Dial in Main 631 992 3221 Dial in






































- Slides: 42

Dial in (Main): (631) 992 -3221 Dial in (Alt): (516) 453 -0031 Attendee Access Code: 813 -189 -484 Slides in “Handout” Tab R&D Committee Workshop Series: RDC Review of Amendments and Continuing Review Moderated by: Soundia Duche, MA, MS Director, Education & Training ORPP&E June 3, 2020

Introductions • Carole Palumbo, Ph. D. , Deputy ACOS/R&D, Director, Human Research Protections Program, VA Boston Healthcare System • Mieke Verfaellie, Ph. D. , Senior Research Career Scientist, R&D Committee Co-Chair, VA Boston Healthcare System • Douglas W. Miller, MPA, Research Committee Coordinator, VA Pacific Islands Health Care System 2

Objectives • Review R&D Committee oversight responsibilities for VA research • Research overseen by a subcommittee or external committee • Research under the sole oversight of the R&D Committee • Discuss R&D Committee review of amendments • Discuss R&D Committee continuing review • Share tools for R&D Committee review of amendments and continuing reviews 3

R&D Committee Responsibilities for the Review and Approval of Research • The R&D Committee is responsible for ensuring the maintenance of high standards within VA’s research program and ensuring that VA research is scientifically valid and complies with regulatory and ethical standards. • All VA research must be approved by the R&D Committee and cannot be initiated until the ACOS/R&D has notified the PI in writing that all approvals are in place. • R&D Committee provides final approval of all VA research. • Approval is granted only after the R&D Committee receives documentation from all applicable subcommittees/external committees of their review and non-contingent approval. 4

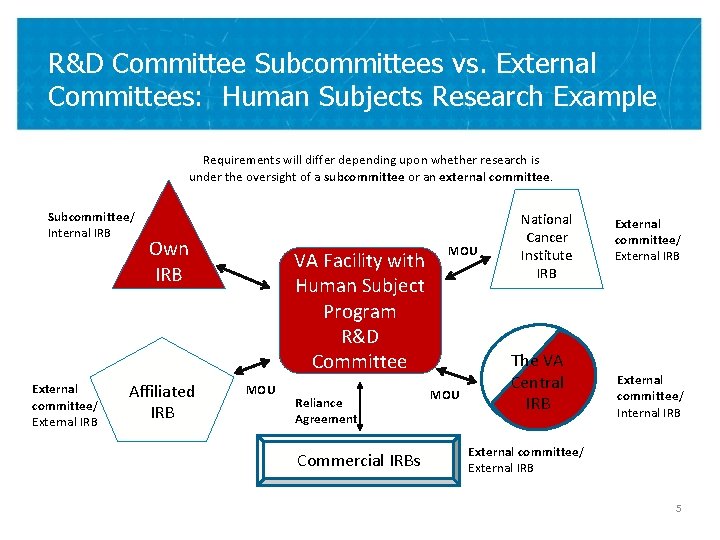
R&D Committee Subcommittees vs. External Committees: Human Subjects Research Example Requirements will differ depending upon whether research is under the oversight of a subcommittee or an external committee. Subcommittee/ Internal IRB External committee/ External IRB Own IRB Affiliated IRB VA Facility with Human Subject Program R&D Committee MOU Reliance Agreement Commercial IRBs MOU National Cancer Institute IRB The VA Central IRB External committee/ External IRB External committee/ Internal IRB External committee/ External IRB 5

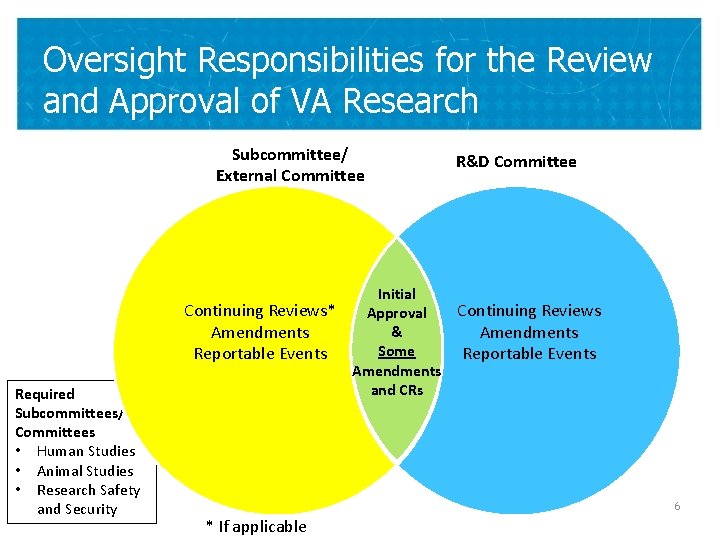
Oversight Responsibilities for the Review and Approval of VA Research Subcommittee/ External Committee Required Subcommittees/ Committees • Human Studies • Animal Studies • Research Safety and Security R&D Committee Initial Continuing Reviews* Continuing Reviews Approval & Amendments Some Reportable Events Amendments and CRs 6 * If applicable

Research Under the Sole Oversight of the R&D Committee Typically, the following research is under the sole oversight of the R&D Committee: • Exempt human subjects research (HSR) • Exempt HSR is research involving human subjects that is exempt from the majority of the requirements of the Common Rule (38 CFR 16). • Research involving data and specimens from non-human subjects, including: • Research that only involves de-identified data/biospecimens or research involving information/biospecimens from deceased individuals. • Research involving animal data only 7

R&D Committee Responsibilities for Approved VA Research • The R&D Committee does not need to approve continuing reviews and amendments of studies that are under the oversight of a subcommittee or external committee. • Sufficient documentation should be provided in the subcommittee/external committee minutes that are provided to the R&D Committee. • Subcommittee minutes are sent to the R&DC for review within 60 calendar days of being finalized. • MOU with external committee should stipulate how R&D Committee will be informed of approved actions and how R&D Committee will gain access to approved documents 8

What is the Best Way to Make Sure that Actions on Research Under the Oversight of a Subcommittee or Committee are Communicated to the R&D Committee? • There is no single best way to ensure that actions on research under the oversight of a subcommittee or committee are communicated to the R&D Committee. Different factors impact when and how actions are communicated and will vary among committees, including use of electronic vs. paper-based systems. • R&D Committee subcommittees are required to provide the R&D Committee with copies of subcommittee minutes within 60 calendar days of the finalization of the minutes. A well-constructed quality assurance program at your site will also help to ensure that the reporting of minutes is happening within the required 60 calendar days of finalization. The minutes must include information on actions approved by the convened committee as well as a list of actions that were approved outside of committee, via an expedited (IRB) or designated member review (IACUC and SRS) process. • The Memorandum of Understanding (MOU) or reliance agreement between the VA facility and the external committee must stipulate how actions approved by the committee will be communicated to the R&D Committee. The MOU or reliance agreement should be reviewed on at least an annual basis to ensure the obligations detailed in the MOU are being met. 9

What is the Best Way to Make Sure that Actions on Research Under the Oversight of a Subcommittee or Committee are Communicated to the R&D Committee (continued)? Elements of the MOU should include: • How the R&D Committee will be made aware of actions approved by the committee, to include a mutually agreed upon time frame for such notification. Notification can take the form of submission of meeting minutes, a list of actions approved, copies of approval notices, or some other means by which the R&D Committee is made aware of actions that have been approved by the committee. • A provision to allow the VA facility access to unredacted copies of meeting minutes within 2 business days of a written request to allow the R&D Committee to review deliberations on VA protocols. • Information on how the R&D Committee will either receive copies of actions and the supporting documents approved by the committee or be able to access those records remotely. VHA Directive 1200. 01, Research and Development Committee: FAQ #19 10

For Studies Overseen by an External Committee, Does the R&D Committee Need to Retain Copies of the Complete Protocol File on All Actions Approved by the External Committee? No. The R&D Committee is not required to physically maintain a copy of the complete protocol file on all actions approved by the external committee. However, the R&D Committee must have the ability to access the protocol file if it does not physically maintain a paper or electronic copy of the complete protocol file. VHA Directive 1200. 02, Paragraph 12. a. (4)(c) requires VA research to maintain and control a copy (paper or electronic) of all approved Research Protocols, amendments, consent document templates, and other documents submitted to a research review committee/subcommittee, and documents related to the actions of the research review committees. VHA Directive 1200. 01, Research and Development Committee: FAQ #20 11

Continued Oversight of VA Research: Duplicative Reviews and Approvals • Having multiple committees retain continued oversight of a study can create confusion, including questions surrounding • When does the study expire? • When can approved changes be implemented? • What if more than one committee requires changes prior to approving the continuing review or amendment? • Does this mean that review by more than one committee should never occur? • NO! There may be times when review by more than one committee is necessary… 12

Examples of When Review by More than One Committee May be Required and/or Appropriate • R&D Committee approval of enrollment of non-Veterans in research that is under the oversight of a subcommittee or committee • Studies that involve activities that are overseen by more than one subcommittee/committee • Amendment to an exempt study that may change the exempt status of the research or require limited IRB review • Requests for R&D Committee review of an amendment that may significantly impact the institution 13

Amendments 14

Amendments to Research under the Sole Oversight of the R&D Committee • Amendments to approved research must be submitted to the R&D Committee for approval (VHA Directive 1200. 01 paragraph 9 d(3)). • Amendments to studies that were initially eligible for designated review can be reviewed by designated review*. Examples include: • Exempt human subjects research protocols • Protocols that do not involve human subjects, biosafety level (BSL-3) or higher containment, use of select agents or nonexempt quantities of select toxins *See VHA Directive 1200. 01 para 9 e for complete list of activities that can be approved by designated review. 15

Can an R&D Committee Chair or Designated Member of the R&D Committee Approve Research Through the Designated Review Process? Yes. VHA Directive 1200. 01, Paragraph 9. e. allows the R&D Committee to review the activities defined in the applicable ORD policy using a designated review process. However, only the R&D Committee Chair or a voting member designated by the Chair can review and approve the activity on behalf of the R&D committee. If the research study is eligible for designated review at initial approval, subsequent actions that are required to be approved by the R&D Committee on the study, to include approval of amendments and continuing reviews, for studies overseen solely by the R&D Committee can also be approved by designated review. VHA Directive 1200. 01, Research and Development Committee: FAQ #22 16

When Does the Designated R&D Committee Reviewer Have to Report the Review Approval to the R&D Committee? • Final initial R&D Committee approval of research by designated review must be reported to the full R&D Committee at its next convened meeting and noted in the minutes (VHA Directive 1200. 01, Paragraph 9. b. (1)). • The R&D Committee’s standard operating procedures (SOPs) should specify how approvals by designated review are communicated to the rest of the committee and the timeframe for communication. • While ORD policy does not currently require reporting of other designated review approvals, such as exempt research, to the convened R&D Committee, ORD recommends that all designated review approvals be reported to the R&D Committee and noted in the minutes. • ORD does not prescribe a specific method for VA facilities to report designated review approvals to the R&D Committee. However, ORD recommends that a minimum of the name of the research study, name of Principal Investigator, the type of designated review action (e. g. , exempt research approval; final approval after ISSO and PO reviews), and date of designated review approval be included in the information reported to the R&D Committee. VHA Directive 1200. 01, Research and Development Committee: FAQ #23 17

When is ACOS/R&D Notification Required for R&D Committee Actions? After the research project has been granted final approval by the R&D Committee, the ACOS/R&D is responsible for notifying investigators, in writing that the research project can be initiated, and the period for which the project is approved (VHA Directive 1200. 01, Paragraph 5. g. (2)). Local policy will dictate timeframes and format for that notification. Does the ACOS/R&D have to notify investigators of approvals of amendments and continuing reviews by the reviewing subcommittees or committees? • No. There is no ORD policy requirement for notification by the ACOS/R&D of subsequent subcommittee or committee actions, such as approval of amendments or continuing reviews, in addition to the notifications sent by the reviewing subcommittees or committees. If a study is not approved by the R&D Committee is the ACOS/R&D required to send a letter to the investigator? • No. There is no ORD policy requirement for the ACOS/R&D to notify the investigator in addition to the R&D Committee’s notification of the study disapproval (VHA Directive 1200. 01, Paragraph 9. b. (3). VHA Directive 1200. 01, Research and Development Committee: FAQs #16 -18 18

For Research Under the Sole Oversight of the R&D Committee, is the R&DC Required to Approve Changes to Investigators? Yes. It is the responsibility of the overseeing committee to ensure the availability of qualified research team members, including investigators, who can conduct the approved research. Changes to investigators, to include Principal Investigators/co-investigators/subinvestigators, constitute amendments to approved research. Amendments to approved research under the sole oversight of the R&D Committee must be submitted to the R&D Committee for approval prior to implementation. VHA Directive 1200. 01, Research and Development Committee: FAQ #24 19

R&D Committee Responsibilities when Reviewing and Approving Research Reviews by the R&D Committee and its subcommittees must ensure the following: (1) Relevance of the research to VA’s mission and the care of Veterans. (d) Security of VA Data and VA sensitive information and storage of data and specimens in accordance with all applicable requirements. (a) Protection of human subjects (including privacy and confidentiality), and the implementation of adequate safety measures for research subjects and personnel. e) Scientific merit of the research proposal. (b) Welfare of and appropriate use and care of animals in research. (f) Availability of required resources, investigator’s time, and appropriate location where the research will be conducted. (c) Security of research laboratories. This includes laboratories utilizing or storing hazardous agents, for example, select agents and toxins, or other hazardous agents, and the security of all BSL-3 research laboratories. (g) Availability of qualified research team members, including investigators, who can conduct the approved research, and prove successful completion of all relevant research -related training requirements. 20 VHA Directive 1200. 01 para 12 a

For Research Under the Sole Oversight of the R&D Committee, is the R&DC Required to Approve Changes to Study Team Members Who are Not Investigators? Yes, if the names of the study team members are named in the protocol, information sheet received by VA subjects or advertisement materials. Changes in study team members who are not investigators on committee applications are not required by ORD policy to be approved by the R&D Committee prior to that individual being permitted to work on the study. Changes in personnel are required to be reported to the R&D Committee annually as part of the R&D Committee’s continuing review requirements (VHA Directive 1200. 01, Paragraph 9. d. (2)(a)(3)). R&D Committee SOPs must specify when such changes in study team members are to be submitted and how they are reviewed. 21

For Research Under the Sole Oversight of the R&D Committee, is the R&DC Required to Approve Changes to Study Team Members Who are Not Investigators (continued)? However, any study team member cannot be a VA study member unless they have the required appointment, qualifications, and training. For example, a VA Investigator cannot add a study team member as a member of the VA research team who does not have a VA appointment. VHA Directive 1200. 02, Paragraph 14. a. (7) requires the VA Principal Investigator to ensure that all research staff are qualified (including but not limited to appropriate training, education, expertise, and credentials) to perform procedures assigned to them during the course of the research. Written procedures must be in place to ensure that all research personnel hold an official VA appointment from HRMS (as a compensated, full-time or part time employee, a WOC, or under an IPA) prior to conducting or being involved in any way in VA research activities, and that the individuals maintain their appointment while conducting or being involved in any way in any VA research activities. VHA Directive 1200. 01, Research and Development Committee: FAQ #25 22

R&D Committee vs. Administrative Review of Changes to Approved Research Local SOPs should describe changes that require R&D Committee approval vs. changes that can be reviewed administratively • • Examples of changes that require approval by the R&D Committee: • Changes to the protocol and research plan • Changes to recruitment materials • Changes to questionnaires/instruments/surveys/interviews • Changes to investigators and study team members named in approved documents • Changes involving data sources, data systems; waivers of HIPAA authorization Examples of changes that can be reviewed administratively • Administrative changes – mailing address; telephone numbers, etc. • Changes to study team members that are not investigators and/or not named in the protocol or information sheet 23

R&D Committee Sample Tools: Amendments • R&D Committee Amendment Application • Non-Veteran Application • R&D Committee Amendment Reviewer Form 24

Continuing Review 25

Research under the Sole Oversight of the R&D Committee: Continuing Review • Investigators are responsible for preparing and submitting information, at least annually or as otherwise required, on all research projects to the appropriate R&D Committee subcommittee or the R&D Committee for continuing review (CR) (VHA Directive 1200. 01 para 5 l(5)). • If the study is under the oversight of another subcommittee/external committee, the R&D Committee is not responsible for continuing review of the study, even if continuing review is not required by that subcommittee/external committee. • Studies under the oversight of an IRB that are subject to the 2018 Requirements/Revised Common Rule and do not require continuing review by the IRB do not require continuing review by the R&D Committee. • Exempt studies that are under the oversight of an exempt subcommittee 26 would not require continuing review by the R&D Committee.

Continuing Review by the R&D Committee: Required Documentation • The following information must be received and reviewed by the R&D Committee: • Scientific progress of the research. • Budget requirements changes impacting the VAMC budget. • Changes in requirements for space, personnel, equipment, and supplies impacting the VAMC. • Summary and impact of any unanticipated problems. • Any issues of serious non-compliance with applicable policies, including privacy and security that have occurred since last approval. (VHA Directive 1200. 01 para 9 d(2)(a)) • Continuing review of studies that were initially eligible for designated review can be reviewed by designated review 27

R&D Committee Sample Tools: Continuing Review • R&D Committee Continuing Review Application • R&D Committee Continuing Review Checklist 28

Continuing Review by the R&D Committee: Approval Period • At initial/final approval, the R&D Committee must set the time frame for continuing review of studies under the sole oversight of the R&D Committee, which may not exceed 365 days. • Thereafter, continuing review must occur annually • Local SOPs should specify how “annually” is defined and how the approval date is calculated for protocols reviewed by the convened committee vs. designated review. • For approval by designated review, the date of approval is the date of final approval by the designated reviewer once all changes have been made (VHA Directive 1200. 01 para 9 d(1)(d)). 29

Lapses/Expiration of R&D Committee Approval • Expiration of R&D Committee approval can occur for multiple reasons. R&D Committee does not approve continuation of the study by the expiration date because: • CR was not submitted in time • CR was not approved in time • CR was approved with modifications and modifications were not received/reviewed prior to expiration date… • If approval expires, the R&D Committee must notify the investigator of the expiration of approval and all study activities must stop, including any data analyses. 30

Lapses/Expiration of R&D Committee Approval (continued) • Local SOPs should define procedures for dealing with lapses/expiration of approval • Typical things to include in SOPs: • When does a study lapse? • What must the PI do if the study lapses? • What must the PI do to resume study activities? • Time frame by which certain actions must be done to avoid further actions? • Lapse in approval is not a reportable event to ORO 31

What is Required of the R&D Committee and the PI if there is a Lapse in Approval for Studies that are under the Sole Oversight of the R&D Committee? A lapse of R&D Committee approval is an expiration of study approval. For studies requiring R&D Committee continuing review, the time frame for R&D Committee approval cannot exceed 365 days (VHA Directive 1200. 01, Paragraph 9. d. (1)(d)). When a study has lapsed R&D Committee approval, the study no longer has any institutional approval to be conducted by the VA Investigators. A study cannot be conducted without approval. 32

What is Required of the R&D Committee and the PI if there is a Lapse in Approval for Studies that are under the Sole Oversight of the R&D Committee (continued)? • The R&D committee must have written policies and procedures for recurring processes (VHA Directive 1200. 01, Paragraph 6. 3. ). For continuing review, the R&D Committee must have written policies and procedures for conducting continuing review, including processes for investigator submission and R&D Committee review procedures. • If approval expires for a study under sole oversight of the R&D Committee, the R&D Committee must notify the investigator that R&D Committee approval has expired and all study activities must stop, including any data analyses. • Any requirements for continuing review submission or review of received materials must be completed before approval can be obtained following expiration of the study’s approval. Once approval is obtained, the study continuation written notification is sent to the 33 PI.

What is Required of the R&D Committee and the PI if there is a Lapse in Approval for Studies that are under the Sole Oversight of the R&D Committee (continued)? • If the requirements for continuing review submission or review are not completed, the study must be closed. The R&D Committee should include in its written policies and procedures time frames by which additional actions such as study closure will occur following expiration of study approval. • ORD wishes to emphasize that R&D Committee written policies and procedures should be put in place to prevent lapses in study approval when the R&D Committee is the sole oversight committee. When a study lapse occurs, the R&D Committee should evaluate the root cause of the lapse in study approval, and if appropriate, initiate correction actions and/or revise policies and procedures to remediate or prevent lapse of R&D Committee continuing review approvals. VHA Directive 1200. 01, Research and Development Committee: FAQ #26 34

Lapses in R&D Committee Continuing Review: SOP Excerpt 35

Lapses in R&D Committee Continuing Review: SOP Excerpt (continued) Courtesy of Providence VA R&D Committee SOP 36

Closing Thoughts 37

Contact Information for Panelists Carole L. Palumbo, Ph. D. Mieke Verfaellie, Ph. D. Deputy Associate Chief of Staff for Research & Development Senior Research Career Scientist Director, Human Research Protections Program R&D Co-Chair VA Boston Healthcare System E-mail: Carole. Palumbo@va. gov E-mail: Mieke. Verfaellie@va. gov Douglas W. Miller Research Committee Coordinator VA Pacific Islands Health Care System E-mail: Douglas. Miller@va. gov 38

Questions 39

Availability of Recording • A recording of this session and the associated handouts will be available on ORPP&E’s Education and Training website approximately one week post-webinar • An archive of all ORPP&E webinars can be found here: https: //www. research. va. gov/programs/orppe/education /webinars/archives. cfm 40

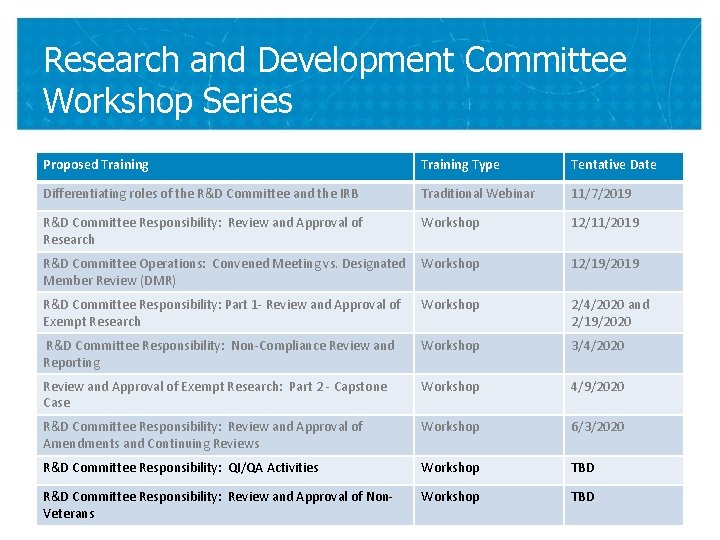
Research and Development Committee Workshop Series Proposed Training Type Tentative Date Differentiating roles of the R&D Committee and the IRB Traditional Webinar 11/7/2019 R&D Committee Responsibility: Review and Approval of Research Workshop 12/11/2019 R&D Committee Operations: Convened Meeting vs. Designated Member Review (DMR) Workshop 12/19/2019 R&D Committee Responsibility: Part 1 - Review and Approval of Exempt Research Workshop 2/4/2020 and 2/19/2020 R&D Committee Responsibility: Non-Compliance Review and Reporting Workshop 3/4/2020 Review and Approval of Exempt Research: Part 2 - Capstone Case Workshop 4/9/2020 R&D Committee Responsibility: Review and Approval of Amendments and Continuing Reviews Workshop 6/3/2020 R&D Committee Responsibility: QI/QA Activities Workshop TBD R&D Committee Responsibility: Review and Approval of Non. Veterans Workshop TBD 41

Important Links • Revised Common Rule (published January 19, 2017) • Pages 7259 to 7274 contain the Text of the Final Rule • VHA Directive 1200. 05 • VHA Directive 1200. 01 • ORD Policies and Guidance Documents • ORPP&E Cyberseminars • ORPP&E’s Single IRB webpage • VA Innovation and Research Review System (VAIRRS) 42