, atom and group transfer chemistry. In this chapter we wii study the roles of metal ions and metalloenzymes.

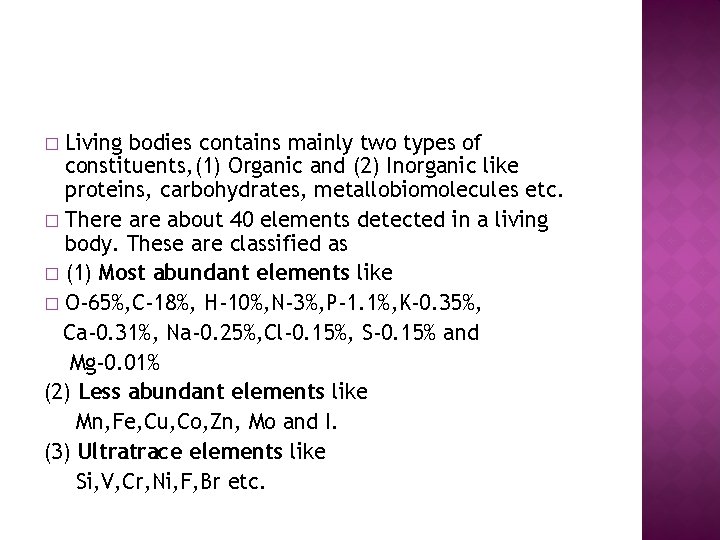
Living bodies contains mainly two types of constituents, (1) Organic and (2) Inorganic like proteins, carbohydrates, metallobiomolecules etc. � There about 40 elements detected in a living body. These are classified as � (1) Most abundant elements like � O-65%, C-18%, H-10%, N-3%, P-1. 1%, K-0. 35%, Ca-0. 31%, Na-0. 25%, Cl-0. 15%, S-0. 15% and Mg-0. 01% (2) Less abundant elements like Mn, Fe, Cu, Co, Zn, Mo and I. (3) Ultratrace elements like Si, V, Cr, Ni, F, Br etc. �

� Depending on the requirements and their role in biological processes, above elements are classified as � (1) Essential Elements : � An element said to be essential when it has a specific role indispensable for maintaining normal living state of tissue or body. � Absence or deficiency of such elements leads to serious hindrance to the normal functioning of living system. � Depending upon absolute amounts of essential elements in the body, they are further divided into � (i) Macro elements and (ii) Micro elements

� The elements that are required to be present in the diet in the amount of more than 1 mg and they constitute 60 -80% of all inorganic minerals in the body are called as macro elements. � These includes 12 elements, i, e. Carbon, hydrogen, oxygen, nitrogen, sodium, potassium, calcium, magnesium, iron, phosphorous, sulphur and chlorine. � The first four elements Carbon, hydrogen, oxygen and nitrogen present in substantial amounts in body tissue as they derived from carbohydrates, proteins and lipids. � About 85% of total oxygen and 70% total Hydrogen occur in the form of water making about 60% of total body weight.

� The elements that are needed by the body in very small amounts in micro or nanograms are called as micro elements. � Micro elements are copper, zinc, cobalt, molybdenum, manganese, iodine and fluorine. � (2) Non-essential elements: � The elements found in the living organism other than essential are considered as non-essential elements. � e, g. Bromine, boron, silicon, argon, nickel, aluminiu m, lead, tin, vanadium, titanium etc. � These are not actually non-essential but their function in the body is not yet properly known.

� Some of the elements are essential at low concentration but at high concentration they are toxic. e, g. Fe, As, Se etc. � Even sodium chloride at higher concentration is toxic � An adult healthy man weighing 70 kgm has 43 kgm water and 4. 3 kgm mineral salts. � Q. Give a brief account of essential and nonessential elements?

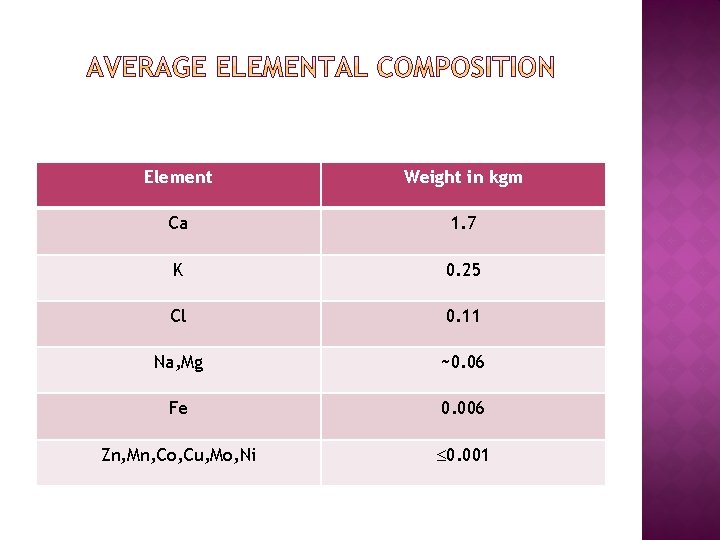
Element Weight in kgm Ca 1. 7 K 0. 25 Cl 0. 11 Na, Mg ~0. 06 Fe 0. 006 Zn, Mn, Co, Cu, Mo, Ni 0. 001

� � � � � This indicates, metal constitutes about 3% of human body weight. Our intrinsic(naturally) biological system depends very much upon metals. Metals are found as free ions or in complex form in various parts of the body and also in blood stream. Some metals present as metal chelates of very high stability. Generally, metal chelates in living systems play two important roles: 1. They act as a catalyst in many metabolic processes which involves: a. )Redox reactions with change in oxidation state of the metal in complex. b. )acid-base reactions in which metal is Lewis acid combining with substrate to accelerate the reaction. Help in storage and transfer of either metal ions or donor molecules and serve as agents for transmission of energy in animal and plant metabolism.

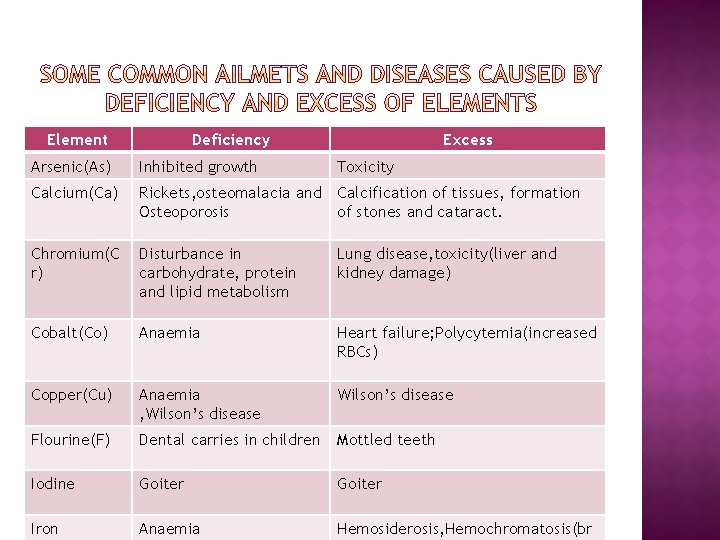
Element Deficiency Excess Arsenic(As) Inhibited growth Toxicity Calcium(Ca) Rickets, osteomalacia and Calcification of tissues, formation Osteoporosis of stones and cataract. Chromium(C r) Disturbance in carbohydrate, protein and lipid metabolism Lung disease, toxicity(liver and kidney damage) Cobalt(Co) Anaemia Heart failure; Polycytemia(increased RBCs) Copper(Cu) Anaemia , Wilson’s disease Flourine(F) Dental carries in children Mottled teeth Iodine Goiter Iron Anaemia Hemosiderosis, Hemochromatosis(br

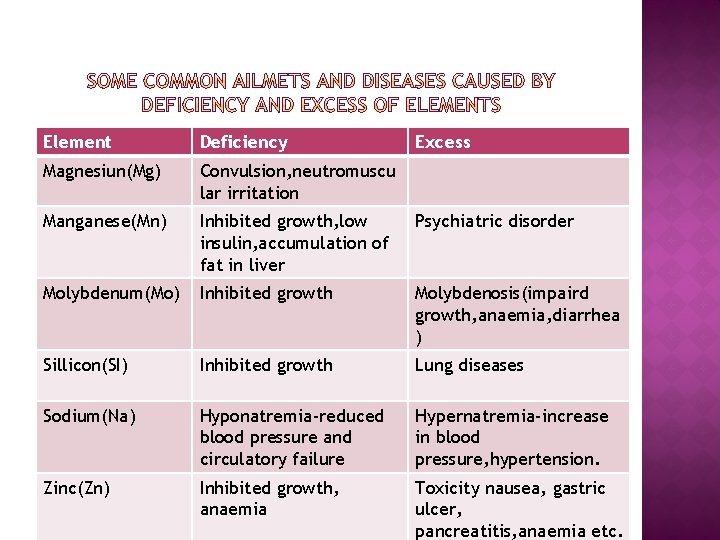
Element Deficiency Excess Magnesiun(Mg) Convulsion, neutromuscu lar irritation Manganese(Mn) Inhibited growth, low insulin, accumulation of fat in liver Psychiatric disorder Molybdenum(Mo) Inhibited growth Molybdenosis(impaird growth, anaemia, diarrhea ) Sillicon(SI) Inhibited growth Lung diseases Sodium(Na) Hyponatremia-reduced blood pressure and circulatory failure Hypernatremia-increase in blood pressure, hypertension. Zinc(Zn) Inhibited growth, anaemia Toxicity nausea, gastric ulcer, pancreatitis, anaemia etc.

� � � � � Role of Sodium and Potassium: The two alkali metals, sodium and potassium are very important and play a vital role in biological systems. These are highly mobile unipositive cations forming soluble salts. They are strongly solvated in water. Our body obtained them from plants, i. e. from fruits and vegetables. Common salt is the most important source of sodium in the diet. Potasssium is present in coffee, tea, cocoa, dried beans, molasses, green leafy vegetables, milk, fish, bananas, oranges, pineapples, potatoes etc. Daily requirement of Na. Cl is minimum 5 -10 gm and of potassium is approx. 4 gm. Our average intake is much above these values. Deficiencies are readily observed. Q. What are sources of sodium and potassium?

� � � � Sodium is a major cation in blood plasma of vertebrates i, e. outside the cells. Potassium is a major cation in cytoplasm i, e. inside the cell. Thus inside the cell and tissue, there is high concentration of K+ and low concentration of Na+ while In fluid, bathing the cells and tissues(blood plasma), there is high concentration of Na+ and low conc. of K+. The difference in Na+ and K+ conc. inside and outside the cell creates a potential difference across the cell membrane. This allows nerve fibres to conduct impulses and muscles to contract. Both the ions are used to maintain constant Osmotic pressure on either sides of cell wall.

� � � They are part of regulatory system of acid-base balance. Both ions are needed for smooth working of muscles and nervous system i, e. they maintain sensitivity of nerves and control muscles. They are charge carriers. Both ions are structure promoters for both protein and polynucleic acid. K+ is especially as enzyme activator. Thus , sodium and potassium are i) charge carriers. Ii) helps to maintain constant osmotic pressure on either side of cell wall. (iii) maintain sensitivity of nerves and control muscles. In the form of compound, Na. Cl is a source of HCl for gastric juices and bicarbonate is generally a buffer in maintaining acidbase balanceof body fluids and in transportation of CO 2. Q. What is the role of sodium and potassium in biological systems?

� � � Sodium : Reduction in fat deposit. Atrophy(waste away) of muscles and testis. Lung infection Retarded bone growth Reduction in osteoid tissue. Reduces blood pressure and causes circulatory failure. Excess sodium increases blood pressure i, e. hypertension. Potassium: Deficiency reduces heart beats Causes scarring of heart muscle, hypertrophy of kidneys and paralysis of muscles. Q. Mention some ailments caused by deficiency or excess of sodium and potassium?

� Most important transition element having a vital role in living systems. � An adult man contains 4 -5 g iron in the body. � About 75% of this is present in the erythrocytes of blood as constituent of haemoglobin. � 20% is stored as non-heme iron in ferretin and hemosoderin etc. � 3. 4% is present in myglobin of muscles. � Rest in other hemoproteins. � An adult man requires about 10 mg iron per day, menstruating women 18 mg and pregnant and lactating woman 40 mg per day.

Our body obtains iron from pulses, leafy vegetables, whole wheat, oat meals, spinach, carrots, bananas, cheese, tomatoes, potatoes, apples, orange and milk. � Non-vegetarian sources of iron are meat, liver, kidney, heart, fish, spleen and egg yolk. � Fe 3+ ions are released from food in the acid medium provided by gastric juice. � Ascorbic acid converts Fe 3+ to Fe 2+. � Iron in ferrous form is absorbed by upper duodenum in the small intestine and the stomach. � Iron is stored in ferritin in liver and bone marrow and also in spleen. � Iron containing proteins participate in two ways. Oxygen transport and electron transfer. � Q. What are sources of Iron? �

Anaemia is most prevalent disorder. � It may caused by inadequate dietary intake or some defects in absorption system. � Other reasons for anaemia are chronic blood loss, repeated pregnancy and lookwarm infection. � Anaemia is characterised by lower haemoglobin content of blood, retarded growth, loss of appetite and slugish metabolic activity. � Another disease is hemosiderosis occurs due to repeated blood transfusion over the years. � Deposition o f iron in liver, spleen, pancreas and skin leads to cirrhosis of liver, pancreate fibrosis and bronze pigmentation of skin. (Hemochromatosis) � Q. Mention some ailments caused by deficiency or excess of Iron? �

� � � � Copper is the third most biologically important transition metal after iron and zinc. Daily consumption of copper is around 2. 5 mg while required amount of adult is 2 mg per day. Our body obtains copper from nuts, dried leguments etc. Cows milk contain 0. 015 to 0. 18 mg/litre while human milk contains 1. 05 mg/litre of copper at beginning of lactation. Copper is mainly distributed in liver, kidneys and intestines. Copper is bound by albumin and histinide and is transported in liver and is transfered to ceruloplasmin. This ceruloplasmin is the major transporter of copper in the human body. What are sources of Copper?

� Transition metals like Mn, Fe, Co, Cu are constituents of metalloproteins and metalloenzymes. � Large amount of copper metalloenzymes are required to maintain high metabolic rate of heart and brain. � Copper occurs in over 30 enzymes and proteins. � It is a constituent of redox enzymes and oxygen transport pigment.

Severe deficiencies of copper causes demineralisation of bones, anaemia, fragility of arteries, hypopigmentation of skin, greying of hair, mycrocardial fibrosis and several other disorders. � Less sufficient absorbance of copper in intenstine causes Menke’’s disease. � This disease is characterise by lower conc. of copper in plasma, urine, anaemia and pigmentation. � It causes kinking of hair due to deficiency of cytochrome oxidase. It becomes fatal in 3 -5 years. � Abnormal copper metabolism give rise Wilson’s disease. � Mention some ailments caused by deficiency or excess of Copper? �

� � � � Metallobiomolecules are biomolecules that contain one or more metallic elements. They are usually natural products and complex coordination compounds. Active sites are involved in biological processes such as electron transfer, catalysis and binding of exogenous molecules. They are classified into following on the basis of their function. 1. Metalloenzyme : Enzymes are large protein molecules which can bind atleast one reactant called as substrate. They catalyse biochemically important reactions. Extremely efficient as catalysts, increasing the rate of reactions about 106 times compared to uncatalysed rate.

� Metal atom occurs at or very near to the active site and play role in the activity of enzyme. � It does not merely participate during the time of existence of enzyme-substrate but it is a permanent part of the enzyme. � Such enzyme is called as metalloenzymes. � Metals found in metalloenzymes are Ca, Mn, Fe, Cu, Zn and Mo. � The most commonly used metals are Zn, Fe and Cu. � Metal ions perform catalytic and structural roles in protein.

� � � � Metal ions have ability to accept electron and hence it act as electrophile. To some extent metal may donate electron and sometimes act as nucleophile. Hence they have tendency to form sigma bond or multiple bonds. Metal ions accepting or donating electrons can activate their electrophiles or nucleophiles and hence act as acidbase catalyst. E, g. 1. Kinases lyases enzymes have metal which activates electrophile. ii) Carbonic anhydrase where metal activate nucleophile. Sometimes enzymes accelerate the rates of chemical reactons by approximation of reactants i, e. Co-ordination sphere of metal may bring together enzyme and substrate.

� Most naturally occurring metal ions are bound by protein. � Iron is most important and most widespread transition metal in living organism � Iron containing protein participate in two main process. � 1. Oxygen transport and � 2. Electron transfer process. � Q. What are metalloenzymes? Describe biological role of metalloenzymes.

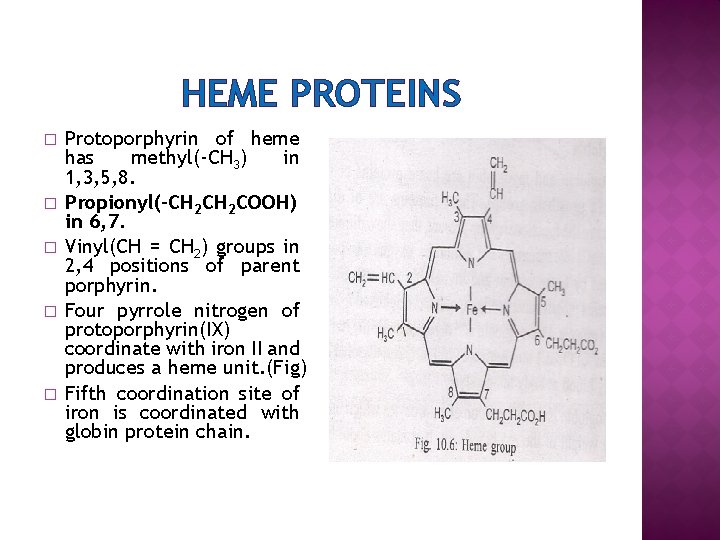
HEME PROTEINS Iron porphyrin complex is known as heme. Ø It is a prosthetic group. Ø Some protein molecule contain a nonpeptide portion which does not have amino group(NHCO-) linkage is called prosthetic group e. g. metal porphyrin complex part in haemoglobin. Ø Different substitution at eight pyrrole position on porphin ring produces protoporphyrin(IX) which is used in hemes(fig) Ø

HEME PROTEINS � � � Protoporphyrin of heme has methyl(-CH 3) in 1, 3, 5, 8. Propionyl(-CH 2 COOH) in 6, 7. Vinyl(CH = CH 2) groups in 2, 4 positions of parent porphyrin. Four pyrrole nitrogen of protoporphyrin(IX) coordinate with iron II and produces a heme unit. (Fig) Fifth coordination site of iron is coordinated with globin protein chain.

HEME PROTEINS � Important functions of heme protein are � i)Transport and storage of oxygen e. g. haemoglobin and myoglobin. � ii)Electron transport e. g. cytochrome. � Iii)Catalysis in redox reaction. e, g. catalase, cytochrome P-450 etc. � All these proteins have heme as prosthetic group but their biological functions are different. � The protein structure controls these properties. � A heme unit with globin protein chain is known as haemoglobin and myoglobin(fig) � Q. What is heme complex.

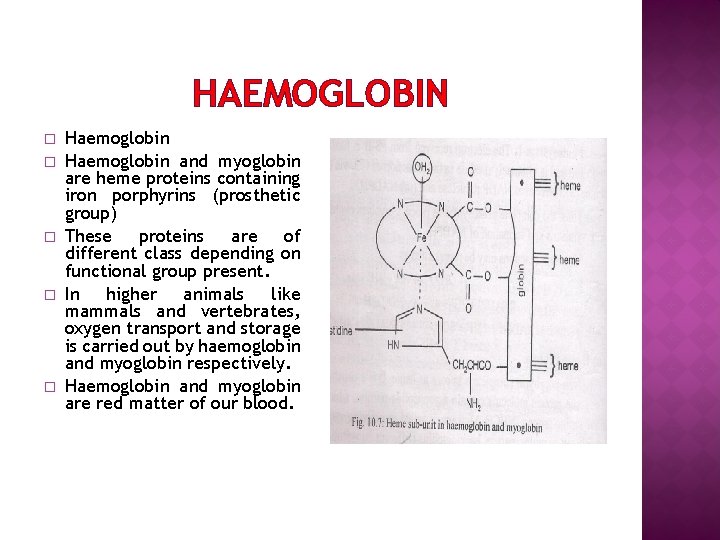
HAEMOGLOBIN � � � Haemoglobin and myoglobin are heme proteins containing iron porphyrins (prosthetic group) These proteins are of different class depending on functional group present. In higher animals like mammals and vertebrates, oxygen transport and storage is carried out by haemoglobin and myoglobin respectively. Haemoglobin and myoglobin are red matter of our blood.

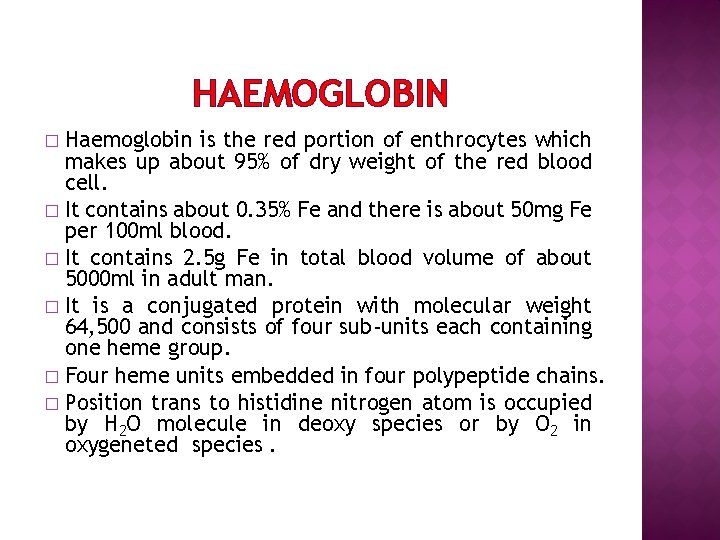
HAEMOGLOBIN Haemoglobin is the red portion of enthrocytes which makes up about 95% of dry weight of the red blood cell. � It contains about 0. 35% Fe and there is about 50 mg Fe per 100 ml blood. � It contains 2. 5 g Fe in total blood volume of about 5000 ml in adult man. � It is a conjugated protein with molecular weight 64, 500 and consists of four sub-units each containing one heme group. � Four heme units embedded in four polypeptide chains. � Position trans to histidine nitrogen atom is occupied by H 2 O molecule in deoxy species or by O 2 in oxygeneted species. �

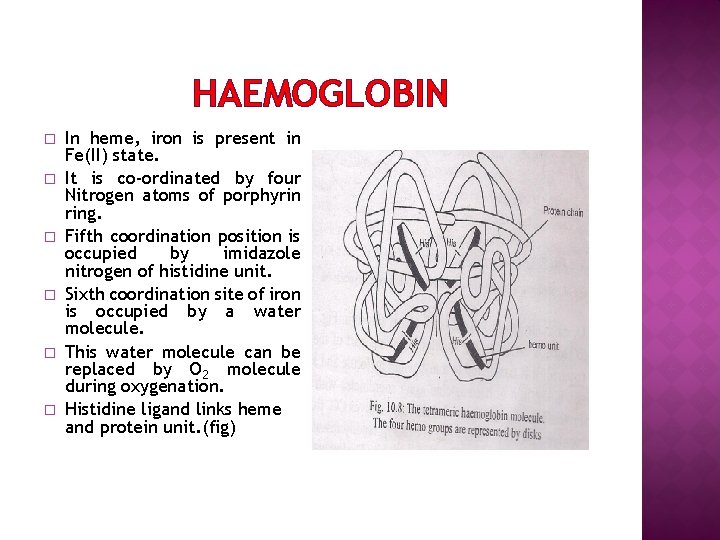
HAEMOGLOBIN � � � In heme, iron is present in Fe(II) state. It is co-ordinated by four Nitrogen atoms of porphyrin ring. Fifth coordination position is occupied by imidazole nitrogen of histidine unit. Sixth coordination site of iron is occupied by a water molecule. This water molecule can be replaced by O 2 molecule during oxygenation. Histidine ligand links heme and protein unit. (fig)

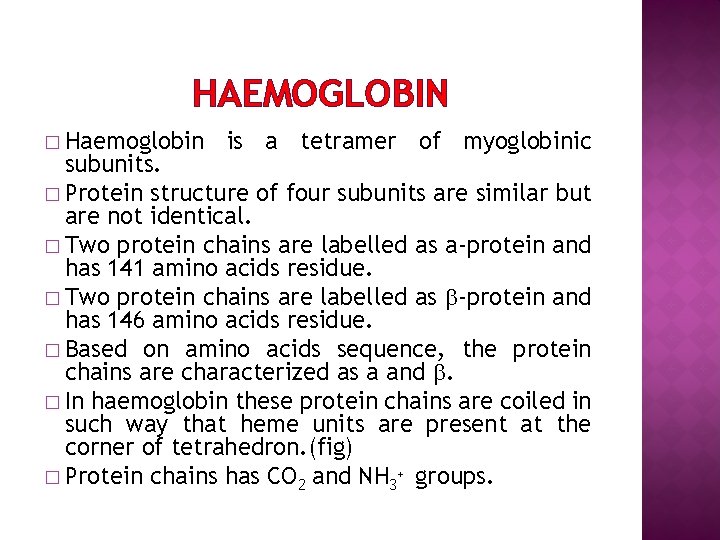
HAEMOGLOBIN � Haemoglobin is a tetramer of myoglobinic subunits. � Protein structure of four subunits are similar but are not identical. � Two protein chains are labelled as a-protein and has 141 amino acids residue. � Two protein chains are labelled as -protein and has 146 amino acids residue. � Based on amino acids sequence, the protein chains are characterized as a and . � In haemoglobin these protein chains are coiled in such way that heme units are present at the corner of tetrahedron. (fig) � Protein chains has CO 2 and NH 3+ groups.

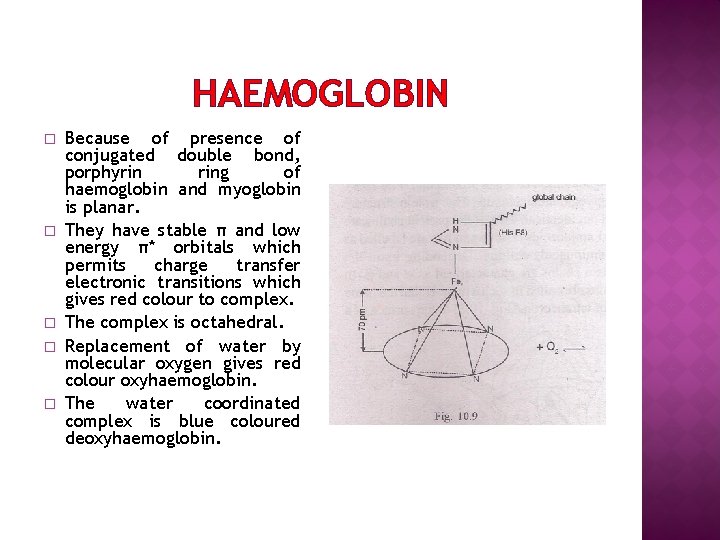
HAEMOGLOBIN � � � Because of presence of conjugated double bond, porphyrin ring of haemoglobin and myoglobin is planar. They have stable π and low energy π* orbitals which permits charge transfer electronic transitions which gives red colour to complex. The complex is octahedral. Replacement of water by molecular oxygen gives red colour oxyhaemoglobin. The water coordinated complex is blue coloured deoxyhaemoglobin.

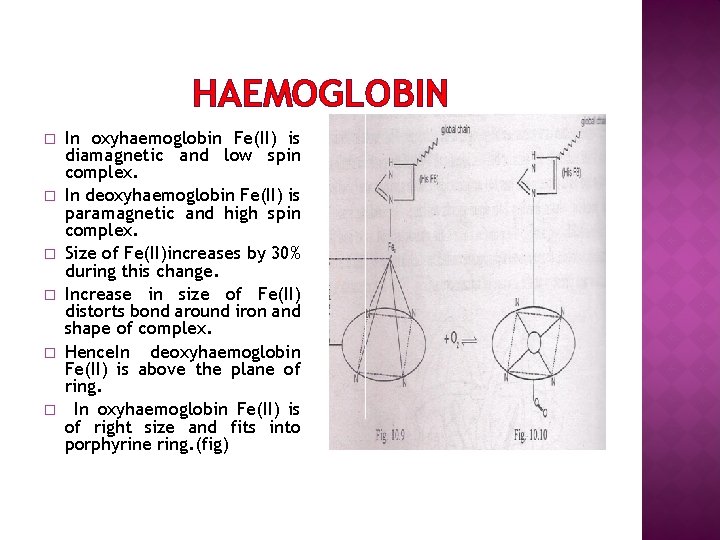
HAEMOGLOBIN � � � In oxyhaemoglobin Fe(II) is diamagnetic and low spin complex. In deoxyhaemoglobin Fe(II) is paramagnetic and high spin complex. Size of Fe(II)increases by 30% during this change. Increase in size of Fe(II) distorts bond around iron and shape of complex. Hence. In deoxyhaemoglobin Fe(II) is above the plane of ring. In oxyhaemoglobin Fe(II) is of right size and fits into porphyrine ring. (fig)

HAEMOGLOBIN � Oxygenated blood is carried to the muscles in various parts of body as per requirement. It is transferred to myoglobin molecule and stored. After oxygen is lost, iron of haemoglobin again coordinates with water molecule. Protein parts of absorbs H+. It indirectly removes CO 2 from tissues by converting CO 2 to HCO 3 and H+. HCO 3 - readily dissolves in blood and H+ is absorbed in protein unit of haemoglobin. Impure blood is taken to heart and from there it reaches the lungs. Here HCO 3 - is converted into CO 2 in lungs and cycle is repeated. Oxygen carrying process by haemoglobin is reversible. Q. Explain the role of iron in haemoglobin and myoglobin. � Q. Give difference in structure of haemoglobin and myoglobin. � � � � �

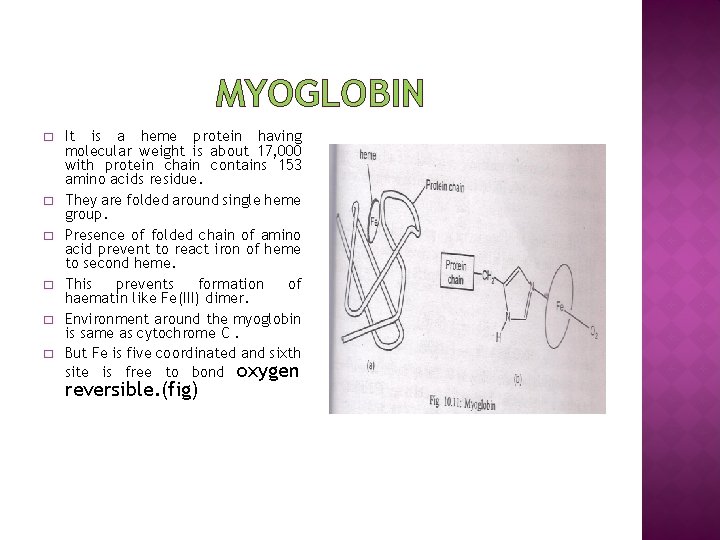
MYOGLOBIN � � � It is a heme protein having molecular weight is about 17, 000 with protein chain contains 153 amino acids residue. They are folded around single heme group. Presence of folded chain of amino acid prevent to react iron of heme to second heme. This prevents formation of haematin like Fe(III) dimer. Environment around the myoglobin is same as cytochrome C. But Fe is five coordinated and sixth site is free to bond oxygen reversible. (fig)

TRANSPORT AND STORAGE OF OXYGEN � The iron of each heme unit can bind one oxygen molecule as one of axial position of iron or has water molecules attached to it. � When dissolved oxygen is present, it occupies this position and change in protein confirmation takes place. � This change in molecular shape helps in binding additional oxygen molecule. � Thus four iron of haemoglobin carry four oxygen molecule. � One oxygen molecule can be added at a time and equilibrium constant value increases with number of O 2 molecule.

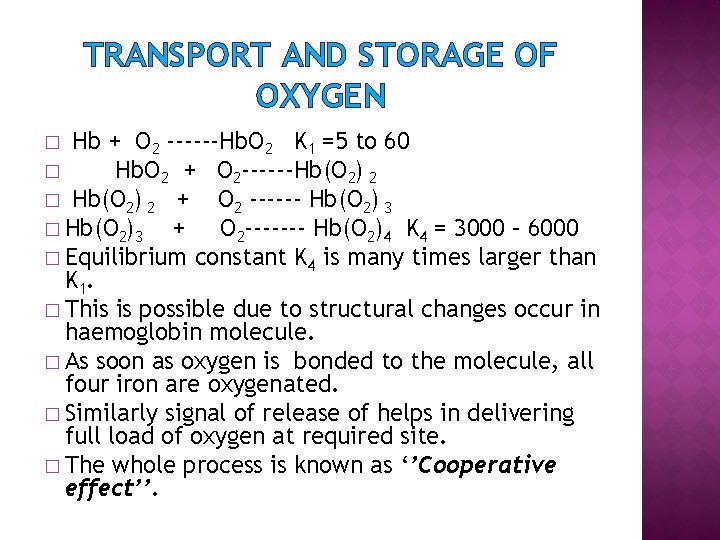
TRANSPORT AND STORAGE OF OXYGEN Hb + O 2 ------Hb. O 2 K 1 =5 to 60 � Hb. O 2 + O 2 ------Hb(O 2) 2 � Hb(O 2) 2 + O 2 ------ Hb(O 2) 3 � Hb(O 2)3 + O 2 ------- Hb(O 2)4 K 4 = 3000 – 6000 � Equilibrium constant K 4 is many times larger than K 1. � This is possible due to structural changes occur in haemoglobin molecule. � As soon as oxygen is bonded to the molecule, all four iron are oxygenated. � Similarly signal of release of helps in delivering full load of oxygen at required site. � The whole process is known as ‘’Cooperative effect’’. �

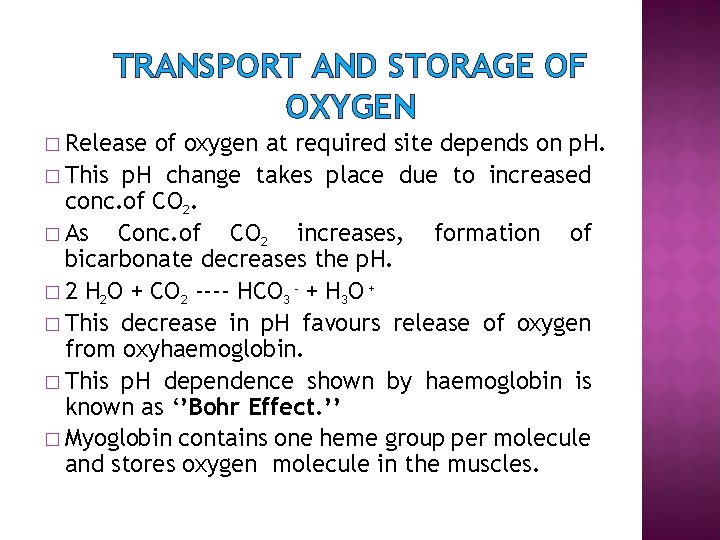
TRANSPORT AND STORAGE OF OXYGEN � Release of oxygen at required site depends on p. H. � This p. H change takes place due to increased conc. of CO 2. � As Conc. of CO 2 increases, formation of bicarbonate decreases the p. H. � 2 H 2 O + CO 2 ---- HCO 3 - + H 3 O + � This decrease in p. H favours release of oxygen from oxyhaemoglobin. � This p. H dependence shown by haemoglobin is known as ‘’Bohr Effect. ’’ � Myoglobin contains one heme group per molecule and stores oxygen molecule in the muscles.

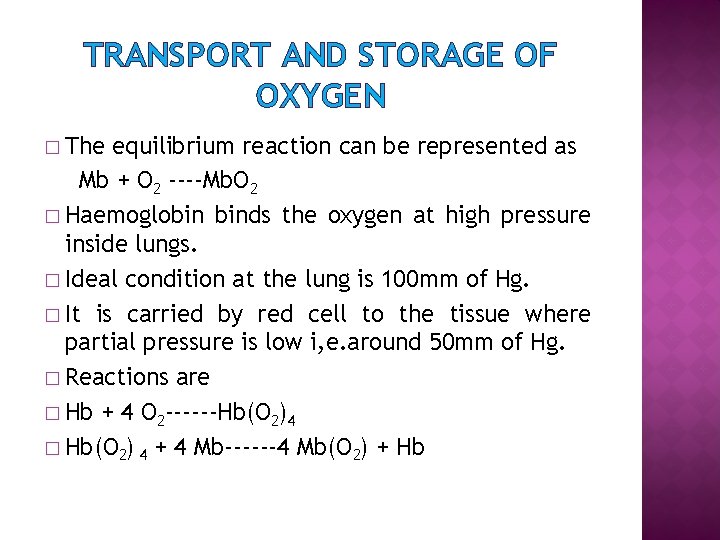
TRANSPORT AND STORAGE OF OXYGEN � The equilibrium reaction can be represented as Mb + O 2 ----Mb. O 2 � Haemoglobin binds the oxygen at high pressure inside lungs. � Ideal condition at the lung is 100 mm of Hg. � It is carried by red cell to the tissue where partial pressure is low i, e. around 50 mm of Hg. � Reactions are � Hb + 4 O 2 ------Hb(O 2)4 � Hb(O 2) 4 + 4 Mb------4 Mb(O 2) + Hb

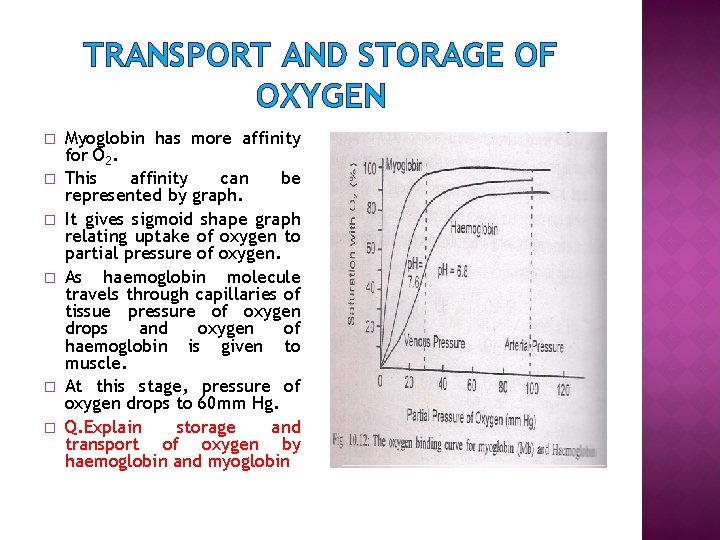
TRANSPORT AND STORAGE OF OXYGEN � � � Myoglobin has more affinity for O 2. This affinity can be represented by graph. It gives sigmoid shape graph relating uptake of oxygen to partial pressure of oxygen. As haemoglobin molecule travels through capillaries of tissue pressure of oxygen drops and oxygen of haemoglobin is given to muscle. At this stage, pressure of oxygen drops to 60 mm Hg. Q. Explain storage and transport of oxygen by haemoglobin and myoglobin

THANK YOU
 Vikas lab thakur complex
Vikas lab thakur complex Fairy
Fairy I u thakur
I u thakur Thakur college of engineering and technology
Thakur college of engineering and technology In treatment
In treatment Kapil krishna thakur
Kapil krishna thakur Saurav thakur
Saurav thakur Promotion from assistant to associate professor
Promotion from assistant to associate professor Dr vikas patel
Dr vikas patel Dr vikas bhatia
Dr vikas bhatia Enokj
Enokj Vikas kumar
Vikas kumar Dr vikas patel
Dr vikas patel Vikas motwani
Vikas motwani Dhara vikas sikkim poster
Dhara vikas sikkim poster Vikas kumar
Vikas kumar 138000/12
138000/12 Gramin sarva shiksha vikas kendra
Gramin sarva shiksha vikas kendra Surajya sarvangin vikas prakalp
Surajya sarvangin vikas prakalp Pmagy nic.in
Pmagy nic.in Dr vikas bansal
Dr vikas bansal Vagad vikas
Vagad vikas Gramin sarva shiksha vikas kendra
Gramin sarva shiksha vikas kendra Organic vs inorganic chemistry
Organic vs inorganic chemistry Functional groups ib chemistry
Functional groups ib chemistry Community based tourism in bangladesh
Community based tourism in bangladesh Professor guhan subramanian
Professor guhan subramanian Professor dr. m. fakhrul islam
Professor dr. m. fakhrul islam The telephone rang inside startlingly
The telephone rang inside startlingly Analise matematica
Analise matematica Professor laura lundy
Professor laura lundy Critical and creative thinking capability
Critical and creative thinking capability Good morning welcome back
Good morning welcome back Professor roger jones
Professor roger jones How to read literature like a professor violence
How to read literature like a professor violence Faber character analysis
Faber character analysis Sube banerjee
Sube banerjee Balaji venkatraman rate my professor
Balaji venkatraman rate my professor Professor brian s peskin
Professor brian s peskin Professor patrizia simondo
Professor patrizia simondo Proper adjective for java
Proper adjective for java Jey saes
Jey saes Lindab professor
Lindab professor