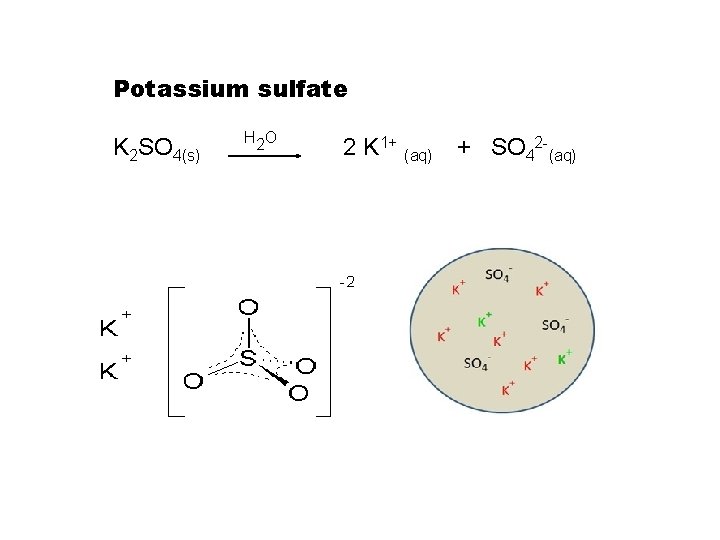
Dissolution of SALTS Potassium sulfate K 2 SO







- Slides: 17

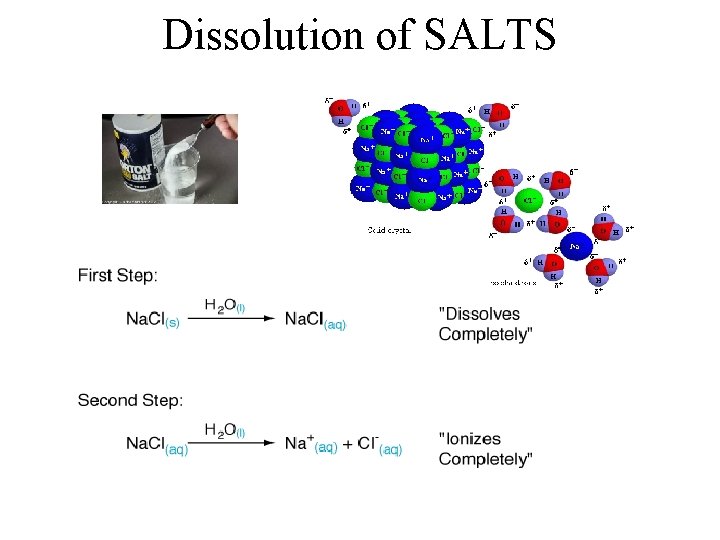
Dissolution of SALTS

Potassium sulfate K 2 SO 4(s) H 2 O 2 K 1+ (aq) + SO 42 -(aq)


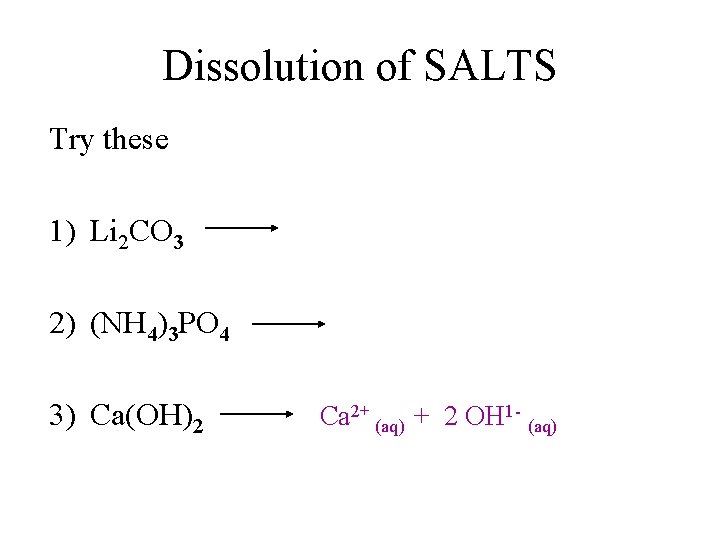
Dissolution of SALTS Try these 1) Li 2 CO 3 2) (NH 4)3 PO 4 3) Ca(OH)2 Ca 2+ (aq) + 2 OH 1 - (aq)

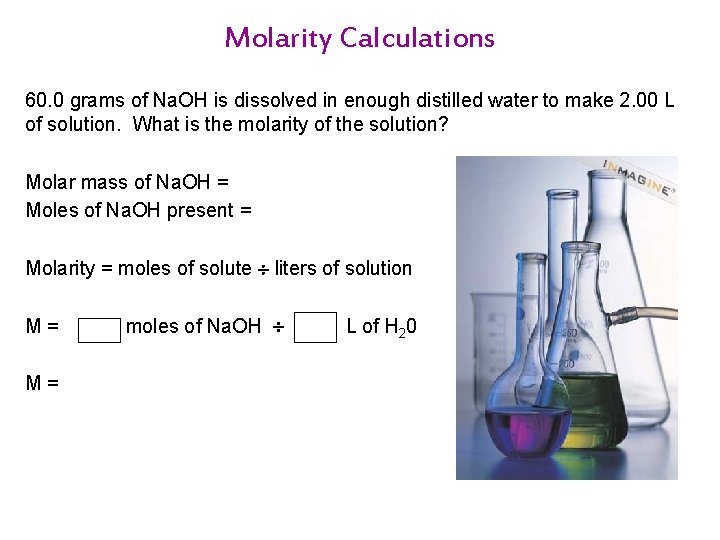
Molarity Calculations 60. 0 grams of Na. OH is dissolved in enough distilled water to make 2. 00 L of solution. What is the molarity of the solution? Molar mass of Na. OH = Moles of Na. OH present = Molarity = moles of solute liters of solution M= M= moles of Na. OH L of H 20

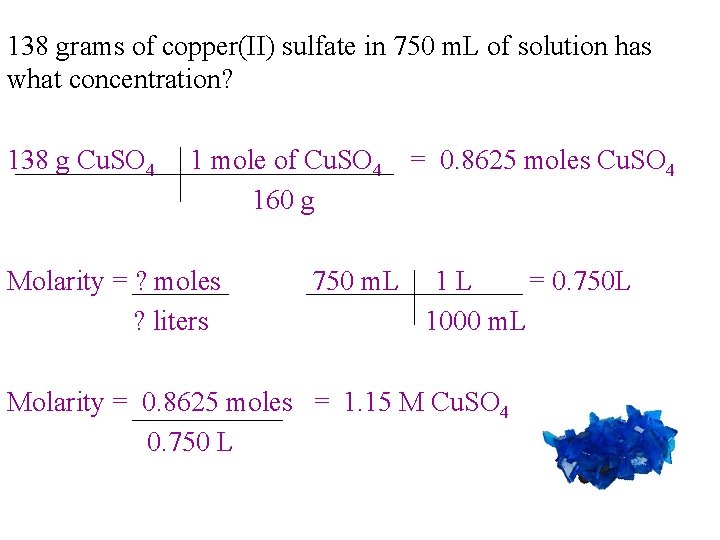
138 grams of copper(II) sulfate in 750 m. L of solution has what concentration? 138 g Cu. SO 4 1 mole of Cu. SO 4 = 0. 8625 moles Cu. SO 4 160 g Molarity = ? moles ? liters 750 m. L 1 L = 0. 750 L 1000 m. L Molarity = 0. 8625 moles = 1. 15 M Cu. SO 4 0. 750 L

You want to make 1. 50 L of 4. 00 M silver nitrate solution. How many grams of silver nitrate are needed? 2

If you want 250 m. L of a 0. 08 M concentrate solution of barium hydroxide, how many grams of barium hydroxide is needed?

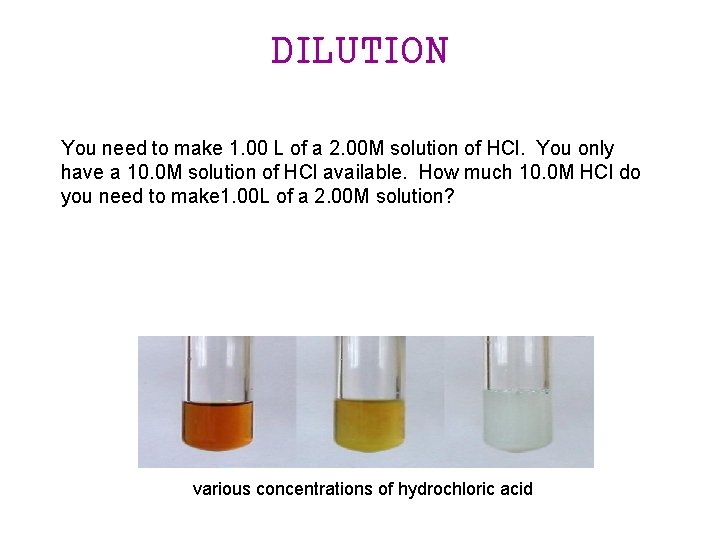
DILUTION You need to make 1. 00 L of a 2. 00 M solution of HCl. You only have a 10. 0 M solution of HCl available. How much 10. 0 M HCl do you need to make 1. 00 L of a 2. 00 M solution? various concentrations of hydrochloric acid

How much 18 M sulfuric acid is needed to make 1250 m. L of a 4. 5 molarity solution? For DILUTION (concentration 1)(volume 1) = (concentration 2)(volume 2) (18 M) (V) = (4. 5 M) (1250 m. L) divide by 18 M V = 312. 5 m. L of 18 M sulfuric acid

If 61 m. L of 8 M hydrobromic acid is mixed with 689 m. L of water, what is the concentration of the resulting solution?

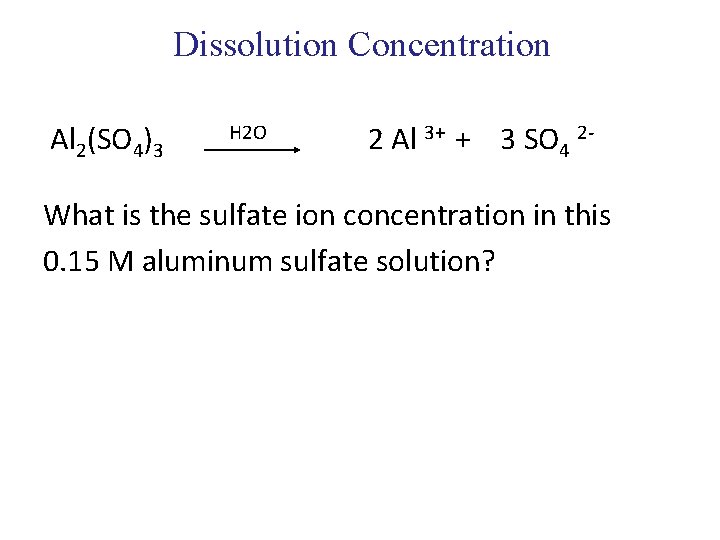
Dissolution Concentration Al 2(SO 4)3 H 2 O 2 Al 3+ + 3 SO 4 2 - What is the sulfate ion concentration in this 0. 15 M aluminum sulfate solution?

Dissolution Concentration What are the concentrations of the two ions that are formed from a 2. 31 M calcium acetate solution?

Concentration of a Gas If the maximum concentration of krypton gas in water is 0. 00089 M at 404 K and 1250 mm. Hg, what volume of gas can potentially be removed from 20 liters of solution?

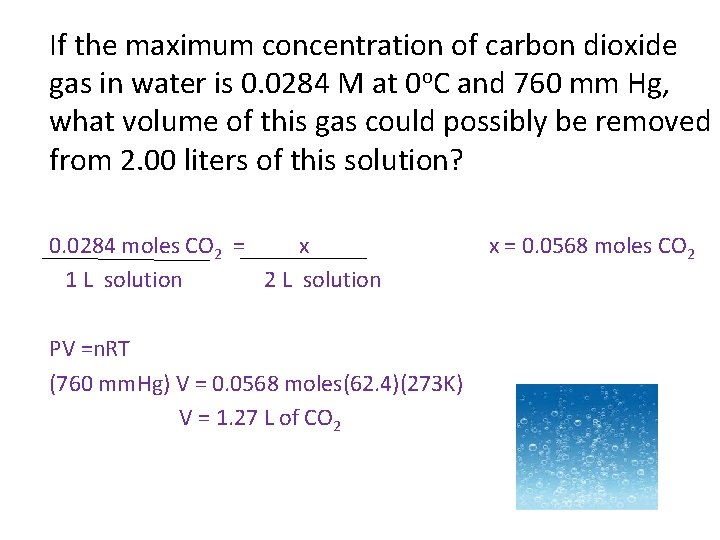
If the maximum concentration of carbon dioxide gas in water is 0. 0284 M at 0 o. C and 760 mm Hg, what volume of this gas could possibly be removed from 2. 00 liters of this solution? 0. 0284 moles CO 2 = x 1 L solution 2 L solution PV =n. RT (760 mm. Hg) V = 0. 0568 moles(62. 4)(273 K) V = 1. 27 L of CO 2 x = 0. 0568 moles CO 2

Molarity with a Chemical Reaction How many milliliters of 18. 0 M H 2 SO 4 are required to react with 250 m. L of 2. 5 M Al(OH)3 to create aluminum sulfate and water? What do we need to solve this problem?

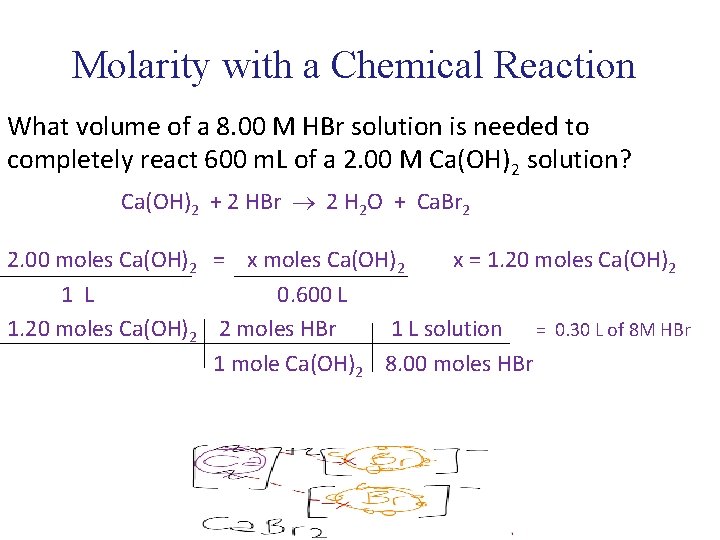
Molarity with a Chemical Reaction What volume of a 8. 00 M HBr solution is needed to completely react 600 m. L of a 2. 00 M Ca(OH)2 solution? Ca(OH)2 + 2 HBr 2 H 2 O + Ca. Br 2 2. 00 moles Ca(OH)2 = x moles Ca(OH)2 x = 1. 20 moles Ca(OH)2 1 L 0. 600 L 1. 20 moles Ca(OH)2 2 moles HBr 1 L solution = 0. 30 L of 8 M HBr 1 mole Ca(OH)2 8. 00 moles HBr
 Copper sulfate and potassium iodide precipitate
Copper sulfate and potassium iodide precipitate Tocolytics
Tocolytics Dic.n
Dic.n Acute fulminating preeclampsia
Acute fulminating preeclampsia Magnesium and copper oxide equation
Magnesium and copper oxide equation Calcium carbonate and nitric acid reaction
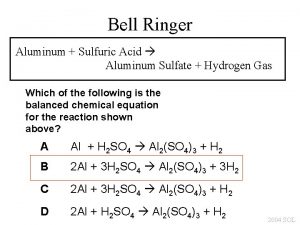
Calcium carbonate and nitric acid reaction Sulphuric acid + aluminium
Sulphuric acid + aluminium Sulfate anion test
Sulfate anion test Sulfate reducing bacteria
Sulfate reducing bacteria Ammonium sulfate cation and anion
Ammonium sulfate cation and anion Mgso4 side effects
Mgso4 side effects Asma rp
Asma rp Ag2scompound name
Ag2scompound name What are spectator ions
What are spectator ions Ionic bonding substances
Ionic bonding substances Ammonium sulfate precipitation
Ammonium sulfate precipitation Baruim sulfate
Baruim sulfate Cristaux de sulfate de cuivre
Cristaux de sulfate de cuivre