DOKUZ EYLUL UNIVERSITY DEPARTMANT OF METALLURGICAL MATERIALS ENGINEERING








- Slides: 24

DOKUZ EYLUL UNIVERSITY DEPARTMANT OF METALLURGICAL & MATERIALS ENGINEERING SYNTHESIS AND CHARACTERIZATION OF BZO DOPED YBCO SUPERCONDUCTING FILMS WITH DIFFERENT TYPES OF PRECURSORS Murat BEKTAŞ Dr. Işıl BİRLİK Dr. Osman ÇULHA Doç. Dr. Mustafa TOPARLI Supervisor: Prof. Dr. Erdal ÇELİK

Content Ø AIM Of THE STUDY Ø INTRODUCTION q Superconductivity q TFA-MOD Technique Ø EXPERIMENTAL STUDIES Characterization of; q YBCO Thin Film Production from Oxide Powder q YBCO Thin Film Production from Acetate-based Presursor Ø CONCLUSION

ØAIM OF THE STUDY • TFA-MOD process using highly purified metal acetates as starting materials are rather expensive and thus it is desirable to find more economic route. • Recently, several attempts to use oxide powders such as commercially available REBCO powder as starting materials have been reported which showed comparable Jc (critical current density) for the YBCO films. • In this study, two different types of Ba. Zr. O 3 doped YBa 2 Cu 3 O 7 -δ (YBCO) superconducting thin films were prepared using commercially available YBCO powder and yttrium, barium and copper acetate on Sr. Ti. O 3 (STO) substrates by TFA-MOD method. • The effect of precursor type on the film structure and superconducting properties were studied.

ØSUPERCONDUCTIVITY

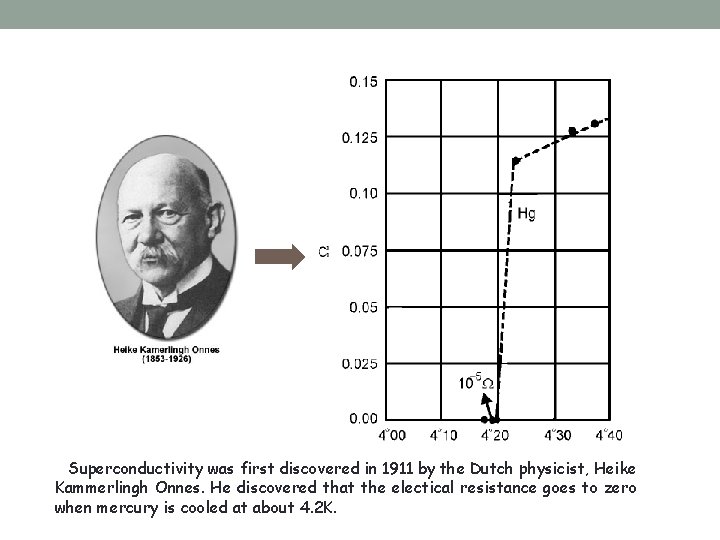
Superconductivity was first discovered in 1911 by the Dutch physicist, Heike Kammerlingh Onnes. He discovered that the electical resistance goes to zero when mercury is cooled at about 4. 2 K.

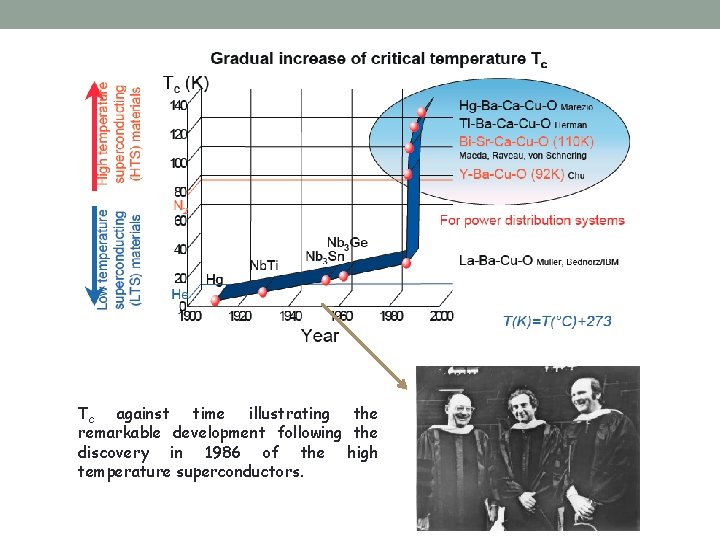
Tc against time illustrating the remarkable development following the discovery in 1986 of the high temperature superconductors.

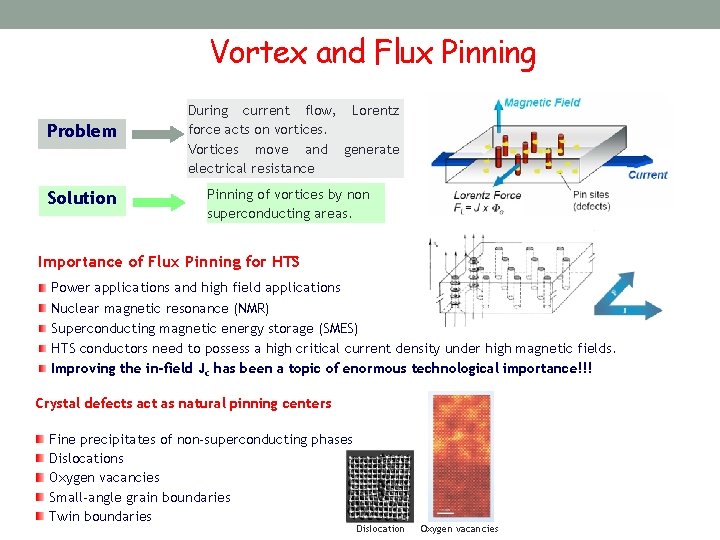
Vortex and Flux Pinning Problem Solution During current flow, Lorentz force acts on vortices. Vortices move and generate electrical resistance Pinning of vortices by non superconducting areas. Importance of Flux Pinning for HTS Power applications and high field applications Nuclear magnetic resonance (NMR) Superconducting magnetic energy storage (SMES) HTS conductors need to possess a high critical current density under high magnetic fields. Improving the in-field Jc has been a topic of enormous technological importance!!! Crystal defects act as natural pinning centers Fine precipitates of non-superconducting phases Dislocations Oxygen vacancies Small-angle grain boundaries Twin boundaries Dislocation Oxygen vacancies

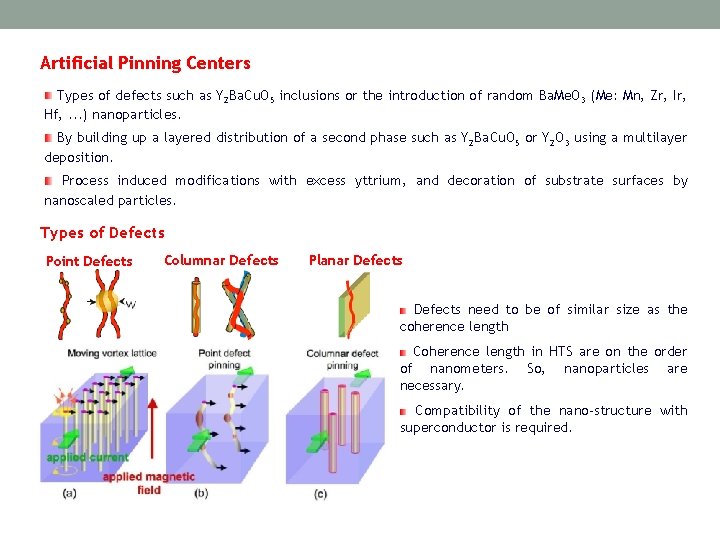
Artificial Pinning Centers Types of defects such as Y 2 Ba. Cu. O 5 inclusions or the introduction of random Ba. Me. O 3 (Me: Mn, Zr, Ir, Hf, . . . ) nanoparticles. By building up a layered distribution of a second phase such as Y 2 Ba. Cu. O 5 or Y 2 O 3 using a multilayer deposition. Process induced modifications with excess yttrium, and decoration of substrate surfaces by nanoscaled particles. Types of Defects Point Defects Columnar Defects Planar Defects need to be of similar size as the coherence length Coherence length in HTS are on the order of nanometers. So, nanoparticles are necessary. Compatibility of the nano-structure with superconductor is required.

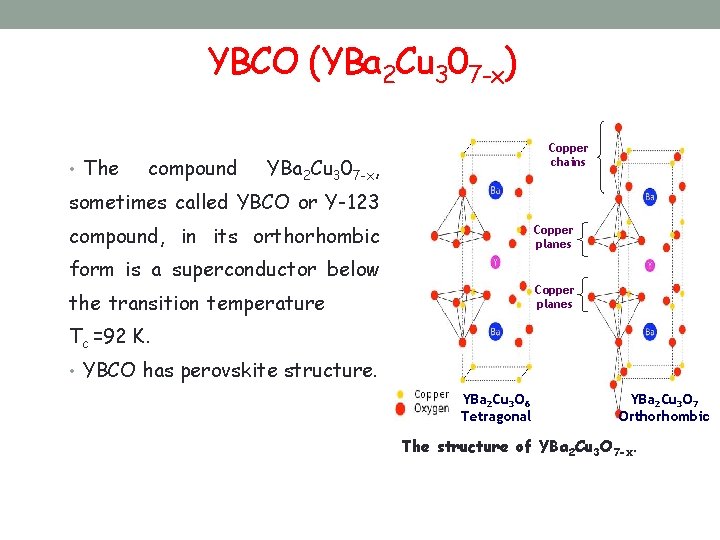
YBCO (YBa 2 Cu 307 -x) • The compound Copper chains YBa 2 Cu 307 -x, sometimes called YBCO or Y-123 compound, in its orthorhombic Copper planes form is a superconductor below Copper planes the transition temperature Tc =92 K. • YBCO has perovskite structure. YBa 2 Cu 3 O 6 Tetragonal YBa 2 Cu 3 O 7 Orthorhombic The structure of YBa 2 Cu 3 O 7 -x.

TFA-MOD Schematic illustration of metal organic deposition using trifluoroacetates (TFA-MOD) for fabricating YBCO superconductors.

ØEXPERIMENTAL STUDIES

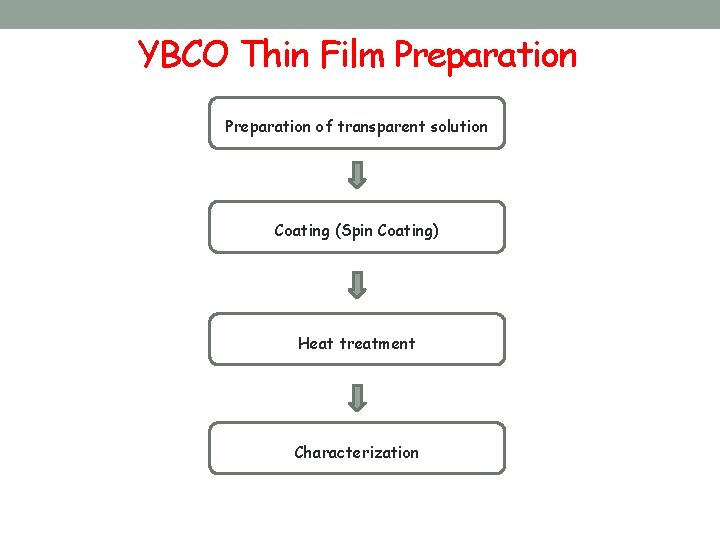
YBCO Thin Film Preparation of transparent solution Coating (Spin Coating) Heat treatment Characterization

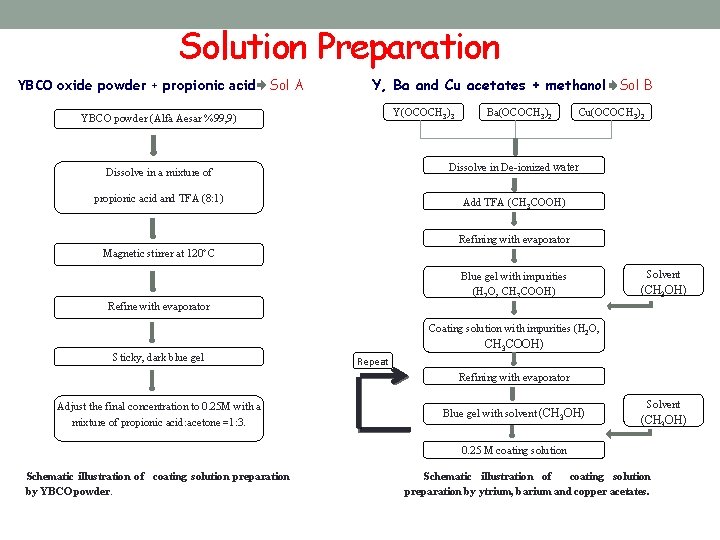
Solution Preparation YBCO oxide powder + propionic acid Sol A Y, Ba and Cu acetates + methanol Sol B Y(OCOCH 3)3 YBCO powder (Alfa Aesar %99, 9) Ba(OCOCH 3)2 Cu(OCOCH 3)2 Dissolve in De-ionized water Dissolve in a mixture of propionic acid and TFA (8: 1) Add TFA (CH 3 COOH) Refining with evaporator Magnetic stirrer at 120°C Solvent Blue gel with impurities (H 2 O, CH 3 COOH) Refine with evaporator (CH 3 OH) Coating solution with impurities (H 2 O, CH 3 COOH) Sticky, dark blue gel Repeat Adjust the final concentration to 0. 25 M with a mixture of propionic acid: acetone =1: 3. Refining with evaporator 0. 25 M coating solution Schematic illustration of coating solution preparation by YBCO powder. Solvent Blue gel with solvent (CH 3 OH) Schematic illustration of (CH 3 OH) coating solution preparation by ytrium, barium and copper acetates.

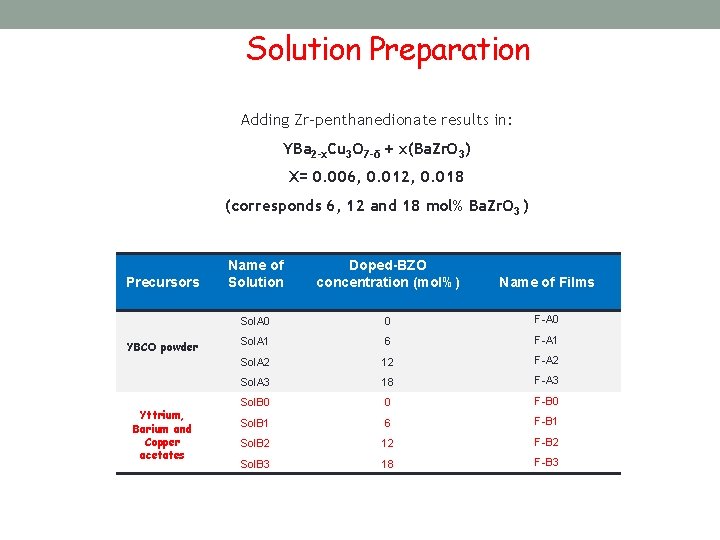
Solution Preparation Adding Zr-penthanedionate results in: YBa 2 -x. Cu 3 O 7 -δ + x(Ba. Zr. O 3) X= 0. 006, 0. 012, 0. 018 (corresponds 6, 12 and 18 mol% Ba. Zr. O 3 ) Precursors YBCO powder Yttrium, Barium and Copper acetates Name of Solution Doped-BZO concentration (mol%) Name of Films Sol. A 0 0 F-A 0 Sol. A 1 6 F-A 1 Sol. A 2 12 F-A 2 Sol. A 3 18 F-A 3 Sol. B 0 0 F-B 0 Sol. B 1 6 F-B 1 Sol. B 2 12 F-B 2 Sol. B 3 18 F-B 3

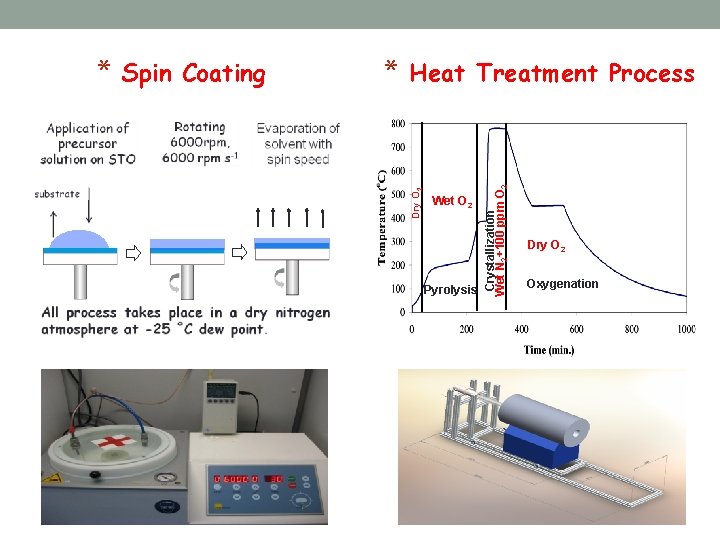
* Heat Treatment Process Wet O 2 Pyrolysis Crystallization Wet N 2+100 ppm O 2 Spin Coating Dry O 2 * Dry O 2 Oxygenation

Characterization of Solutions & YBCO Films ØSolution characterization; Viscosity and contact angle, • DTA-TG (Differential Thermal Analysis-Thermal Gravimetric Analysis), • Ø YBCO film characterization; • XRD (X-Ray Diffractometer), • SEM (Scanning Electron Microscopy) ØPhysical properties ; • Inductive Tc measurement • Inductive Jc measurement

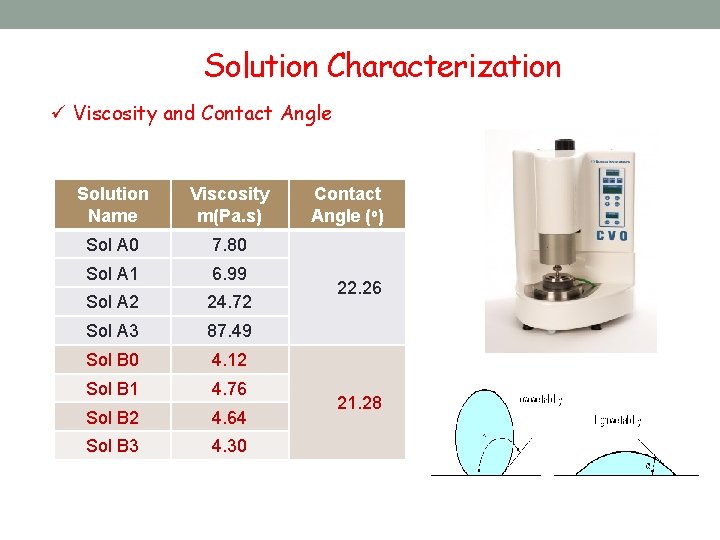
Solution Characterization ü Viscosity and Contact Angle Solution Name Viscosity m(Pa. s) Sol A 0 7. 80 Sol A 1 6. 99 Sol A 2 24. 72 Sol A 3 87. 49 Sol B 0 4. 12 Sol B 1 4. 76 Sol B 2 4. 64 Sol B 3 4. 30 Contact Angle (o) 22. 26 21. 28

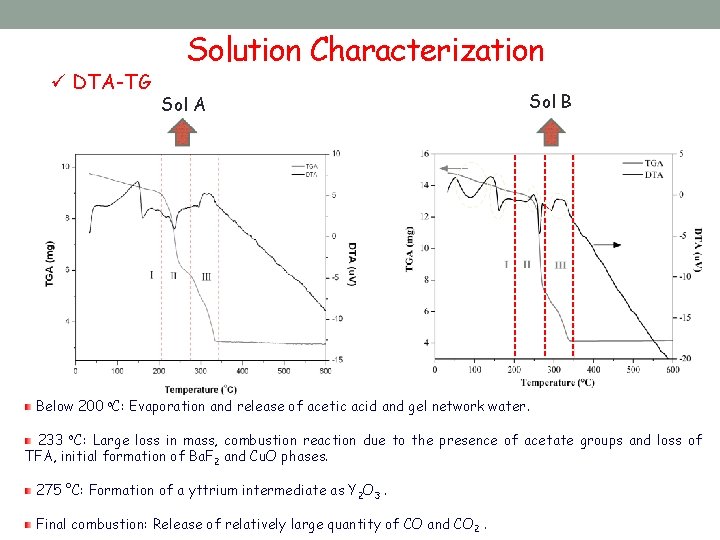
ü DTA-TG Solution Characterization Sol A Sol B Below 200 o. C: Evaporation and release of acetic acid and gel network water. 233 o. C: Large loss in mass, combustion reaction due to the presence of acetate groups and loss of TFA, initial formation of Ba. F 2 and Cu. O phases. 275 °C: Formation of a yttrium intermediate as Y 2 O 3. Final combustion: Release of relatively large quantity of CO and CO 2.

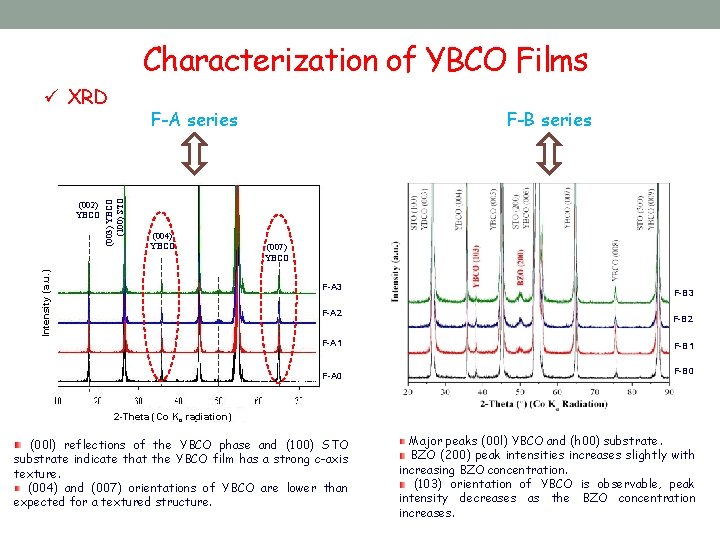
Characterization of YBCO Films ü XRD (003) YBCO (100) STO (004) YBCO Intensity (a. u. ) (002) YBCO F-A series F-B series (007) YBCO F-A 3 F-A 2 F-B 3 F-B 2 F-A 1 F-B 1 F-A 0 F-B 0 2 -Theta (Co Kα radiation) (00 l) reflections of the YBCO phase and (100) STO substrate indicate that the YBCO film has a strong c-axis texture. (004) and (007) orientations of YBCO are lower than expected for a textured structure. Major peaks (00 l) YBCO and (h 00) substrate. BZO (200) peak intensities increases slightly with increasing BZO concentration. (103) orientation of YBCO is observable, peak intensity decreases as the BZO concentration increases.

Characterization of YBCO Films ü SEM F-A series F-B series (a) (b) (c) (d) Surface morphologies of (a) F-A 0, (b) F-A 1, (c) F-A 2 and (d) F-A 3 films. Surface morphologies of (a) F-B 0, (b) F-B 1, (c) F-B 2 and (d) F-B 3 films.

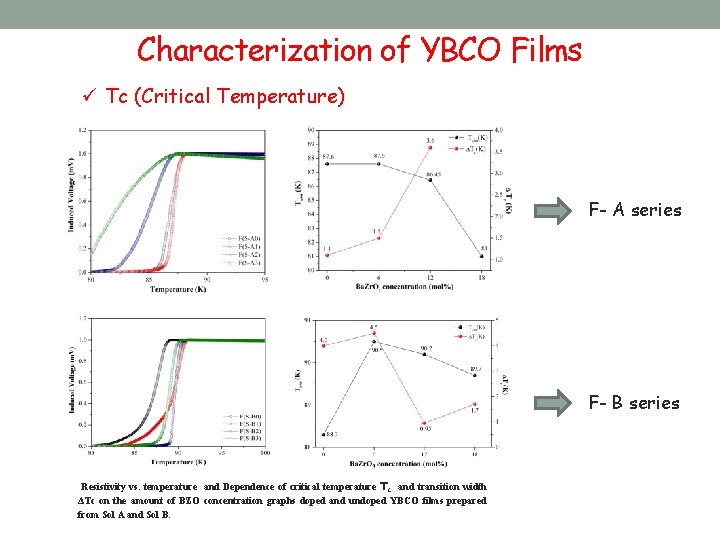
Characterization of YBCO Films ü Tc (Critical Temperature) F- A series F- B series Resistivity vs. temperature and Dependence of critical temperature Tc and transition width ΔTc on the amount of BZO concentration graphs doped and undoped YBCO films prepared from Sol A and Sol B.

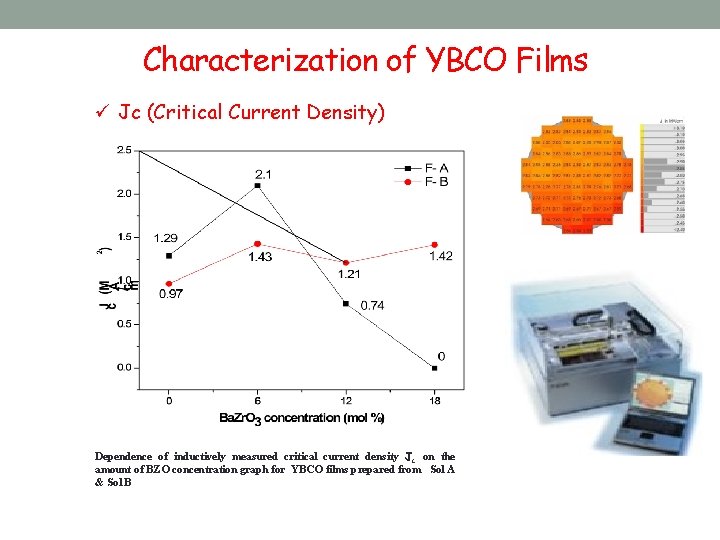
Characterization of YBCO Films ü Jc (Critical Current Density) Dependence of inductively measured critical current density Jc on the amount of BZO concentration graph for YBCO films prepared from Sol A & Sol B

Conclusion ü YBCO superconducting thin films were successfully prepared from YBCO powder and yttrium, barium, copper acetate precursors via TFA-MOD method on STO single crystal substrates and BZO was incorporated into the structures of them as artificial pinning centers. ü According to SEM images, YBCO films prepared from Sol. A exhibit better surface morphology and all of them are generally formed by c-axis oriented grains. BZO doped YBCO films present a denser surface structure with decreasing porosity compared with the undoped YBCO films. On the other hand, 18 mol% BZO doped sample surface possesses bigger sized grains in comparison to the fine grains of 6 and 12 mol% BZO doped sample surfaces. ü As a result of Jc measurements, 6 mol% BZO doped YBCO sample prepared from Sol. A (YBCO powder) has the highest Jc value.

Thanks for your attention… ACKNOWLEDGEMENT TO; TUBITAK-109 M 054 Leibniz Enstitute For Solid State and Materials Research Dresden
 Dokuz eylül yazılım
Dokuz eylül yazılım Malzeme bilimi (ders notlari dokuz eylül)
Malzeme bilimi (ders notlari dokuz eylül) Deuzem giriş
Deuzem giriş Deymler
Deymler Emitter injection efficiency formula
Emitter injection efficiency formula Otto hoffman's method
Otto hoffman's method Metallurgical testing
Metallurgical testing Cant stop the feeling trolls go noodle
Cant stop the feeling trolls go noodle Useful and harmful materials at home pictures
Useful and harmful materials at home pictures Man made materials
Man made materials Adopting and adapting teaching materials
Adopting and adapting teaching materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Manufacturing processes for engineering materials
Manufacturing processes for engineering materials Heat conductivity examples
Heat conductivity examples What is the engineering materials
What is the engineering materials Optical properties of engineering materials
Optical properties of engineering materials Materials engineering science processing and design
Materials engineering science processing and design What is the engineering materials
What is the engineering materials Engineering materials
Engineering materials Materials for engineering
Materials for engineering Integrated computational materials engineering
Integrated computational materials engineering Divya nayar iit delhi
Divya nayar iit delhi Integrated computational materials engineering
Integrated computational materials engineering Civil engineering source
Civil engineering source What is system design in software engineering
What is system design in software engineering